Abstract
The micro-arc oxidation film on TC4 alloy was prepared using a multifunctional micro-arc oxidation (MAO) power source. The effect of nano-titania colloid addition on the growth process, microstructure, and comprehensive mechanical properties of titanium alloy micro-arc oxidation films was studied by gradient concentration. The results show that, with the increase in titanium dioxide colloid content, the intensity of the discharge phenomenon during the MAO process increases, and the average current density gradually increases. The film thickness is gradually increased from 111.06 μm to 159.8 μm, and the roughness first decreases and then increases. The XRD results show that the rutile content in the film layer is increased with titanium dioxide colloid in the electrolyte. This resulted in an increase in the density of the film, and lead to the corrosion resistance of film improved. When the concentration of titanium dioxide colloid is 1.5 g/L, the comprehensive performance of the film is the best.
1. Introduction
As a new type of metal material, titanium alloy has received extensive attention in many fields owing to its high specific strength, high thermal stability, and good biocompatibility. Titanium alloys play an important role in the aerospace, medical, automotive, and marine industries [1]. However, the low wear resistance, low surface hardness, and poor thermal conductivity of titanium alloys limit its application and development [2]. In order to improve the mechanical properties of titanium alloys in special environments and expand the application scope of titanium alloys, it is necessary to treat the surface of the titanium alloy while maintaining the good plastic toughness of the internal metal, so as to improve the surface hardness, wear resistance, corrosion resistance, and other special characteristics functional requirements. Among various surface treatment methods, micro-arc oxidation has the advantages of simple operation, low cost, and no pollution. The film layer grows an oxide layer on the substrate in situ, and its bonding force with the substrate is higher [3,4]. The membrane layer can also have special properties by adjusting the electrical parameters and electrolyte. Therefore, it is of great interest to domestic and foreign researchers and has broad application prospects.
Among them, electrical parameters and electrolyte composition are the main factors affecting the performance of the micro-arc oxidation film. The current research direction is mostly to adjust the electrical parameters and adjust the composition of the electrolyte [5]. Additives can affect or participate in the process of micro-arc oxidation, thereby affecting the performance of the film [6]. However, there are relatively few reports on adding colloidal additives to electrolytes. This is because the nano-titania colloid has higher surface activity and dispersibility, and the anatase crystal has higher chemical activity at high temperature, which makes it easier to participate in micro-arc oxidation [7]. The anatinite-type nano-titania colloid was added to the silicate electrolyte system in order to improve the mechanical properties of the micro-arc oxidation film in this experiment.
As the main component of the film is titanium dioxide, the addition of nano-titanium dioxide colloidal additives will not introduce other impurities, and it is difficult for the micro-arc oxidation film to undergo chlorination and oxidation reactions [8]. In addition, there is no potential difference between the nano-titania colloid and the micro-arc oxide film, which makes the electrochemical corrosion of the micro-arc oxide film more difficult [9,10].
The thickness, structure, wear resistance, and corrosion resistance of the nano-titania colloid on the micro-arc oxidation film of TC4 alloy were systematically studied.
2. Materials and Methods
The TC4 titanium alloy is processed into a circular sheet with a thickness of φ35 and a thickness of 3 mm by wire cutting, which is polished step by step with sandpaper, and ultrasonically cleaned with an acetone solution and an ethanol solution to remove the oil stain and oxide film on the surface. Using a multifunctional micro-arc oxidation power supply, the electrolyte is 16 g/L Na2SiO3, 2.0 g/L Na2EDTA, 10.0 g/L Na2HPO4, and titanium dioxide colloids with different concentrations in the treatment solution, and the concentration is shown in Table 1. During the experiment, the forward and negative voltages were kept at 420 and 80 V, respectively; the frequency was 100 Hz; the duty cycle was 50%; and the oxidation time was 30 min. When the concentration of nano-titania colloid was higher than 3 g/L, the film layer appeared as “ablation” and the surface roughness was higher. Therefore, the maximum concentration of titania colloid was 3 g/L in this paper. The sample is connected to the anode through a wire, and the stainless steel container is the cathode. The temperature of the treatment solution is controlled below 30 °C by cooling circulating water, and the electrolyte solution is stirred during the micro-arc oxidation process. After the micro-arc oxidation is over, the power supply is turned off and the sample is taken out after the voltage drops to 0 V.

Table 1.
The specimens’ code MAO parameters.
The oxidized samples were washed with deionized water, dried, and measured with an OXFORD INSTRUMENTS CMI 233 magnetic induction/eddy current dual-purpose thickness gauge (10 measurement points were taken on both sides, and the average results were taken). The morphology and thickness of the cross-section were observed and proved by scanning electron microscope. The surface roughness was observed by means of a laser confocal microscope (LSM700); three X-axes and three Y-axes with equal spacing were selected for line scanning in the area of 1.3 mm × 1.3 mm of the sample, and the average value was taken. The microscopic morphology of the surface was observed by means of scanning electron microscope, and the element content and distribution in the film layer were analyzed by EDS scanning of the area. The phase structures in the films were carried out on an X-ray diffractometer (PW1700, CuKα radiation) with a scan angle of 20°–80° and a scan rate of 2°/min. The relative content of the phase was calculated using the RIR method.
The adhesion between the film layer and the substrate was tested with an automatic adhesion scratching instrument (a total of six scratches were made on the front and back sides, and the average result was taken). The sudden change in the electrical signal was used to record the adhesion of the film layer when it was scratched. The wear resistance was tested with a DZ-322TABER rotary wear tester, the pressure load was 750 g, the speed was 50 r/min, the mass loss was measured 50 times per revolution, and a total of 8 (400 r) repetitions of wear were carried out. The corrosion resistance of the film layer passed the YWS-250 salt spray box developed by Beijing Zhongke Environmental Testing Instrument Co., Ltd., Beijing, China. The test method adopts the national standard GB-T10587-2006, in which the concentration of NaCl is (5%), and the temperature of the salt spray process is controlled at 35 ± 2 °C. The entire salt spray process was carried out for 720 h, and the surface was observed at intervals.
3. Results
3.1. Growth Process of MAO Film
Figure 1 shows the effect of different titanium dioxide colloid concentrations on the current density during the micro-arc oxidation process. It can be seen from Figure 1a that, with the addition of titanium dioxide colloid, the current density during the micro-arc oxidation process is significantly increased, and the time at which the current density peak appears is significantly earlier. It is shown that the addition of TiO2 colloid reduces the critical voltage, and the primary oxide film is more easily broken down, resulting in a higher current density and a direct impact on the film formation rate. Higher current densities result in faster film formation rates [11]. This is because the nano-TiO2 particles in the electrolyte are negatively charged. Owing to the strong electric field, these particles can easily move to the titanium alloy positive electrode under the action of electrophoresis [12,13,14]. These nano-titania particles are attached to the nascent oxide film, promoting the process of “breakdown-growth-rebreakdown” of the film [3]. The nucleation site is increased on the surface to promote the nucleation and growth of the titanium dioxide film. After 200 s, there was no significant difference in current density. At this time, the oxidation process further penetrated into the deep layer, and the titanium dioxide colloid in the electrolyte was isolated by the new oxide film, making it difficult to participate in the micro-arc oxidation process. With the increase in the film thickness, the film is difficult to break down again, and the film thickness growth becomes slower [15]. Therefore, in the later stage of the reaction, the nano-titania colloid has almost no effect on the current density.
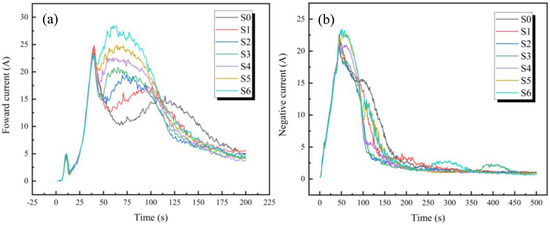
Figure 1.
Current density of micro-arc oxidation: (a) Forward current; (b) Negative current.
3.2. Characteristics of Film
3.2.1. Phase Compositions
Figure 2 is the X-ray (XRD) pattern of the micro-arc oxidation film of TC4 titanium alloy. There are sharp diffraction peaks of crystals in the figure, which proves that the micro-arc oxidation film is crystalline, and there are two phases in the film, namely anatase-phase titanium dioxide and rutile-phase titanium dioxide. With the increase in the titanium dioxide colloid concentration, the diffraction peaks of anatase did not change significantly, while the characteristic peaks of rutile became more and more obvious, and the intensity of the diffraction peaks increased, indicating that the content of rutile in the film increased. The rutile content gradually increases by the phase mass fraction calculation. When the concentration of titanium dioxide colloid is 0, rutile accounts for about 49.9%. When the concentration of titanium dioxide colloid is 3 g/L, rutile accounts for about 69.1%, an increase of about 20%. The rutile phase is more stable and requires higher energy to form, so it shows that the addition of titanium dioxide colloid causes the micro-arc oxidation process to generate a larger current density and higher temperature, which is more conducive to the formation of rutile phase and the current in the micro-arc oxidation process. The density results are consistent.
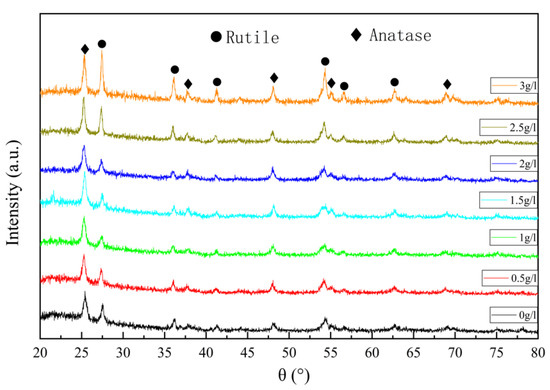
Figure 2.
X-ray (XRD) pattern of the micro-arc oxidation film of TC4 titanium alloy.
3.2.2. Micro Morphology
Figure 3 shows the surface topography of the micro-arc oxidation film of TC4 titanium alloy. It can be seen from Figure 3a that, when the titanium dioxide colloid is not added, the oxide film has discharge micropores with large diameters, and the size of the discharge micropores is not uniform, the average size is about 30 μm, and there are large-sized microcracks. When the concentration of titanium dioxide colloid is lower than 1 g/L, with the increase in additive concentration, the number of discharge micropores increases significantly, and the size of micropores tends to decrease. It shows that the addition of titanium dioxide colloid increases the discharge position on the surface and the number of breakdown points. The molten metal generated after the breakdown forms a discharge channel under the rapid cooling of the electrolyte, thereby forming a porous oxide on the surface. When the concentration of titanium dioxide colloid is higher than 1 g/L, with the increase in the concentration of titanium dioxide colloid, the size of discharge micropores keeps increasing, and the size gap keeps increasing. This is because, in the continuous increase in the discharge position, there are more nucleation positions in a smaller area, and more micro-arcs are generated, so the area where the micro-arcs gather generates higher energy, and the micro-arc oxidation process is more intense. After a large amount of molten metal is ejected from the aggregated micropores, the heat is quickly removed by the electrolyte, and large undulating micropores are formed on the surface. These large-sized micropores are formed in large quantities in the early stage of micro-arc oxidation, and then repeatedly break down, resulting in large internal stress and the formation of micro-cracks [16]. It can be seen from the figure that the width and length of the microcracks are increasing continuously. This is because the titanium dioxide colloid increases the current density in the process of micro-arc oxidation, resulting in higher energy generated on the surface, and the metal melt ejected from the micro-holes gradually increases, resulting in greater internal stress during rapid cooling, causing the crack width and length to increase continuously.
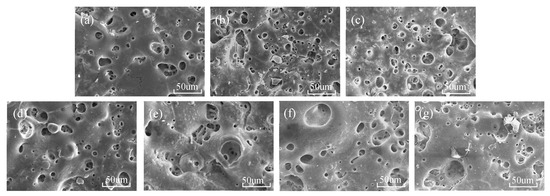
Figure 3.
Surface morphology of MAO coatings: (a) S0; (b) S1; (c) S2; (d) S3; (e) S4; (f) S5; and (g) S6.
The surface EDS results are shown in Table 2. It lists the element content (in mass fraction) on the surface of the micro-arc oxidation film in different concentrations of titanium dioxide colloidal electrolyte. It can be seen that the micro-arc oxide films in different concentrations of electrolytes are composed of three main elements, Ti, Si, and O, and the content of O is the highest, about 36%, while the content of Ti is about 20%. It has been proven that a large amount of oxides, mainly titanium dioxide [17], are generated under the high temperature and high pressure of micro-arc oxidation. The content of Ti and O has no obvious effect with the increase in TiO2 colloid concentration. Si comes from SiO32− in the electrolyte. The Si content increased significantly after adding the additive and the content reached the highest level when the titanium dioxide colloid concentration was 1.5 g/L. From the change in the content in EDS, it can be judged that the addition of titanium dioxide colloid can promote the movement of SiO32− in the electrolyte to the positive electrode and participate in the formation of oxide film.

Table 2.
Surface element content (wt%) of micro-arc oxidation films in different concentrations of titanium dioxide colloidal electrolytes.
Figure 4 shows the cross-sectional morphology of the oxide film. It can be seen from the figure that, when the titanium dioxide colloid is not added, the thickness of the film layer is about 120 μm. The oxide film on the side of the film layer close to the substrate is denser and, the closer it is to the surface, the more micropores and the larger the diameter of the micropores. This is because, in the process of oxidation, the outer layer makes direct contact with the electrolyte, the cooling rate is fast, and its shape is mostly irregular, which means that bulges form easily and envelop air. On the side close to the metal, a denser oxide film can form more easily under the action of external pressure. With the increase in the concentration of titanium dioxide additive, the film layer becomes dense at first, and becomes loose when the concentration is higher than 1.5 g/L, which is because of the participation of nano-titanium dioxide colloid in the micro-arc oxidation process. When the content is small, part of the holes [17] left by the reaction can be filled. When the concentration of the additive is too high, it will accumulate around the original pores, and it cannot easily combine well with the matrix, resulting in looseness and pores [18]. It can be seen from the cross-sectional view that, when the concentration of titanium dioxide colloid is 1.5 g/L, the density of the film layer is the highest, there are fewer micropores, and the surface of the film layer is relatively smooth. Figure 4 is the EDS line scan analysis diagram of the cross section of the S3 sample. It can be seen from the figure that the element distribution in the longitudinal direction of the film layer is relatively uniform, and there is no obvious element segregation in the longitudinal direction. When the concentration of titanium dioxide colloid is higher than 1.5 g/L, the thickness of the film begins to increase significantly and, when the concentration is 3 g/L, the thickness reaches 150 μm. With the increase in the titanium dioxide colloid concentration, larger pores and cracks appeared in the film layer, the surface became rougher, and the density decreased significantly.
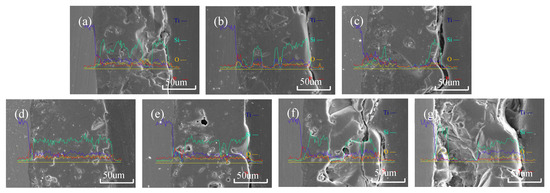
Figure 4.
Line scan of MAO coatings with different ZrO2 additions: (a) S0; (b) S1; (c) S2; (d) S3; (e) S4; (f) S5; and (g) S6.
3.2.3. Thickness and Roughness
Figure 5 shows the variation trend of the thickness and roughness of the micro-arc oxide film. As can be seen from the figure, when the titanium dioxide colloid is not added, the average thickness of the oxide film is 111.06 μm. With the increase in the titanium dioxide colloid concentration, its thickness increases, and the growth rate trend is first slow and then fast. When the titanium dioxide colloid concentration is 3 g/L, the thickness reaches the maximum value of 159.8 μm. This is related to the concentration of nano-titania in the electrolyte. When the concentration is low, the current density is low, the energy generated is low, the molten metal ejected from the substrate is relatively small, and the film layer is relatively thin. When the concentration increases, some nano-titanium dioxide will adhere to the surface of the substrate under the action of electrophoresis, which increases the discharge probability of the surface, promotes the occurrence of discharge melting and cooling nucleation, and increases the amount of molten metal ejected, which forms after cooling. The oxide film thickness increases. At first, the film thickness increases slowly, and the surface roughness continues to decrease. When the titanium dioxide colloid concentration is 1.5 g/L, the surface roughness reaches the lowest Ra = 17.318 μm. Then, the film thickness increases faster and the roughness becomes larger and larger. When the titanium dioxide colloid concentration is 3 g/L, the roughness reaches the maximum Ra = 42.414 μm. Because of the addition of titanium dioxide colloid, the content of rutile in the film increases. The hardness of the rutile phase is higher than that of the anatase phase, and when the two phases cool and shrink simultaneously, cracks are easily generated at the interface. As the film is thicker, the cooling rates of the surface and the interior are not the same during the rapid cooling process, resulting in greater internal stress, which is accompanied by the initiation and propagation of cracks. Figure 6 shows a 3D topography of the oxide film. In the Figure 6a, the surface undulation is large and the maximum height difference reaches 400 μm. Among them, the height difference of Figure 6c is the smallest, only 200 μm, indicating that the film layer has less fluctuation and is more flat.
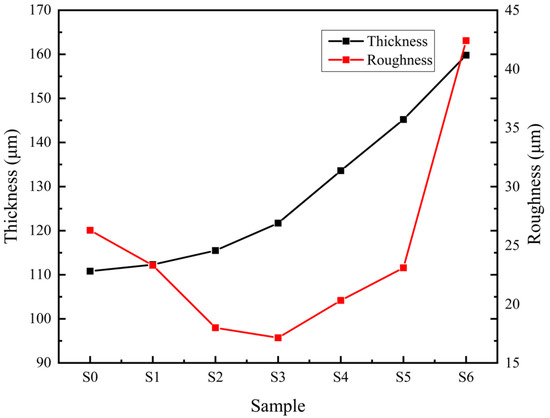
Figure 5.
Thickness and roughness of MAO coatings.
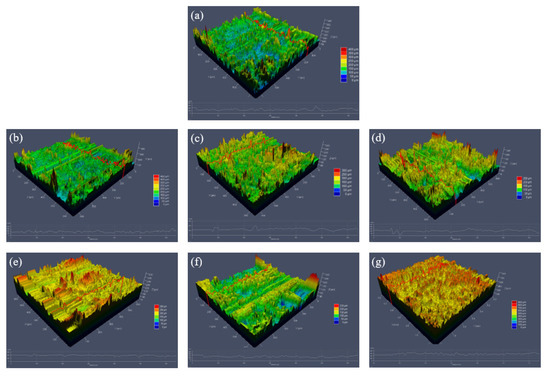
Figure 6.
3Dmorphology of MAO coatings: (a) S0; (b) S1; (c) S2; (d) S3; (e) S4; (f) S5; and (g) S6.
3.3. Resistance of Film
3.3.1. Adhesion Force
Figure 7 is the scratch diagram of the micro-arc oxidation film under different titanium dioxide colloid concentrations. The figure shows that the load applied by the diamond scribing needle continues to increase until the film is scratched, exposing the titanium alloy matrix, and the critical load of film failure is recorded through the mutation of the electrical signal. When no titania colloid was added, during the process of film damage, the phenomenon of film peeling occurred as a result of the diamond scribing, indicating that the bonding force of the film at this time was poor [19]. With the increase in TiO2 colloid content, the ploughing phenomenon occurs around the scratches, and the spalling phenomenon is significantly reduced. At the same time, it can be observed that the scratch lines gradually become tortuous, as shown in Figure 7f. This is because the rutile content in the film layer is continuously increasing, and the hardness of rutile is relatively large. When the diamond scribing needle encounters the rutile phase, the force exerted by the diamond scribing needle is less than the binding force between the rutile phase and the titanium alloy matrix, and it cannot be removed from the matrix, resulting in a brief change in scratch direction. This shows that the addition of titanium dioxide colloid causes the film to have better erosion resistance and a stronger bonding force with the substrate. When the content of titanium dioxide colloid is 3 g/L, the adhesion between the film and the substrate reaches its highest level, at 80.9 N. At this time, the adhesion is at the highest level, as shown in Figure 8, but when the applied force exceeds its adhesion, the bonding force between the film layers is higher than the bonding force between the film layer and the substrate, and a large area of peeling occurs, as shown in Figure 7g. It shows that the distribution of rutile phase in the film is not uniform, the brittleness of the film is enhanced [20], and the erosion resistance of the film is poor.
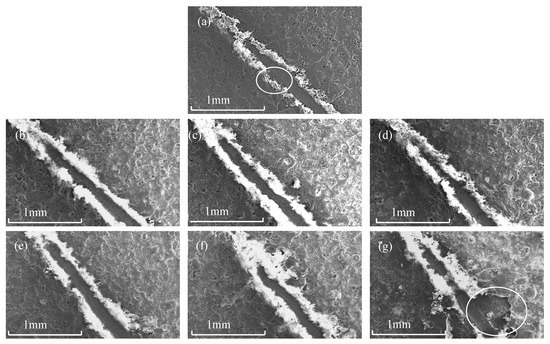
Figure 7.
Scratch morphology of MAO coating: (a) S0; (b) S1; (c) S2; (d) S3; (e) S4; (f) S5; and (g) S6.
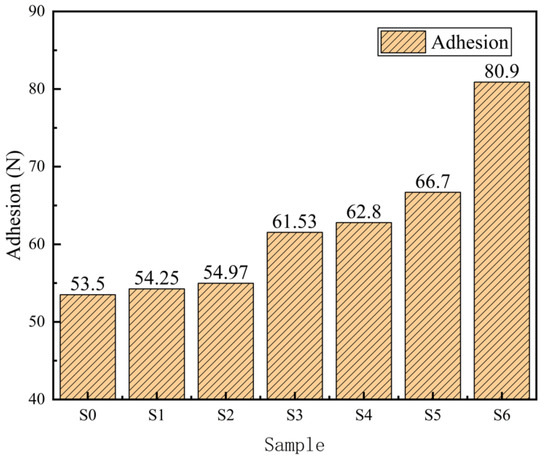
Figure 8.
Adhesion force of MAO coating.
3.3.2. Wear Resistance
Figure 9 and Figure 10 show the surface morphology and wear amount curve of the sample after wear under the rotary wear tester. It can be seen from the surface morphology that the oxide film on the surface has been worn away when no titanium dioxide colloid is added, more silver-white TC4 titanium alloy substrates are exposed, and the wear resistance of the film layer is relatively poor. With the increase in titanium dioxide colloid content, the exposed crescent shape of the TC4 titanium alloy matrix becomes smaller and smaller, indicating that the wear resistance of the film layer is better. It can be seen from the wear curve in Figure 10 that the wear without adding titanium dioxide colloid is the most serious case, and the addition of titanium dioxide colloid can significantly improve the wear resistance of the film. This is related to the content of the rutile phase with higher hardness in the film layer and the density of the film layer. The higher the rutile content, the higher the overall hardness, the smaller the mass loss during the wear process, and the better the wear resistance [21]. The higher the density, the fewer holes and cracks, and the better the wear resistance. This is because, at an appropriate concentration, the nano-titania particles play the role of filling voids and transferring loads, which can improve the wear resistance of the matrix [17]. However, when the concentration is too high, the film layer becomes loose and porous, the density is reduced, and the wear resistance is deteriorated. Among them, S4 has the best wear resistance. It can also be seen from the cross-sectional scan of the film layer that the density of S4 is relatively high, and the content of rutile is higher than that of S0. When the content of titanium dioxide colloid is higher than 2 g/L, the content of rutile continues to increase, but, because of the more intense oxidation process, the resulting film is thicker, and the structure is looser and accompanied by a large number of cracks, so the wear resistance shows a decreasing trend.

Figure 9.
Wear morphology of MAO coating: (a) S0; (b) S1; (c) S2; (d) S3; (e) S4; (f) S5; and (g) S6.
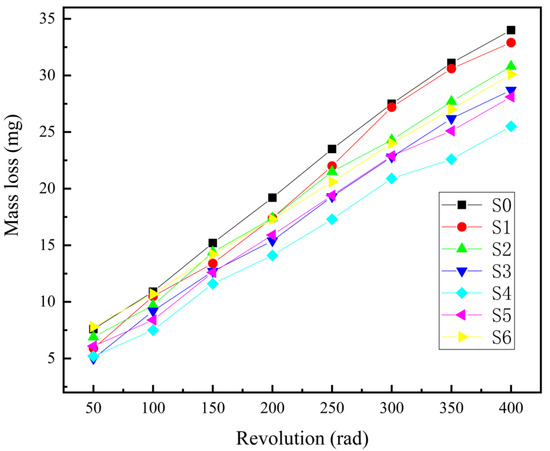
Figure 10.
Wear curves of ceramic layer samples.
3.3.3. Corrosion Resistance
The oxide film on the surface of titanium alloy has good corrosion resistance, because the main component of the oxide layer is titanium dioxide, which has stable chemical properties and can protect the substrate from corrosion. However, most failures due to corrosion occur under special circumstances. Therefore, the most destructive atmospheric corrosion is simulated by salt spray corrosion, and the corrosion resistance of titanium dioxide colloid to the micro-arc oxidation film of TC4 titanium alloy is analyzed by comparing the corrosion conditions after corrosion. Salt spray corrosion mainly occurs because chloride ions can be adsorbed in voids and cracks, replacing oxygen molecules in the oxide layer, turning insoluble oxides into soluble chlorides, and making the original chemically stable film active. The chloride ion can also pass through the oxide layer to undergo electrochemical reaction with the metal matrix. The denser the structure of the film layer, the better the protection of the metal matrix and the stronger the corrosion resistance [22]. Corrosion resistance can be compared by the number of corrosion points [23]. Figure 11 shows the surface morphology of the samples subjected to salt spray corrosion for different periods of time. It can be seen that, after the addition of titanium dioxide colloid, the phenomenon of pitting corrosion on the surface of the film is reduced, and the pitting corrosion occurs for a longer time. It shows that the addition of titanium dioxide colloid can significantly improve the corrosion resistance of the micro-arc oxidation film. This is because the composition of the additive is titanium dioxide, which is consistent with the composition of the film layer and no chlorination reaction and oxidation reaction, and there is no potential difference with the film layer, so electrochemical corrosion cannot easily occur. Secondly, the titanium dioxide colloid can increase the thickness of the film layer, protect the metal matrix inside, and make it more difficult to react with the outside world [24]. In addition, the titanium dioxide colloid can also increase the rutile content in the film layer. Compared with anatase, the chemical properties of rutile are more stable and the lattice structure is denser, which is easier to block the contact reaction between chloride ions and the metal matrix, thereby improving the TC4 titanium alloy corrosion resistance.
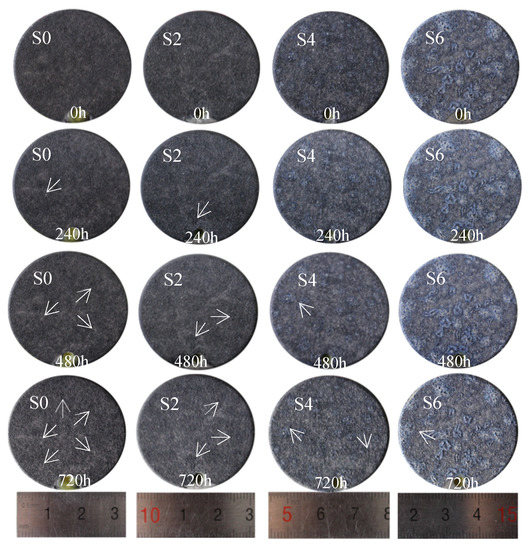
Figure 11.
MAO coating after different times by salt spray corrosion.
4. Conclusions
- (1)
- After adding nano titanium dioxide colloid to the electrolyte, the current density of the oxidation process is increased, and the proportion of rutile in the film layer is increased. When the concentration of titanium dioxide reaches 3 g/L, the rutile content increases by 20% compared with when it is not added.
- (2)
- Titanium dioxide colloid can increase the thickness of the film and improve the adhesion of the micro-arc oxidation film. When the concentration of titanium dioxide reaches 3 g/L, the adhesion reaches a maximum value of 80.9 N and the thickness reaches a maximum value of 159.8 μm.
- (3)
- With the increase in the concentration of titanium dioxide colloid, the diameter of the micropores in the film, the density of the film, the surface roughness, and the wear resistance all show a trend of first decreasing and then increasing. When the concentration of titanium dioxide colloid is 1.5 g/L, the best comprehensive mechanical properties are achieved.
Author Contributions
Writing—original draft, P.J.; Conceptualization, K.L.; Methodology, W.C. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the University Science Foundation for Young Science and Technology Talents in Inner Mongolia Autonomous Region of China (Grant No. NJYT22078), National Natural Science Foundation of China (Grant No. 51964035), and Inner Mongolia Natural Science Foundation (Grant No. 2018LH05034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Q.-M.; Zhang, Z.-H.; Liu, S.-F.; Yang, H.-Y. Application and development of titanium alloys in aerospace and military hardware. J. Iron. Steel Res. 2015, 27, 4. [Google Scholar]
- Dong, K.-H.; Song, Y.-W.; Han, E.-H. Research progress on the preparation of wear-resistant Micro-arc Oxidation coatings on titanium alloys. Surf. Technol. 2021, 50, 57–65. [Google Scholar]
- Qi, Y.-M.; Peng, Z.-J.; Liu, B.-X.; Liang, B.; Liang, J.; Wang, P. Fabrication and wear resistance of hard Micro Arc Oxidation coatings on Ti alloys. Surf. Technol. 2019, 48, 82–84. [Google Scholar]
- Luo, Q.; Cai, Q.-Z.; Li, X.-W.; Pan, Z.-H.; Li, Y.-J.; Chen, X.-D.; Yan, Q.-S. Preparation and characterization of ZrO2/TiO2 composite photocatalytic film by micro-arc oxidation. Trans. Nonferrous. Met. Soc. China 2013, 23, 2945–2950. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Sun, X.-F.; Song, W.; Qiu, J.; Li, Z.-M.; Li, D.-M. Application of additives in Micro -Arc Oxidation of aluminum alloy. Mater. Prot. 2022, 55, 187–191. [Google Scholar]
- Gong, Y.-B.; Wang, P.; Xu, Z.-X.; Wu, T.; Gong, Z.-Y.; Yang, B.; Hu, J.; Xiong, D.; Liu, J.-W. Effects of phytce acid additives on the characteristics of Micro-arc Oxidation coatings on 6061 aluminum alloy. Surf. Technol. 2021, 50, 381–386. [Google Scholar]
- Zhao, R. Preparation, Characterization and Application of TIO2 Nanomaterials Based Anatase. Master’s Thesis, Jilin University, Jilin, China, 2015. [Google Scholar]
- Travkin, V.V.; Pshirkov, V.F.; Kolachev, B.A. Thermodynamic analysis of the chemical mechanism for the hot-salt corrosion of titanium alloys. Mater. Sci. 1979, 15, 134–137. [Google Scholar] [CrossRef]
- Xie, H.; Wu, X.-W.; Liu, B.; Liu, Y.; Shi, Z.-Y.; Zhang, Y.-H.; Zhang, C.-D. Research Progress in the Galvanic Corrosion Behavior of Titanium Alloy/Other Metals in Marine Environment. Mater. Prot. 2022, 55, 12. [Google Scholar]
- Miao, J.; Wang, Q.-F.; Tao, W.; Jiu, F.-S. The corrosion behavior of Ti/TiO2 composite coating. In Proceedings of the 2016 National Corrosion Electrochemistry and Testing Methods Academic Exchange Conference, Qingdao, China, 13–15 July 2016. [Google Scholar]
- Yu, S.-N.; Wu, H.-H.; Chen, G.-Y.; Yuan, X.; Li, L. Effect of Al(OH)3 sol concentration on characteristics of microarc oxidation coatings of titanium alloy. Acta. Phys. Sin. 2011, 60, 5. [Google Scholar]
- Bahramian, A.; Raeissi, K.; Hakimizad, A. An investigation of the characteristics of Al2O3/TiO2 PEO nanocomposite coating. Appl. Surf. Sci. 2015, 351, 13–26. [Google Scholar] [CrossRef]
- Hong-Xia, J.I. Effects of nano-additive TiO2 on performance of micro-arc oxidation coatings formed on 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 406–411. [Google Scholar]
- Holmberg, J.P.; Ahlberg, E.; Bergenholtz, J.; Hassellöv, M.; Abbas, Z. Surface charge and interfacial potential of titanium dioxide nanoparticles: Experimental and theoretical investigations. J. Colloid Interface Sci. 2013, 407, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-N.; Suo, X.-B.; Qiu, J.; Zui, H. Effects of n-SiO2 on Growth Dynamics of Alumina Coatings Formed on 7A52 Aluminum Alloy by Micro-arc Oxidation. J. Aeronaut. Mater. 2012, 32, 4. [Google Scholar]
- Guo, J.-Q.; Guo, E.-J.; Feng, Y.-C.; Wang, L.-P.; Jing, W.-Y. Effect of Na2WO4concentration on the properties of Micro-arc Oxidation coating of ZM6 magnesium alloy. J. Harbin Univ. Sci. Technol. 2021, 26, 6. [Google Scholar]
- Xue, W.; Chao, W.; Chen, R.; Deng, Z. Structure and properties characterization of ceramic coatings produced on Ti–6Al–4V alloy by microarc oxidation in aluminate solution. Mater. Lett. 2002, 52, 435–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Du, H.-Q.; Zeng, L.; Song, R.-G. Effects of nano-additive TiO2 on properties of MAO ceramic coatings Fformed on ZL101A aluminum alloy. In Proceedings of the 11th China Iron and Steel Annual Conference 1994–2010, Jinzhou, China, 4 November 2020; China Academic Journal Electronic Publishing House: Beijing, China. [Google Scholar]
- Zeng, Z.; Xiao, F.; Miao, J.-G.; Du, J. Test and evaluation of superhard aluminum 7A04 micro-arc oxide film-based adhesion based on scratch test. Mater. Prot. 2017, 50, 93–96. [Google Scholar]
- Wei, T.-B.; Zhang, X.-J.; Wang, B.; Tian, J.; Yan, F.-Y. Effect of current density on growth and adhesion of aluminum alloy Micro-arc Oxidation Film. Mater. Prot. 2004, 37, 4–6. [Google Scholar]
- Li, Z.-W.; Di, S.-C. Influence of nano-TiO2 particles on the microstructure and wear resistance of microarc oxidationcoatings on 2214 aluminum alloy. Mater. Rev. 2018, 32, 89–94. [Google Scholar]
- Cao, Y.-X.; Wang, M.-J.; Zhou, F.; Yang, H.-F. Effect of KOH concentration on the growth process and corrosion resistance of Micro-arc Oxidation (MAO) coatings on LA103Z Mg-Li alloy. Surf. Technol. 2021, 50, 348–355. [Google Scholar]
- Yu, S.-H.; Shen, H.-W.; Li, J.-W.; Chen, Q. Researching on corrosion properties of salt spray test in comparison methods. Shanghai Coat. 2021, 59, 61–64. [Google Scholar]
- Zhang, Y.; Liu, X.; Zeng, L.; Li, H.-L.; Song, R.-G. Effects of different nano-additives on corrosion resistance of Micro-arc Oxidation films. In Proceedings of the 12th China Iron and Steel Annual Conference 1994–2010, Beijing, China, 15–16 October 2019; China Academic Journal Electronic Publishing House: Beijing, China, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).