Abstract
Manganese dioxide (MnO2)-based nanostructures are promising electrode materials for supercapacitors (SCs) due to their low cost, eco-friendly nature, and high theoretical capacitance. However, the conductivity of MnO2 is poor, which is a big problem when trying to achieve the desired capacitance value. Herein, hexagonal-phase MnO2 nanoparticles (NPs) are directly grown on a 3D conductive carbon cloth (CC) (denoted as MnO2-NPs@CC) as a binder-free electrode through a simple and scalable hydrothermal strategy. The results show that MnO2-NPs@CC with a large specific surface area and high porosity could be employed as a positive electrode material for high-performance SCs. Owing to these attractive properties, the MnO2-NPs@CC electrode delivers a high specific capacitance of 660 F/g at a current density of 2 A/g in 6 M KOH aqueous electrolytes. Moreover, the MnO2-NPs@CC electrode demonstrates excellent cycling stability with high capacitance retention of 92.8% over 10,000 cycles. Such remarkable findings suggest that MnO2-NPs@CC with enhanced electrochemical performance is a favorable electrode material for next-generation high-performance SCs.
1. Introduction
The rapidly growing technological and scientific requirements for renewable and green energy sources have drawn great attention to the design of ultramodern energy devices with higher specific energy and power density levels [1,2]. Supercapacitors (SCs) are attractive as energy storage devices owing to their high capability and long life span [3,4]. SCs have two major categories based on the charge storage mechanism: electrical double-layer capacitors (EDLCs) and pseudocapacitors (PCs). The EDLCs operate via the adsorption or desorption of electrolyte ions at the electrode–electrolyte interface [5,6,7]. On the other hand, to construct PCs, mostly transition metal oxides (TMOs) and conducting-polymer-based materials are used, which operate through Faraday redox reactions at the electrode surface, thereby exhibiting much higher energy density levels compared to EDLCs.
As compared to carbon-based materials [8] and conducting polymers [9], TMOs show remarkably high capacitance levels due to the rapid Faraday redox reactions; thus, TMOs are the best choice for electrode materials in SCs [10]. Furthermore, TMOs, such as NiO [11], Fe2O3 [12], RuO2 [13], Co3O4 [14], and MnO2 [15], have been widely explored for use in SCs. Among them, MnO2 has the characteristics of being eco-friendly, cost-effective, as well as having a high theoretical capacitance (~1380 F/g). Recently, different types of MnO2 crystal structures have been prepared and examined for SC applications. The electrochemical performance of such materials depends on the crystal structure, grain size, morphology, and surface area [16]. Out of the various polymorphs of MnO2, hexagonal MnO2 (δ-MnO2) has garnered much interest due to its layered architecture and excellent electrochemical performance [17,18]. The hexagonal δ-MnO2 can effectively improve the electrochemical performance due to the rapid charge transport within the nano-architecture during the charge or discharge process. For example, δ-MnO2/C has been synthesized and employed as an electrode for SCs, and displayed a specific capacitance of 520 F/g at 5 mV/s, with a remarkable cycling performance of 80.1% for 5000 cycles [19]. However, δ-MnO2 is limited in SC electrodes due to its bad cycling performance, low specific capacitance, and low conductivity (about 10−5–10−6 S/cm) [20,21]. The low surface area (due to MnO2 agglomeration) and low conductivity of MnO2 ultimately limits the specific capacitance of MnO2-based electrodes for SCs [22]. Enhancing the conductivity and maximizing the MnO2 utilization, as well as maintaining the MnO2 nanoparticles; (MnO2-NPs) unique morphology, have become the basic criteria in the design of electrodes for SCs with remarkable performance [23]. In addition, an efficient method used to enhance the conductivity of MnO2 is to directly grow in into conducting substrates and avoid the use of binders, which block the active sites and reduce the electrical conductivity [24].
Herein, we designed hexagonal-phase MnO2-NPs that were directly grown on a carbon cloth using a simple and scalable hydrothermal strategy with an appropriately controllable size, morphology, and large surface area, along with better conductivity as an SC electrode. MnO2-NPs incorporated on CC can produce more electrochemically active sites, which can support the electron and ion transport in the charging–discharging process, thus providing excellent cycling performance. Furthermore, the MnO2-NPs contributing significantly to enhancing the capacitance. The present MnO2-NPs@CC as a positive electrode for SCs delivers an excellent gravimetric capacitance of 660 F/g at 2 A/g and retains 92.8% of the capacitance over 10,000 cycles. These excellent results indicate that MnO2-NPs@CC with boosted electrochemical properties is a promising cathode choice for next-generation SCs.
2. Experimental Section
2.1. Pre-Treatment of CC
The piece of CC was washed ultrasonically first in acetone, ethanol, and distilled water for 10 min to completely abolish the layer of oxides and oil stains on the CC. To dry the CCs, the cleaned CCs were then left for 12 h at 60 °C for further use.
2.2. Synthesis of MnO2-NPs@CC
The MnO2-NPs precursor was synthesized through a simple and scalable hydrothermal strategy, in which the CC was used as a substrate, and MnO2-NPs were grown on the CC surface. Figure 1 shows a schematic illustration of the fabrication process of MnO2-NPs. Initially, 3 mg of KMnO4 was dissolved in 30 mL of distilled water, then 10 mL of hydrochloric acid (HCl) was slowly added. Afterwards, the above solution was sonicated and a purple solution was obtained. The cleaned CC substrate was put with the side wall of the Teflon-lined stainless steel autoclave and the purple solution was moved into a 70 mL autoclave that was sealed and then left for hydrothermal reaction for 12 h in an oven at 135 °C. After cooling down, the resulting precipitate was washed many times with distilled water and dried for 12 h at 90 °C.
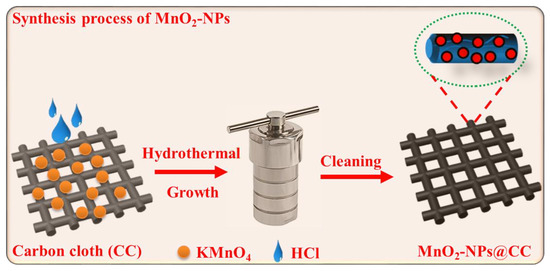
Figure 1.
Schematic representation of a typical synthesis procedure of MnO2-NPs@CC.
2.3. Physical Characterizations
The morphology of MnO2-NPs@CC was investigated using a field-emission scanning electron microscope (FESEM, HITACHI SU8220, Hillsboro, OR, USA). An X-ray diffractometer (X’Pert Pro Analytical) with monochromatic radiation (Cu-Kα; λ = 0.15406 nm, step speed 0.02°) was used to investigate the crystal structure. The Raman spectrum (HJY Lab RAM Aramics 70 France) with a wavelength of 514.5 nm was used to investigate the chemical bonds. The BET surface area and N2 adsorption–desorption were measured at 77.5 K using Micromeritics (ASAP2460, Version 5.12, Boynton Beach, FL, USA).
2.4. Electrochemical Characterization
The electrochemical performance investigations were conducted using an electrochemical work station (CHI 660E, Wuhan, China) in a three-electrode configuration in a 6 M KOH aqueous electrolyte solution. The as-prepared MnO2-NPs@CC measuring 1 × 1 cm2 (1.0 mg/cm2 mass loading density) was employed as the working electrode. The reference and counter electrodes were Ag/AgCl and platinum wire, respectively. The cyclic voltammetry (CV) was measured at a potential window range of 0.0–0.8 V, the galvanostatic charge–discharge (GCD) measurements were performed in the same voltage range (0.0–0.8 V) with diverse current rates (2 to 32 A/g), and the electrochemical impedance spectroscopy (EIS) was performed using a fresh electrode in the frequency range of 0.001 to 100 kHz in open-circuit conditions with an applied amplitude of 5 mV. ZSim-view software was used to simulate the electrical equivalence circuit.
3. Results and Discussion
The morphological architecture of the as-prepared MnO2-NPs@CC was investigated via FE-SEM. The low magnification FE-SEM images (Figure 2a,b) indicate that the interconnected nanoparticles with higher density and rough textures are uniformly distributed onto the cylindrical surface of every fiber of the CC. The high magnification FE-SEM image (Figure 2c) further confirms and provides the detailed architecture of the regular distribution of the well-established MnO2-NPs with an average size of 26 nm.
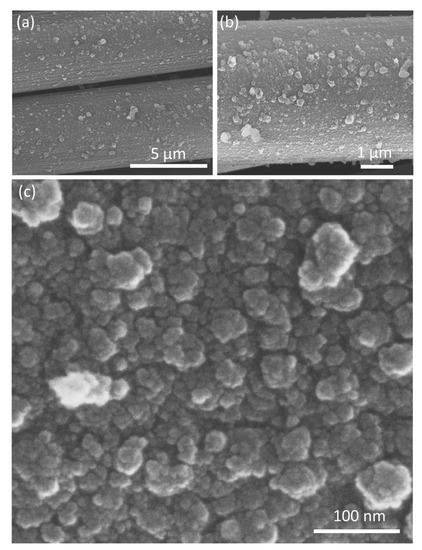
Figure 2.
FESEM images of MnO2-NPs@CC. (a,b) low- and (c) high-magnification.
The XRD spectrum of MnO2-NPs@CC is shown in Figure 3a. The characteristic peaks belong to the hexagonal phase of MnO2, with sharp and intense peaks that are well matched with JCPDS No. 71-0071, which shows the successful formation of hexagonal MnO2-NPs onto the CC. The broad residual peak that appears at about 2θ = 26.5° is related to the carbon cloth substrate, which corresponds to JCPDS No. 41–1487. Figure 3b shows the crystal structure of the as-prepared MnO2, in which every Mn atom is strongly bonded to six O-atoms and also connected to another Mn atom to form a hexagonal structure. The hexagonal structure with 2 × 2 tunnels is beneficial for fast ion and electron transfer and enhances the stability of the electrode [25].
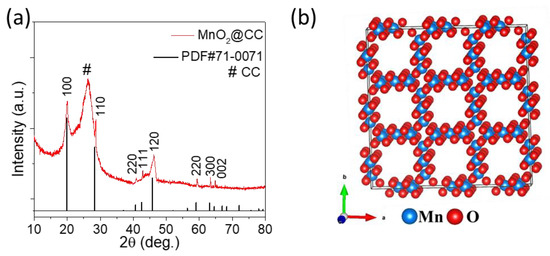
Figure 3.
(a) XRD spectrum of MnO2-NPs@CC. (b) Hexagonal crystal structure of MnO2 with 2 × 2 tunnels.
To further study the chemical bonds, Raman spectroscopy was performed, and Figure 4 exhibits the Raman spectrum of MnO2-NPs@CC. The location of the band at 647 cm−1 is associated with oscillations of the lattices of Mn-O and is consistent with the previous reports [26,27]. In addition, the Raman spectrum shows two more peaks corresponding to the higher-intensity G-band along with the lower-intensity D-band, which demonstrate the excellent graphitization degree of the CC substrate. In the present case, the G-band (1598 cm−1) is more intense than the D-band (3131 cm−1); thus, the homogeneous and dominant contents of the graphitic structure exist. The ID/IG ratio of MnO2-NPs@CC is 0.68, indicating that the carbon in the MnO2-NPs@CC is coupled with a partial graphitic layer and provides excellent conductive support to MnO2-NPs. Thus, the MnO2-NPs@CC electrode shows better conductivity and enhanced electrochemical performance [28].

Figure 4.
Raman spectrum of MnO2-NPs@CC.
The specific surface area of MnO2-NPs@CC was investigated via nitrogen (N2) sorption measurements. The adsorption–desorption isotherms of MnO2-NPs@CC show a type IV curve with a clear hysteresis loop in the of range 0.4–1.0 (P/Po), as shown in Figure 5a. The figure shows typical characteristics of capillary condensation due to the mesopores in MnO2-NPs@CC [29]. The surface area of the mesoporous MnO2-NPs@CC computed using the BET method was 29 m2/g; thus, MnO2-NPs@CC may show excellent electrochemical performance because of its large surface area, meaning it can offer a better active site for the occurrence of redox reactions. The BJH profile for porosity is illustrated in Figure 5b, where the mean size of the pores was estimated to be 12.46 nm, demonstrating the presence of large pores. This porous architecture is believed to be beneficial for storing more energy due to the mesoporous channels and large surface area being suitable for ion diffusion, which are the two main characteristics needed for electrode materials to achieve better electrochemical performance.
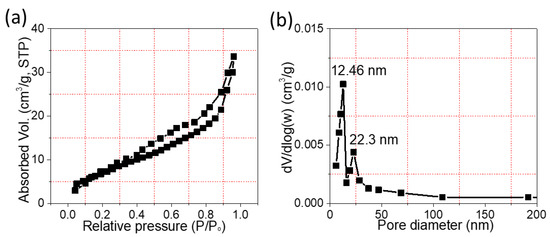
Figure 5.
(a) The N2 sorption isotherms of MnO2-NPs. (b) BJH profile of the pore size distribution.
The MnO2-NPs@CC was investigated as an electrode for SC, and the electrochemical behavior was examined in a 6 M potassium hydroxide (KOH) solution in a three-electrode configuration. First, we tested the pristine CC substrate to find the effect on the performance of the MnO2-NPs@CC electrode. The comparative CV curves of the pristine CC and MnO2-NPs@CC electrode are displayed in Figure 6a. The area under the CV curve of the pristine CC is much lower than that of the MnO2-NPs@CC electrode at the same scan rate (5 mV/s). Furthermore, the GCD measurements were conducted and the comparative GCD curves are displayed in Figure 6b, which indicates that the capacitance generated from the pristine CC is tiny. Based on the CV and GCD results, we neglected the capacitance of the CC in the calculation. The CV curves for the MnO2-NPs@CC electrode within a voltage range of 0.0–0.8 V and at various scan rates (5–30 mV/s) are exhibited in Figure 6c. The CV profiles reveal rectangular shapes without additional redox peaks, even when scan rate was increased to 30 mV/s, which indicates the excellent electrochemical performance of the MnO2-NPs@CC [30]. The wide open spaces in the MnO2-NPs@CC electrode provide easy paths to diffuse the electrolyte ions in the interior section of the active material. The NPs grown on the CC surface offer a good surface area and rapid electron transfer channels. The GCD measurements of the MnO2-NPs@CC electrode at various current rates (2–32 A/g) are exhibited in Figure 6d. The linear and symmetric shapes of the GCD curves further confirm the good charge–discharge features and excellent reversibility. In addition, IR drops of 0.025 and 0.15 V were observed at 2 and 32 A/g, which indicated the quick I–V response with a small internal resistance from the active material. The major cause of an IR drop may be possibly due to charge transfer resistance or internal resistance of the active material or contact resistance at the electrode–electrolyte interface. The specific capacitance (Csp) for the MnO2-NPs@CC electrode was determined from the GCD profiles using Equation (1):
where m (g) indicates the mass, I(A) represents the current, Δt(s) indicates the discharge time, and ΔV(V) represents the potential window [31]. The specific capacitance was 660 F/g with a current rate of 2 A/g (Figure 6e). By increasing the current rate from 2 to 32 A/g, the MnO2-NPs@CC electrode showed 58% capacitance retention, which indicated the excellent rate capability. The capacitance decreased at higher current rates owing to the short time for the diffusion of electrolyte ions into the interior section of the electrode material, so the reduced diffusion decreased the capacitance. The electrochemical impedance spectroscopy (EIS) experiment was performed to investigate the conductivity and diffusion performance of the MnO2-NPs@CC electrode. The Nyquist plot consists of a semi-circular portion in a high-frequency region and a quasi-vertical line in a low-frequency region (Figure 6f). This linear portion corresponds to the remarkable electrochemical capacitive response of MnO2-NPs@CC and the semicircle’s radius indicates the charge transfer resistance (Rct = 2.52 Ω). The intercept with the horizontal axis depicts the combination of equivalent series resistance and the electrode’s intrinsic and electrolytic resistance (Rs = 0.69 Ω). The electrical equivalent circuit diagram consists of the resistor in series with the parallel combination of the resistor and the capacitor and simulates a semicircle, while the Warburg element (W) shows a low-frequency linear part (Figure 6f, inset). These results demonstrate that the MnO2-NPs@CC electrode has remarkable electrochemical properties.
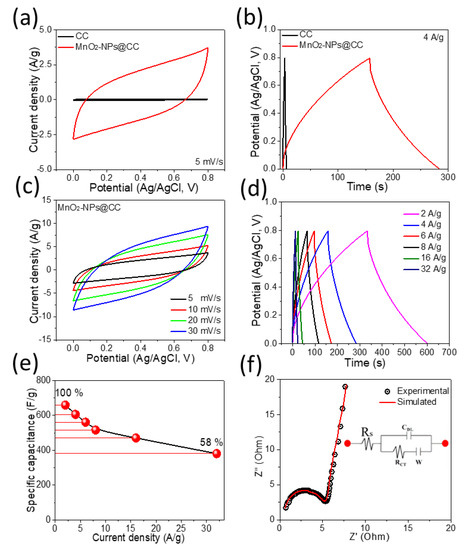
Figure 6.
The electrochemical measurements of the MnO2-NPs@CC electrode: (a,b) comparative CV and GCD curves of MnO2-NPs@CC and pristine CC; (c,d) CV and GCD curves of MnO2-NPs@CC; (e) capacitance as a function of the current density; (f) Nyquist plot with an electrical equivalent circuit.
The cycling performance is also a paramount parameter for the characterization of SC electrodes. A long-term cyclic performance measurement of the MnO2-NPs@CC cathode was studied at a current density of 16 A/g (Figure 7a). The capacitance decreased slowly and reached 92.8% of the initial value after 10,000 GCD cycles, showing excellent electrochemical stability.
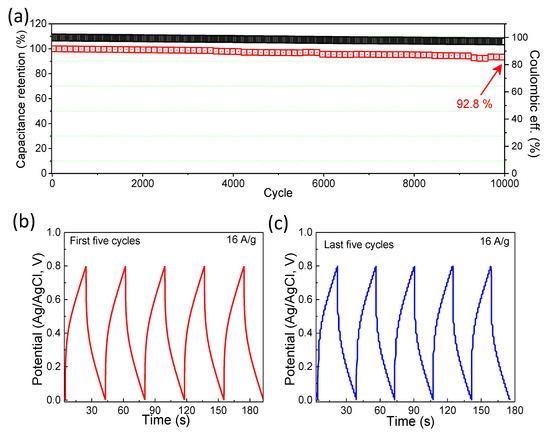
Figure 7.
(a) Cycling performance of MnO2-NPs@CC and CD characteristics for the (b) initial five cycles and (c) last five cycles.
In addition, typical GCD curves for the initial and last five cycles are displayed in Figure 7b,c, respectively, which demonstrate stable and similar shapes for both the initial and last cycles and the excellent stability of the MnO2-NPs@CC electrode. Such exceptional contributions are mainly associated with prominent nanoparticles, such as the morphology of the hexagonal-phase MnO2. Table 1 shows a comparison of the overall performance of the MnO2-NPs@CC electrode, which was compared with similar cathode materials reported previously based on the various forms of MnO2 and its composites with carbon and graphene.

Table 1.
A comparison of MnO2-based supercapacitor electrodes reported in previously published studies.
4. Conclusions
In summary, the hexagonal-phase MnO2-NPs supported on CC were synthesized via a simple and scalable hydrothermal strategy and their electrochemical performance in aqueous KOH electrolyte solution was investigated. The MnO2-NPs@CC, with its large surface area combined with a highly conductive CC support, provides an efficient path for electron and ion transfer and more electrochemical reaction sites. Owing to these characteristics, the MnO2-NPs@CC cathode shows a high specific capacitance of 660 F/g at 2 A/g and an excellent rate capability of 58% at a 32-fold current density. Furthermore, the MnO2-NPs@CC exhibits remarkable cycling stability performance of 92.8% over 10,000 GCD cycles. These results prove that the MnO2-NPs@CC-based electrode is promising and has tunable electrochemical performance, highlighting its potential for SC applications.
Author Contributions
Conceptualization, Q.A. and M.S.J.; methodology, Q.A.; software, A.M.; validation, N.U.H., A.I. and Z.U.R.; formal analysis, N.U.H., A.I. and Z.U.R.; investigation, M.A.B., N.U.H.; resources, A.I.; data curation, Z.U.R.; writing—original draft preparation, Q.A. and M.S.J.; writing—review and editing, M.A.B., N.U.H., A.I. and Z.U.R.; visualization, M.A.B.; supervision, M.S.J. and L.W.; project administration, L.W., M.S.J.; funding acquisition, M.A.B., M.S.J. and L.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 61975171) and Supercomputing Center of Lanzhou University. M.A.B. expresses appreciation to the Deanship of Scientific Research at King Khalid University Saudi Arabia through the research groups program under grant number R.G.P. 2/196/43.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the first author Qasim Abbas, who is responsible for the performed experiments.
Acknowledgments
We acknowledge the scientific and conceptualization input from the Department of Intelligent Manufacturing, Yibin University, China, and the technical and experimental support from the School of Physical Science and Technology, Lanzhou University, China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Veluswamy, P.; Sathiyamoorthy, S.; Chowdary, K.H.; Muthusamy, O.; Krishnamoorthy, K.; Takeuchi, T.; Ikeda, H. Morphology dependent thermal conductivity of ZnO nanostructures prepared via a green approach. J. Alloys Compd. 2017, 695, 888–894. [Google Scholar] [CrossRef]
- Pandiyarasan, V.; Archana, J.; Pavithra, A.; Ashwin, V.; Navaneethan, M.; Hayakawa, Y.; Ikeda, H. Hydrothermal growth of reduced graphene oxide on cotton fabric for enhanced ultraviolet protection applications. Mater. Lett. 2017, 188, 123–126. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Kumar, Y.; Chandra, R. Sputtered Synthesis of MnO2 Nanorods as Binder Free Electrode for High Performance Symmetric Supercapacitors. Electrochim. Acta 2016, 222, 1761–1769. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, J.; Li, C. Mesoporous Carbon Incorporated Metal Oxide Nanomaterials as Supercapacitor Electrodes. Adv. Mater. 2012, 24, 4197–4202. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward Flexible Polymer and Paper-Based Energy Storage Devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Shinde, S.S.; Lokhande, C.D. Architectured Morphologies of Chemically Prepared NiO/MWCNTs Nanohybrid Thin Films for High Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.W.; Xu, W.N.; Wang, Y.; Zhang, B.; Luo, S.; Zhou, X.Y.; Zhang, C.L.; Gu, X.; Hu, C.G. Porous Fe2O3 nanospheres anchored on activated carbon cloth for high-performance symmetric supercapacitors. Nano Energy 2019, 57, 379–387. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, D.; Guo, J.; Xu, M.; Sun, H.; Li, J.; Wang, L.; Shen, L. Maximized Energy Density of RuO2//RuO2 Supercapacitors through Potential Dependence of Specific Capacitance. ChemElectroChem 2020, 7, 928–936. [Google Scholar] [CrossRef]
- Yang, J.; Xu, X.; Zhou, X.; Jiang, S.; Chen, W.; Shi, S.; Wang, D.; Liu, Z. Ultrasmall Co3O4 Nanoparticles Confined in P, N-Doped Carbon Matrices for High-Performance Supercapacitors. J. Phys. Chem. C 2020, 124, 9225–9232. [Google Scholar] [CrossRef]
- Kour, S.; Tanwar, S.; Sharma, A. A review on challenges to remedies of MnO2 based transition-metal oxide, hydroxide, and layered double hydroxide composites for supercapacitor applications. Mater. Today Commun. 2022, 32, 104033. [Google Scholar] [CrossRef]
- Baral, A.; Satish, L.; Zhang, G.; Ju, S.; Ghosh, M.K. A Review of Recent Progress on Nano MnO2: Synthesis, Surface Modification and Applications. J. Inorg. Organomet. Polym. Mater. 2021, 31, 899–922. [Google Scholar] [CrossRef]
- Gupta, S.P.; Kakade, B.A.; Sathe, B.R.; Qiao, Q.; Late, D.J.; Walke, P.S. Thermally Driven High-Rate Intercalated Pseudocapacitance of Flower-like Architecture of Ultrathin Few Layered δ-MnO2 Nanosheets on Carbon Nano-Onions. ACS Appl. Energy Mater. 2020, 3, 11398–11409. [Google Scholar] [CrossRef]
- Xu, B.; Yu, L.; Sun, M.; Ye, F.; Zhong, Y.; Cheng, G.; Wang, H.; Mai, Y. One-pot hydrothermal synthesis of novel 3D starfish-like δ-MnO 2 nanosheets on carbon fiber paper for high-performance supercapacitors. RSC Adv. 2017, 7, 14910–14916. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, H.; Sun, Q.; Zhou, C.; Zhang, X.; Ge, L.; Ma, X. Synthesis of δ-MnO2/C assisted with carbon sheets by directly carbonizing from corn stalk for high-performance supercapacitor. Mater. Lett. 2021, 285, 129116. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Wolverton, C. Relative stability of normal vs. inverse spinel for 3d transition metal oxides as lithium intercalation cathodes. Phys. Chem. Chem. Phys. 2013, 15, 6486–6498. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.-L.; Li, G.-R.; Guo, R.; Ding, L.-X.; Tong, Y.-X. Design and Synthesis of MnO2/Mn/MnO2 Sandwich-Structured Nanotube Arrays with High Supercapacitive Performance for Electrochemical Energy Storage. Nano Lett. 2012, 12, 3803–3807. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jiang, J.; Li, H.; Zhao, X.S. A high-performance asymmetric supercapacitor fabricated with graphene-based electrodes. Energy Environ. Sci. 2011, 4, 4009–4015. [Google Scholar] [CrossRef]
- Hou, D.; Tao, H.; Zhu, X.; Li, M. Polydopamine and MnO2 core-shell composites for high-performance supercapacitors. Appl. Surf. Sci. 2017, 419, 580–585. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Nagaraju, G.; Ko, Y.H.; Cha, S.M.; Im, S.H.; Yu, J.S. A facile one-step approach to hierarchically assembled core–shell-like MnO2@MnO2 nanoarchitectures on carbon fibers: An efficient and flexible electrode material to enhance energy storage. Nano Res. 2016, 9, 1507–1522. [Google Scholar] [CrossRef]
- Toufiq, A.M.; Wang, F.; Li, Y.J.N. Influence of SiO2 on the structure-controlled synthesis and magnetic properties of prismatic MnO2 nanorods. Nanotechnology 2013, 24, 415703. [Google Scholar] [CrossRef]
- Liu, M.; Ma, X.; Gan, L.; Xu, Z.; Zhu, D.; Chen, L. A facile synthesis of a novel mesoporous Ge@C sphere anode with stable and high capacity for lithium ion batteries. J. Mater. Chem. A 2014, 2, 17107–17114. [Google Scholar] [CrossRef]
- Javed, M.S.; Dai, S.; Wang, M.; Guo, D.; Chen, L.; Wang, X.; Hu, C.; Xi, Y. High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres. J. Power Sources 2015, 285, 63–69. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Shaheen, N.; Hussain, S.; Li, J.; Shah, S.S.A.; Abbas, Y.; Ahmad, M.A.; Raza, R.; Mai, W. An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra. J. Mater. Chem. A 2019, 7, 946–957. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Lu, P.; Li, K.; Song, S.; Xue, D. Crystallization design of MnO2 towards better supercapacitance. CrystEngComm 2012, 14, 5892–5897. [Google Scholar] [CrossRef]
- Ramesh, S.; Yadav, H.M.; Karuppasamy, K.; Vikraman, D.; Kim, H.-S.; Kim, J.-H.; Kim, H.S. Fabrication of manganese oxide@nitrogen doped graphene oxide/polypyrrole (MnO2@NGO/PPy) hybrid composite electrodes for energy storage devices. J. Mater. Res. Technol. 2019, 8, 4227–4238. [Google Scholar] [CrossRef]
- Liu, C.-S.; Huang, C.-L.; Fang, H.-C.; Hung, K.-Y.; Su, C.-A.; Li, Y.-Y. MnO2-based carbon nanofiber cable for supercapacitor applications. J. Energy Storage 2021, 33, 102130. [Google Scholar] [CrossRef]
- Wu, T.; Wang, C.; Mo, Y.; Wang, X.; Fan, J.; Xu, Q.; Min, Y. A ternary composite with manganese dioxide nanorods and graphene nanoribbons embedded in a polyaniline matrix for high-performance supercapacitors. RSC Adv. 2017, 7, 33591–33599. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yang, D.; Li, J. Supercapacitor performances of MnO2 and MnO2/ reduced graphene oxide prepared with various electrodeposition time. Vacuum 2020, 178, 109455. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Ansari, M.O.; Cho, M.H. Manganese dioxide nanorods intercalated reduced graphene oxide nanocomposite toward high performance electrochemical supercapacitive electrode materials. J. Colloid Interface Sci. 2017, 506, 613–619. [Google Scholar] [CrossRef]
- Racik, M.; Manikandan, A.; Mahendiran, M.; Madhavan, J.; Raj, M.V.A.; Mohamed, M.G.; Maiyalagan, T. Hydrothermal synthesis and characterization studies of α-Fe2O3/MnO2 nanocomposites for energy storage supercapacitor application. Ceram. Int. 2020, 46, 6222–6233. [Google Scholar] [CrossRef]
- Shah, H.U.; Wang, F.; Javed, M.S.; Saleem, R.; Nazir, M.S.; Zhan, J.; Khan, Z.U.H.; Farooq, M.U.; Ali, S. Synthesis, characterization and electrochemical properties of α-MnO2 nanowires as electrode material for supercapacitors. Int. J. Electrochem. Sci. 2018, 13, 6426–6435. [Google Scholar] [CrossRef]
- Chang, X.; Zhai, X.; Sun, S.; Gu, D.; Dong, L.; Yin, Y.; Zhu, Y. MnO2/g-C3N4 nanocomposite with highly enhanced supercapacitor performance. Nanotechnology 2017, 28, 135705. [Google Scholar] [CrossRef]
- Zhang, Y.; Xuan, H.; Xu, Y.; Guo, B.; Li, H.; Kang, L.; Han, P.; Wang, D.; Du, Y. One-step large scale combustion synthesis mesoporous MnO2/MnCo2O4 composite as electrode material for high-performance supercapacitors. Electrochim. Acta 2016, 206, 278–290. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).