Effect of Cu Coating on Microstructure and Properties of Al/Steel Welding–Brazing Joints Obtained by Cold Metal Transfer (CMT)
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Electroplating Method
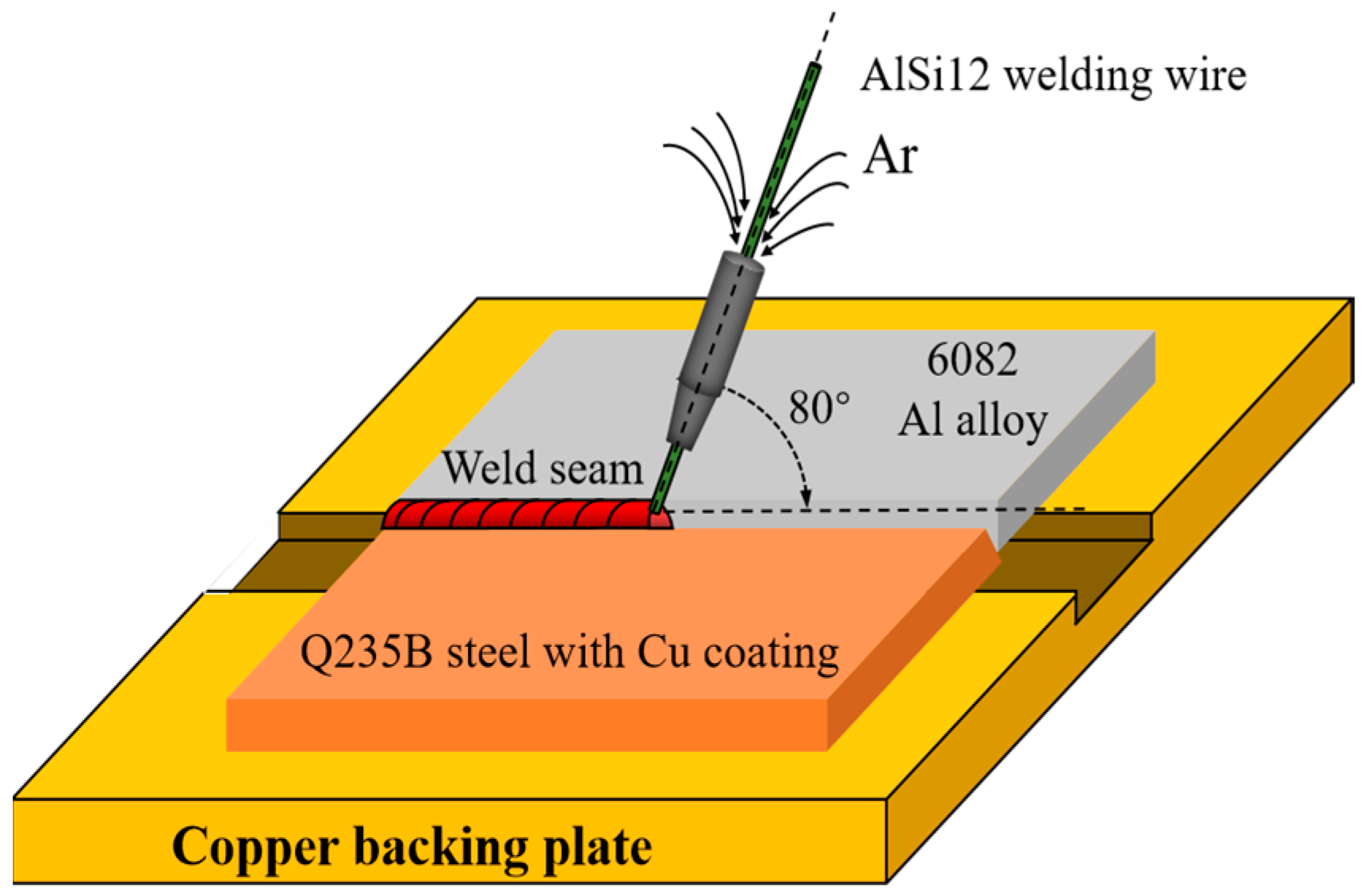
2.2. CMT Welding–Brazing Process
2.3. Characterization Methods
2.4. Electrochemical Measurements
3. Results and Discussion
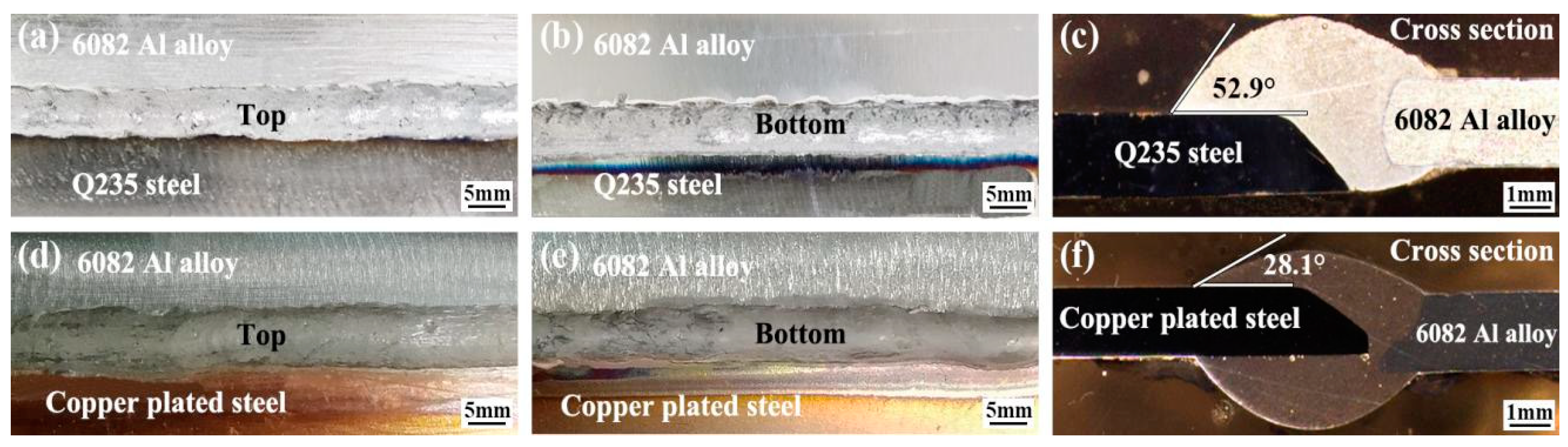
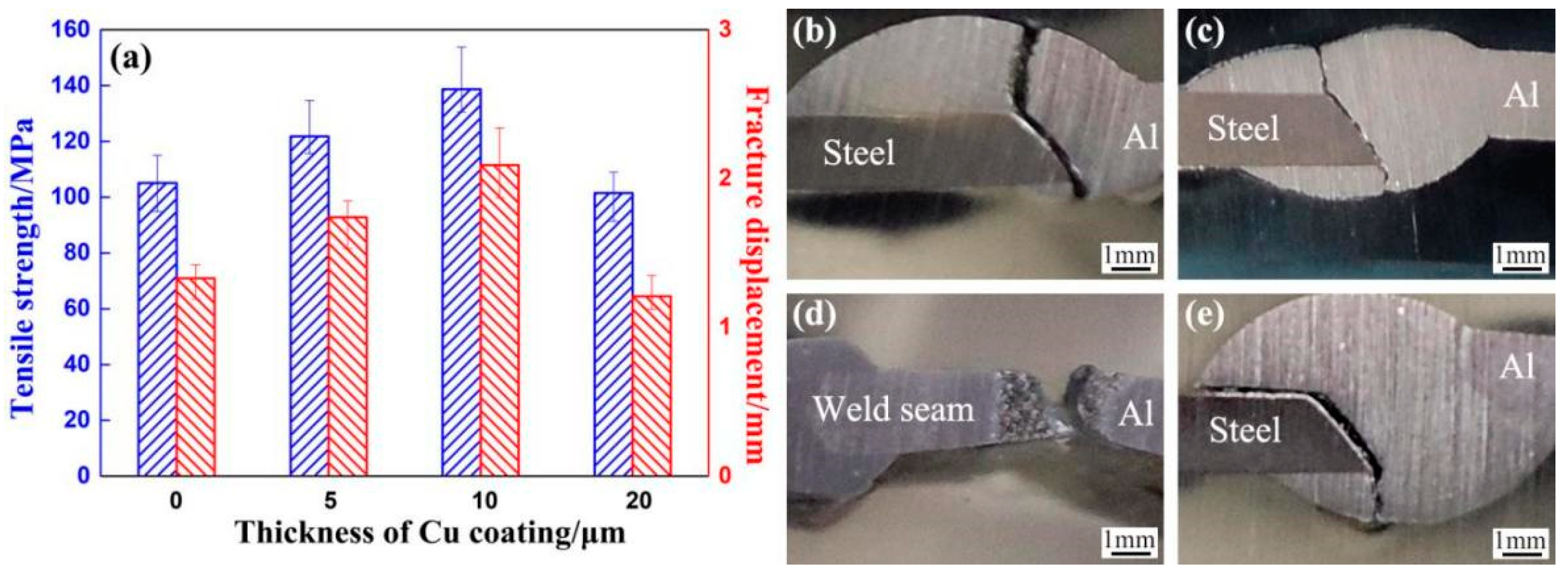
3.1. Effect of Cu Coating on Macro-Morphology of Al Alloy/Steel CMT Welding–Brazing Joints
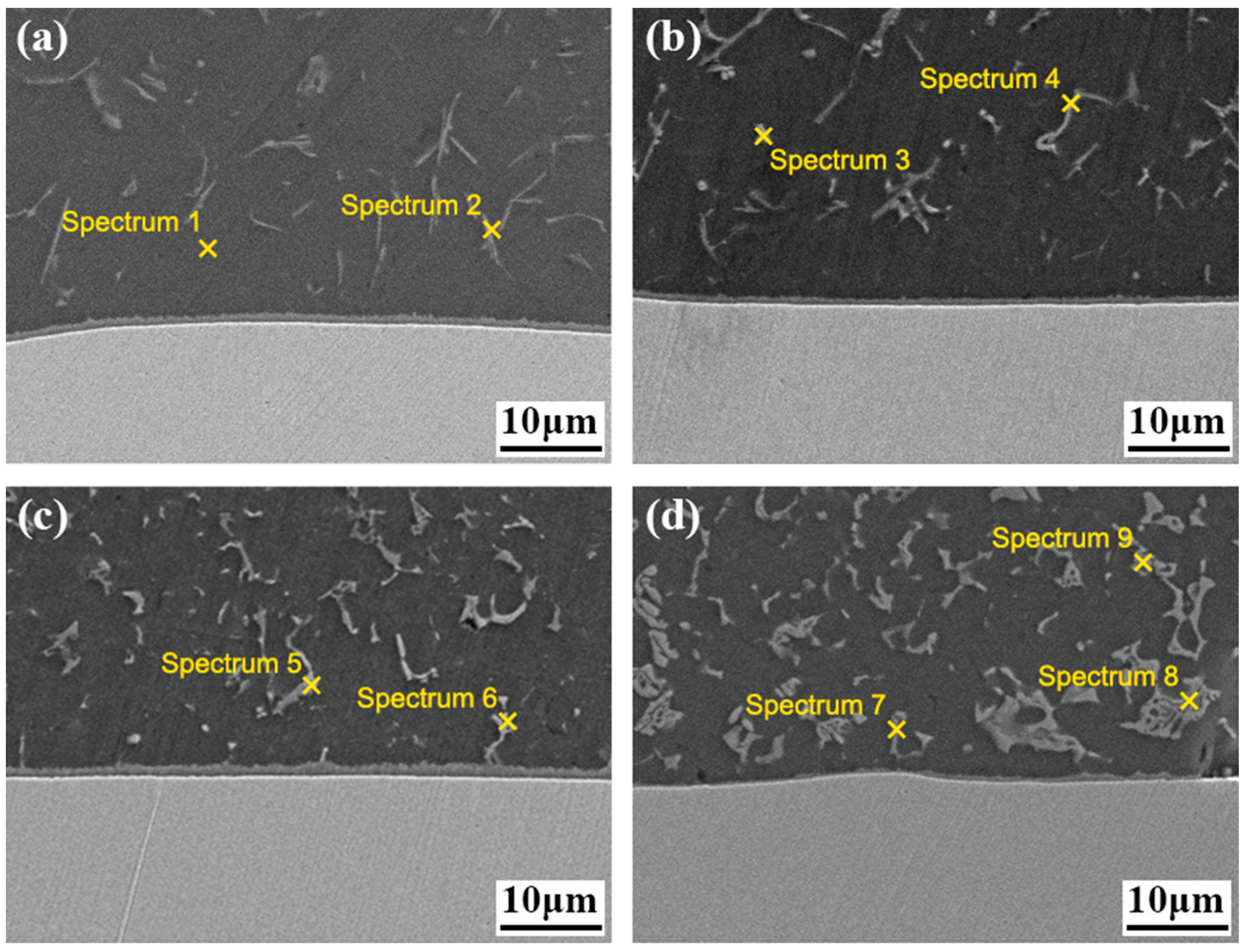
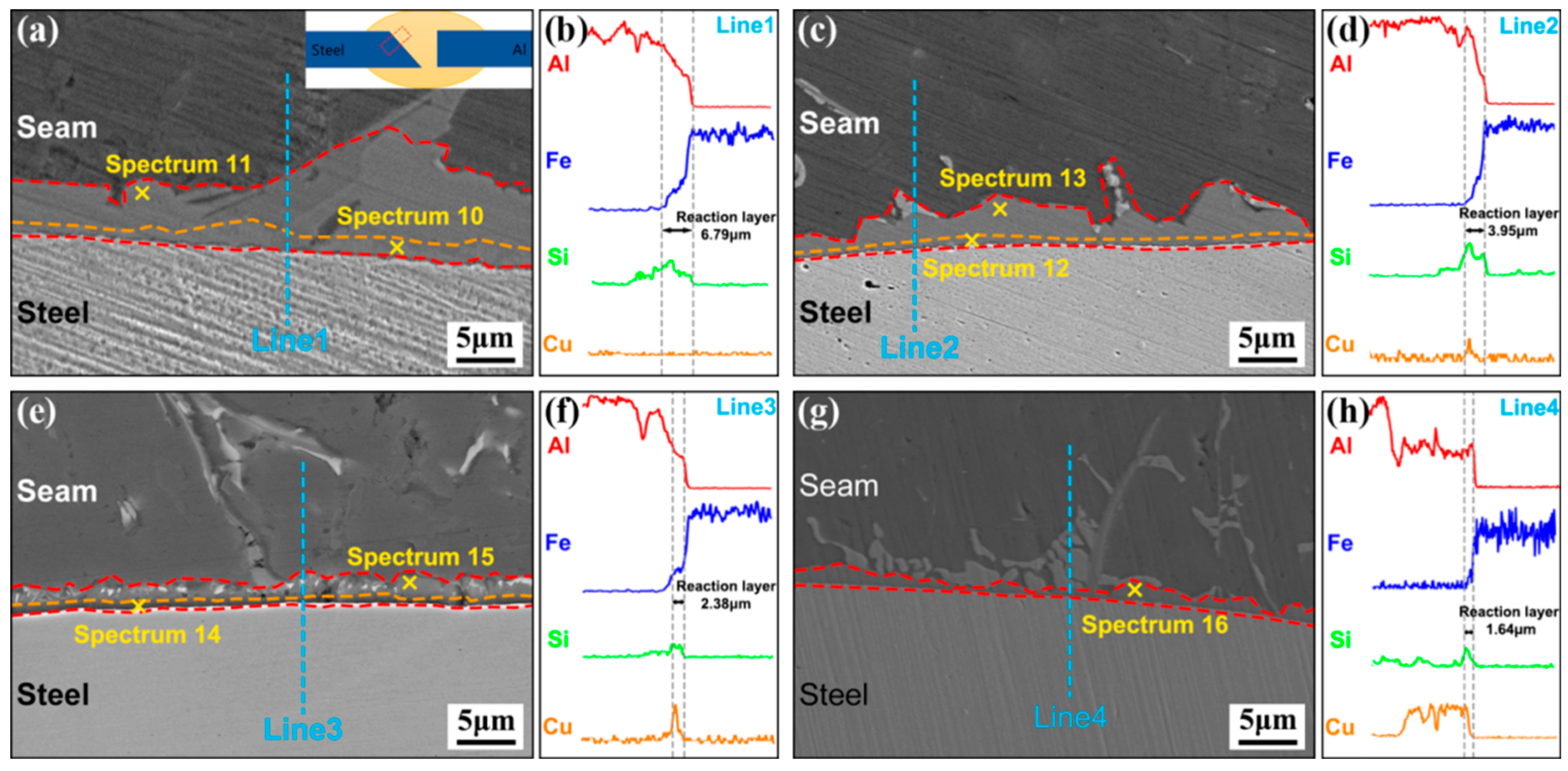
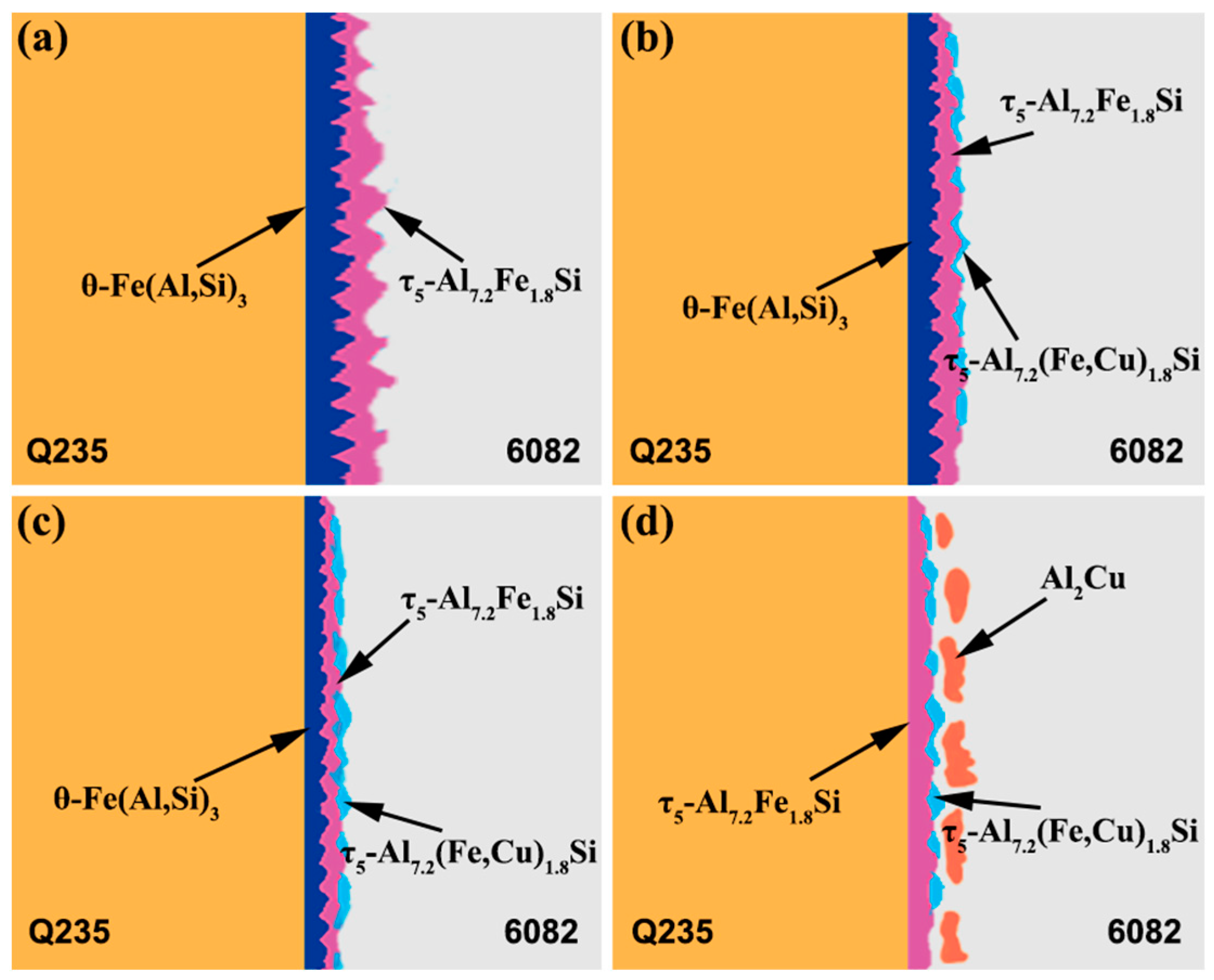
3.2. Influence of Cu Coating on Microstructure and Interface Reaction Layer Composition of Al Alloy/Steel CMT Welding–Brazing Joints
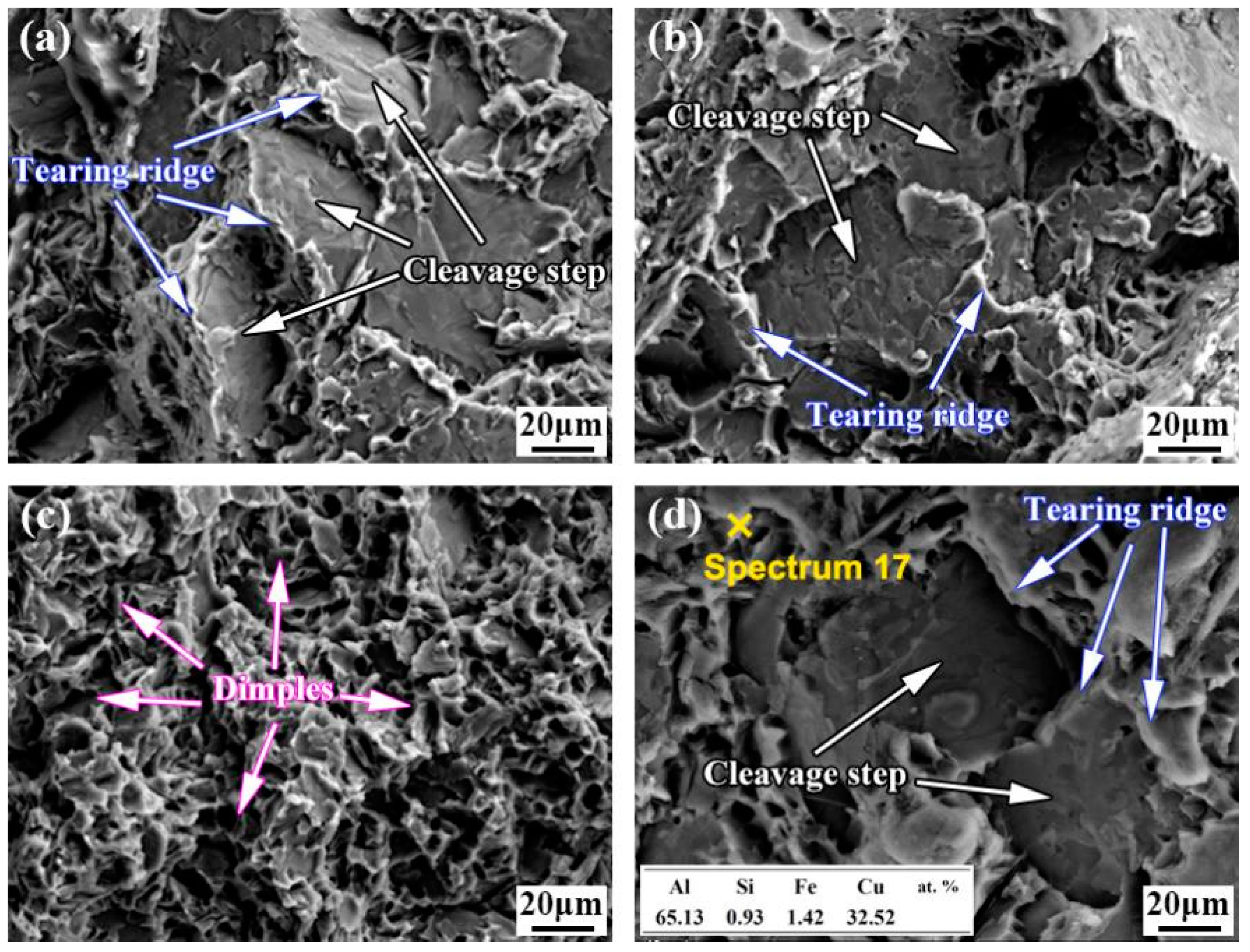
3.3. Effect of Cu Coating on Tensile Properties of Al Alloy/Steel CMT Welding–Brazing Joints
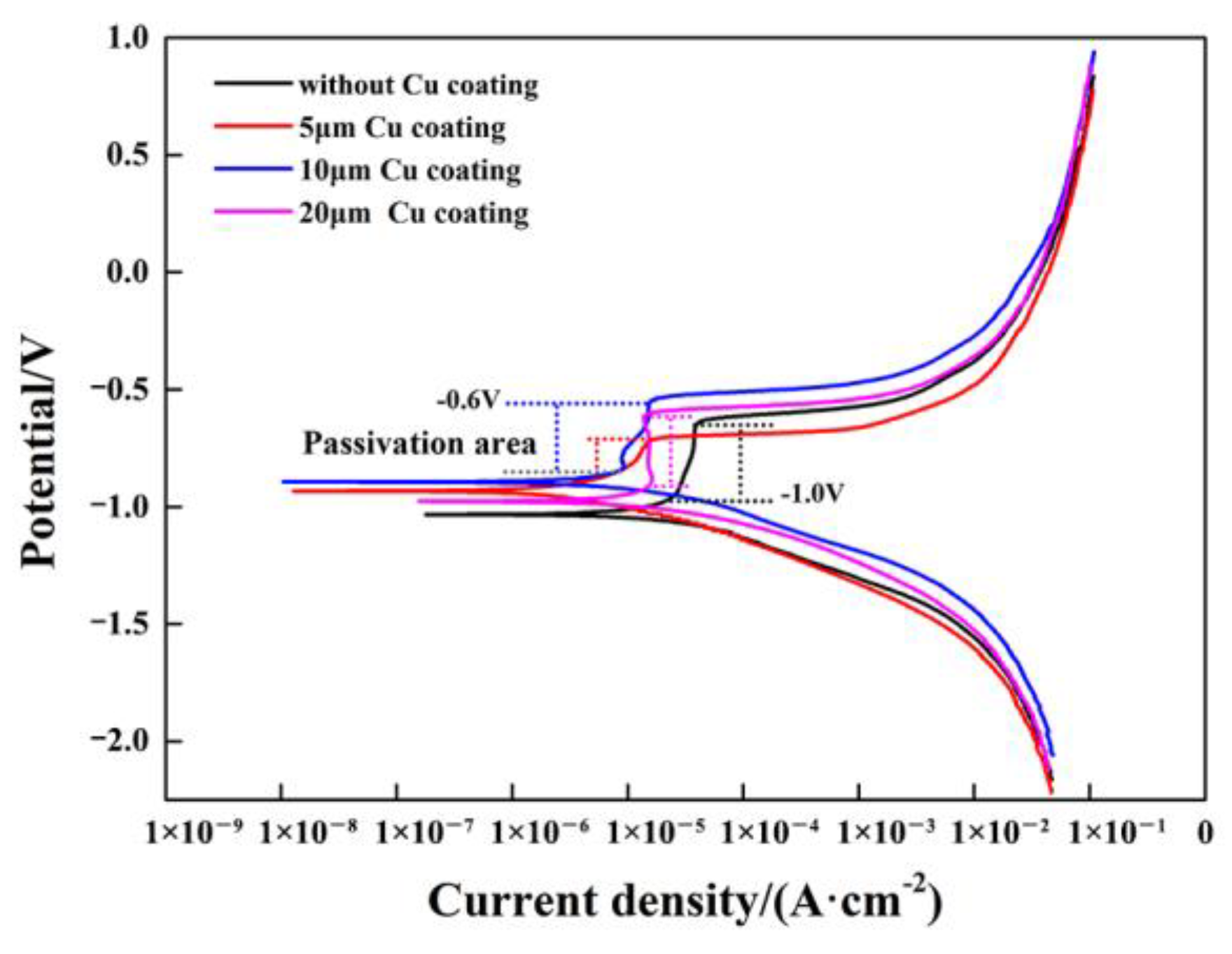
3.4. Effect of Cu Coating on Corrosion Resistance of Al Alloy/Steel CMT Welding–Brazing Joints
4. Conclusions
- A Cu coating with an appropriate thickness could improve the wettability and spreadability of AlSi12 on the surface of steel, which resulted in the excellent formation of Al alloy/steel CMT welding–brazing joints.
- The addition of the Cu element could inhibit the reaction of Al and Fe and reduce the thickness of interfacial brittle IMCs. When the Cu coating thickness was no more than 10 μm, the microstructure of the Al alloy/steel joint was composed of θ-Fe(Al,Si)3, τ5-Al7.2Fe1.8Si and τ5-Al7.2(Fe,Cu)1.8Si at the interfacial reaction layer and α-Al solid solution and τ5-Al7.2Fe1.8Si in the welded seam. As the Cu coating thickness increased to 20 μm, θ-Fe(Al,Si)3 disappeared and Al2Cu appeared in the joint.
- With the increase in the Cu coating on the surface of the steel, the thickness of the IMCs decreased and the content of brittle θ-Fe(Al,Si)3 was reduced, which improved the tensile strength and corrosion resistance of the Al/steel joints. When the thickness of the Cu coating on the surface of steel was 10 μm, the tensile strength of the Al/steel joint reached the maximum of 138.7 MPa and the corrosion resistance of the joint reached the optimal level. However, when the thickness of the Cu coating increased to 20 μm, the IMC thickness reached the minimum, but Cu reacted with Al to form a brittle Al2Cu phase under high Cu content, which deteriorated the tensile strength and corrosion resistance of the joint.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, T.J.; Dong, F.L.; Ding, F.J.; Zhao, J.B.; Wang, S.L. Application of aluminum alloy in automotive lightweight. Hot Work. Technol. 2020, 49, 1–6. [Google Scholar]
- Agudo, L.; Eyidi, D.; Schmaranzer, C.H.; Arenholz, E.; Jank, N.; Brucker, J.; Pyzalla, A.R. Intermetallic FexAly-phases in a steel/Al-alloy fusion welds. J. Mater. Sci. 2007, 42, 4205–4214. [Google Scholar] [CrossRef]
- Hao, D.S.; Zhang, H. Application and development of alloy elements in Aluminum/Steel welding. J. Mater. Sci. 2021, 50, 1–5. [Google Scholar]
- Wang, H.Y.; Feng, B.Q.; Song, G.; Liu, L.M. Laser-arc hybrid welding of high-strength steel and aluminum alloy joints with brass filler. Mater. Manuf. Processes 2017, 33, 735–742. [Google Scholar] [CrossRef]
- Yang, X.D.; Shi, Y.; Liu, J. Effect of Cu foil on laser butt welding quality of Aluminum/Steel dissimilar metals joint. J. Mech. Eng. 2014, 50, 143–149. [Google Scholar] [CrossRef]
- Saleh, M.I.; Khan, T.I.; Roven, H.J. Transient liquid phase bonding of AA-6063 to UNS S32304 using Cu interlayer. Procedia Chem. 2016, 19, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H.; Chen, Y.H.; Kang, J.D.; Amirkhiz, B.S.; Nadeau, F. Friction stir lap welding of aluminum alloy to advanced high strength steel using a cold-spray deposition as an interlayer. Mater. Lett. 2018, 239, 212–215. [Google Scholar] [CrossRef]
- Mathieu, A.; Shabadi, R.; Deschamps, A.; Suery, M.; Matteï, S.; Grevey, D.; Cicala, E. Dissimilar material joining using laser (aluminum to steel using zinc-based filler wire). Opt. Laser Technol. 2007, 39, 652–661. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Yazdian, N.; Wang, H.P.; Carlson, B.; Kovacevic, R. Effect offiller wire composition on performance of Al/galvanized steeljoints by twin spot laser welding-brazing method. J. Manuf. Process. 2018, 31, 20–34. [Google Scholar] [CrossRef]
- Xia, H.B.; Zhao, X.Y.; Tan, C.W.; Chen, B.; Song, X.G.; Li, L.Q. Effect of Si content on theinterfacial reactions in laser welded-brazed Al/steel dissimilarbutted joint. J. Mater. Process. Technol. 2018, 258, 9–21. [Google Scholar] [CrossRef]
- Shi, L.T.; Kang, J.D.; Shalchi-Amirkhiz, B.; Sigler, D.R.; Haselhuhn, A.S.; Carlson, B.E. Effect of coatingtype on microstructure and mechanical behavior of resistancespot welds of thin X626 aluminum sheet to low carbon steel. J. Mater. Process. Technol. 2019, 264, 438–447. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.K.; Jin, Q.S. Research status of welding-brazing of aluminum/steel dissimilar metals. Electr. Weld. Mach. 2018, 48, 109–114. [Google Scholar]
- Wu, S.H.; Chen, S.j. Study on Welding of Steel/Aluminum Dissimilar Metal. Hot Work. Technol. 2012, 41, 170–173. [Google Scholar]
- Chen, Y.L.; Qi, Y.F.; Liu, X. Microstructure and mechanical properties of AlSiCu10-10 filler meter brazing 6063 aluminum alloy. China Weld. 2018, 27, 52–56. [Google Scholar]
- Song, J.L.; Lin, S.B.; Yang, C.L.; Ma, G.C. Analysis of intermetallic layer in dissimilar TIG welding-brazing butt joint of aluminium alloy to stainless steel. Sci. Technol. Weld. Join. 2010, 15, 213–218. [Google Scholar] [CrossRef]
- Pu, J.; Wu, M.F.; Yuan, Y. Study of microstructure and grain boundary penetration behavior in 6063 Al alloy contact reactive brazing. Rare Metal. Mat. Eng. 2007, S3, 168–171. [Google Scholar]
- Song, J.L.; Lin, S.B.; Yang, C.L.; Ma, G.C. Characteristics of precoating TIG welding-brazing joint of aluminum alloy to stainless steel. Chin. J. Nonferrous Met. 2009, 19, 1209–1215. [Google Scholar]
- Lin, S.B.; Song, J.L.; Yang, C.L.; Ma, G.C. Micrstructure analysis of interfacial layer with tungsten inert gas welding-brazing joint of aluminum alloy/stainless steel. Acta Metall. Sin. 2009, 45, 1211–1216. [Google Scholar]
- Kang, M.J.; Kim, C. Joining Al 5052 alloy to aluminized steel sheet using cold metal transfer process. Mater. Des. 2015, 81, 95–103. [Google Scholar] [CrossRef]
- Schubert, E.; Klassen, M.; Zerner, I.; Walz, C.; Sepold, G. Light-weight structures produced by laser beam joining for future applications in automobile and aerospace industry. J. Mater. Process. Technol. 2001, 115, 2–8. [Google Scholar] [CrossRef]
- Kang, D.H.; Lee, H.W. Study of the correlation between pitting corrosion and the component ratio of the dual phase in duplex stainless steel welds. Corros. Sci. 2013, 74, 396–407. [Google Scholar] [CrossRef]
- Gu, Y.F.; Li, J.; Shi, Y.; Huang, J.K.; Fan, D. Corrosion property of arc welding brazed joint between aluminum and steel. Chin. J. Nonferrous Met. 2016, 26, 758–765. [Google Scholar]
- Tan, L.; Allen, T.R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Zhang, M.X.; Guan, Y.Q.; Zheng, Y.P. The corrosion behaviors of ADC12 aluminum alloy in vapour. J. Chin. Soc. Corros. Prot. 2002, 4, 229–232. [Google Scholar]

| Elements | Cr | Mn | C | Mg | Si | Ni | Ti | Zn | Fe | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 6082 | - | 0.4 | - | 0.1 | 0.8 | - | 0.15 | 0.2 | 0.5 | Bal. |
| Q235B | 0.7 | 0.2 | 0.2 | - | 0.2 | 0.02 | - | - | Bal. | - |
| ER4047F | - | 0.15 | - | 0.1 | 12.15 | - | 0.1 | 0.2 | 0.21 | Bal. |
| Parameters | Value |
|---|---|
| Shielding gas flow, L/min | 18 |
| Wire feeding speed, m/min | 5.5 |
| Welding speed, mm/min | 350 |
| Welding voltage, V | 12.1 |
| Welding current, A | 107 |
| Test Point | Element Ratio (at.%) | Possible Phase | |||
|---|---|---|---|---|---|
| Al | Si | Fe | Cu | ||
| 1 | 97.23 | 0.92 | 1.85 | — | α-Al |
| 2 | 69.15 | 11.43 | 19.42 | — | τ5-Al7.2Fe1.8Si |
| 3 | 97.02 | 1.32 | 0.69 | 0.97 | α-Al |
| 4 | 70.08 | 10.63 | 13.97 | 5.32 | τ5-Al7.2(Fe,Cu)1.8Si |
| 5 | 98.01 | 0.85 | 0.23 | 0.91 | α-Al |
| 6 | 72.05 | 8.67 | 11.33 | 7.95 | τ5-Al7.2(Fe,Cu)1.8Si |
| 7 | 97.01 | 0.98 | 0.21 | 1.8 | α-Al |
| 8 | 58.54 | 1.11 | 1.37 | 38.98 | Al2Cu |
| 9 | 73.40 | 7.45 | 8.26 | 10.89 | τ5-Al7.2(Fe,Cu)1.8Si |
| 10 | 63.28 | 10.34 | 26.38 | — | θ-Fe(Al,Si)3 |
| 11 | 73.54 | 10.17 | 16.29 | — | τ5-Al7.2Fe1.8Si |
| 12 | 62.12 | 9.28 | 28.47 | 0.13 | θ-Fe(Al,Si)3 |
| 13 | 71.32 | 10.41 | 17.44 | 0.83 | τ5-Al7.2(Fe,Cu)1.8Si |
| 14 | 62.26 | 11.81 | 25.44 | 0.49 | θ-Fe(Al,Si)3 |
| 15 | 75.34 | 8.58 | 15.23 | 0.85 | τ5-Al7.2(Fe,Cu)1.8Si |
| 16 | 69.81 | 9.23 | 20.01 | 0.95 | τ5-Al7.2(Fe,Cu)1.8Si |
| Cu Coating/μm | Potential/V | Current Density/(A·cm−2) |
|---|---|---|
| 0 | −1.0802 | 4.09 × 10−5 |
| 5 | −1.038 | 4.58 × 10−6 |
| 10 | −0.933 | 4.21 × 10−6 |
| 20 | −1.0708 | 7.34 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wu, M.; Pu, J.; Shan, Q.; Sun, Y.; Wang, S.; Hermann, S.K.U.G. Effect of Cu Coating on Microstructure and Properties of Al/Steel Welding–Brazing Joints Obtained by Cold Metal Transfer (CMT). Coatings 2022, 12, 1123. https://doi.org/10.3390/coatings12081123
Zhang C, Wu M, Pu J, Shan Q, Sun Y, Wang S, Hermann SKUG. Effect of Cu Coating on Microstructure and Properties of Al/Steel Welding–Brazing Joints Obtained by Cold Metal Transfer (CMT). Coatings. 2022; 12(8):1123. https://doi.org/10.3390/coatings12081123
Chicago/Turabian StyleZhang, Chao, Mingfang Wu, Juan Pu, Qi Shan, Yubo Sun, Shuiqing Wang, and Seseke Koyro Ulrich Georg Hermann. 2022. "Effect of Cu Coating on Microstructure and Properties of Al/Steel Welding–Brazing Joints Obtained by Cold Metal Transfer (CMT)" Coatings 12, no. 8: 1123. https://doi.org/10.3390/coatings12081123
APA StyleZhang, C., Wu, M., Pu, J., Shan, Q., Sun, Y., Wang, S., & Hermann, S. K. U. G. (2022). Effect of Cu Coating on Microstructure and Properties of Al/Steel Welding–Brazing Joints Obtained by Cold Metal Transfer (CMT). Coatings, 12(8), 1123. https://doi.org/10.3390/coatings12081123






