Temperature Dependence of Photochemical Degradation of MAPbBr3 Perovskite
Abstract
:1. Introduction
2. Experimental
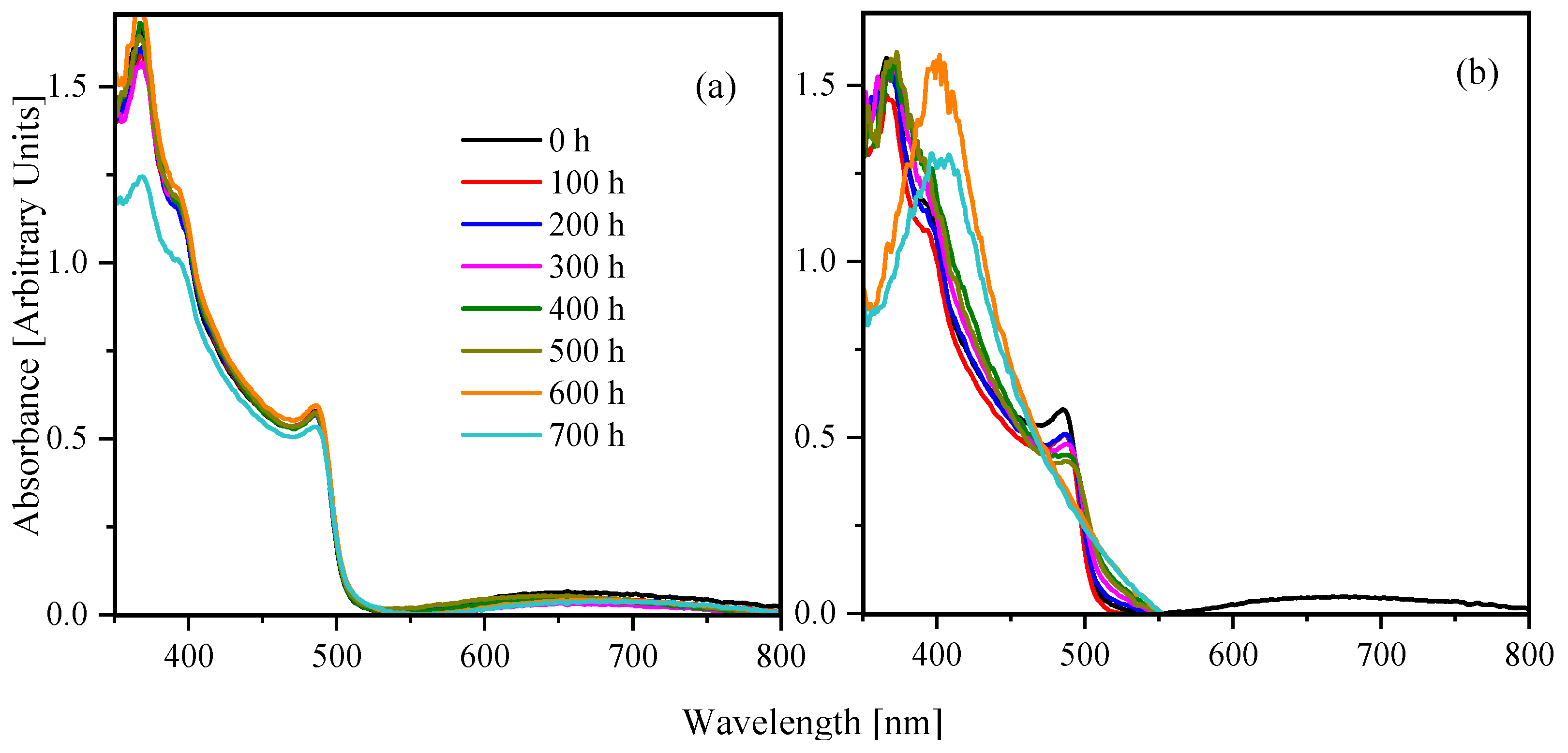
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, L.; Diguna, L.J.; Samy, O.; Muqoyyanah, M.; Abu Bakar, S.; Birowosuto, M.D.; El Moutaouakil, A. Hybrid Organic–Inorganic Perovskite Halide Materials for Photovoltaics towards Their Commercialization. Polymers 2022, 14, 1059. [Google Scholar] [CrossRef]
- Shi, M.; Li, R.; Li, C. Halide perovskites for light emission and artificial photosynthesis: Opportunities, challenges, and perspectives. EcoMat 2021, 3, e12074. [Google Scholar] [CrossRef]
- Jacak, J.E.; Jacak, W.A. Routes for Metallization of Perovskite Solar Cells. Materials 2022, 15, 2254. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, Y.; Cai, X.; Zhou, W.; Shen, Y.; Shen, K.; Wang, J.; Gao, X.; Zhidkov, I.S.; Tang, J. Minimizing Optical Energy Losses for Long-Lifetime Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2021, 31, 2105813. [Google Scholar] [CrossRef]
- Heo, J.H.; Song, D.H.; Im, S.H. Planar CH3NH3PbBr3 Hybrid Solar Cells with 10.4% Power Conversion Efficiency, Fabricated by Controlled Crystallization in the Spin-Coating Process. Adv. Mater. 2014, 26, 8179–8183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, Y.; Yang, M.; Choi, S.; Zhu, K.; Luther, J.M.; Beard, M.C. Low surface recombination velocity in solution-grown CH3NH3PbBr3 perovskite single crystal. Nat. Commun. 2015, 6, 7961. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Fang, Y.; Mulligan, P.; Chuirazzi, W.; Fang, H.-H.; Wang, C.; Ecker, B.R.; Gao, Y.; Loi, M.A.; Cao, L.; et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 2016, 10, 333–339. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 2013, 1, 5628. [Google Scholar] [CrossRef]
- Pont, S.; Bryant, D.; Lin, C.-T.; Aristidou, N.; Wheeler, S.; Ma, X.; Godin, R.; Haque, S.A.; Durrant, J.R. Tuning CH3NH3Pb(I1−xBrx)3 perovskite oxygen stability in thin films and solar cells. J. Mater. Chem. A 2017, 5, 9553–9560. [Google Scholar] [CrossRef]
- Wang, C.; Ecker, B.R.; Wei, H.; Huang, J.; Gao, Y. Environmental Surface Stability of the MAPbBr 3 Single Crystal. J. Phys. Chem. C 2018, 122, 3513–3522. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Correa-Baena, J.-P.; Saracco, G.; Grätzel, M.; Hagfeldt, A.; Turri, S.; Gerbaldi, C. Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers. Science 2016, 354, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 Degradation Rates and Mechanisms in Controlled Humidity Environments Using in Situ Techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Corsini, F.; Griffini, G. Recent progress in encapsulation strategies to enhance the stability of organometal halide perovskite solar cells. J. Phys. Energy 2020, 2, 31002. [Google Scholar] [CrossRef]
- Ramasamy, E.; Karthikeyan, V.; Rameshkumar, K.; Veerappan, G. Glass-to-glass encapsulation with ultraviolet light curable epoxy edge sealing for stable perovskite solar cells. Mater. Lett. 2019, 250, 51–54. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Malucelli, G.; Frigione, M.; Maffezzoli, A. UV-curable epoxy systems containing hyperbranched polymers: Kinetics investigation by photo-DSC and real-time FT-IR experiments. Polym. Test. 2009, 28, 157–164. [Google Scholar] [CrossRef]
- Abdelmageed, G.; Mackeen, C.; Hellier, K.; Jewell, L.; Seymour, L.; Tingwald, M.; Bridges, F.; Zhang, J.Z.; Carter, S. Effect of temperature on light induced degradation in methylammonium lead iodide perovskite thin films and solar cells. Sol. Energy Mater. Sol. Cells 2018, 174, 566–571. [Google Scholar] [CrossRef]
- Misra, R.K.; Aharon, S.; Li, B.; Mogilyansky, D.; Visoly-Fisher, I.; Etgar, L.; Katz, E.A. Temperature- and Component-Dependent Degradation of Perovskite Photovoltaic Materials under Concentrated Sunlight. J. Phys. Chem. Lett. 2015, 6, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Akbulatov, A.; Ustinova, M.; Shilov, G.; Dremova, N.; Zhidkov, I.; Kurmaev, E.; Frolova, L.; Shestakov, A.; Aldoshin, S.; Troshin, P. Temperature Dynamics of MAPbI3 and PbI2 Photolysis: Revealing the Interplay between Light and Heat, Two Enemies of Perovskite Photovoltaics. J. Phys. Chem. Lett. 2021, 12, 4362–4367. [Google Scholar] [CrossRef] [PubMed]
- Zhidkov, I.S.; Boukhvalov, D.W.; Akbulatov, A.F.; Frolova, L.A.; Finkelstein, L.D.; Kukharenko, A.I.; Cholakh, S.O.; Chueh, C.-C.; Troshin, P.A.; Kurmaev, E.Z. XPS spectra as a tool for studying photochemical and thermal degradation in APbX3 hybrid halide perovskites. Nano Energy 2021, 79, 105421. [Google Scholar] [CrossRef]
- Zhidkov, I.; Boukhvalov, D.; Kukharenko, A.; Finkelstein, L.; Cholakh, S.; Akbulatov, A.; Juarez-Perez, E.; Troshin, P.; Kurmaev, E. Influence of Ion Migration from ITO and SiO2 Substrates on Photo and Thermal Stability of CH3NH3SnI3 Hybrid Perovskite. J. Phys. Chem. C 2020, 124, 14928–14934. [Google Scholar] [CrossRef]
- Zhidkov, I.S.; Poteryaev, A.I.; Kukharenko, A.I.; Finkelstein, L.D.; Cholakh, S.O.; Akbulatov, A.F.; Troshin, P.A.; Chueh, C.-C.; Kurmaev, E.Z. XPS evidence of degradation mechanism in CH3NH3PbI3 hybrid perovskite. J. Phys. Condens. Matter 2020, 32, 95501. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Sample | C | O | N | Pb | Br | Si | Sb | N:Pb | Br:Pb |
|---|---|---|---|---|---|---|---|---|---|
| MAPbBr3 as prepared | 34.9 | – | 11.6 | 19.0 | 33.7 | – | 0.8 | 0.61 | 1.77 |
| photo 10 °C, 700 h | 72.4 | 9.9 | 2.2 | 5.7 | 9.8 | – | – | 0.38 | 1.71 |
| photo 60 °C, 700 h | 66.9 | 10.0 | 1.8 | 9.0 | 11.8 | 0.5 | – | 0.20 | 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhidkov, I.S.; Akbulatov, A.F.; Ustinova, M.I.; Kukharenko, A.I.; Frolova, L.A.; Cholakh, S.O.; Chueh, C.-C.; Troshin, P.A.; Kurmaev, E.Z. Temperature Dependence of Photochemical Degradation of MAPbBr3 Perovskite. Coatings 2022, 12, 1066. https://doi.org/10.3390/coatings12081066
Zhidkov IS, Akbulatov AF, Ustinova MI, Kukharenko AI, Frolova LA, Cholakh SO, Chueh C-C, Troshin PA, Kurmaev EZ. Temperature Dependence of Photochemical Degradation of MAPbBr3 Perovskite. Coatings. 2022; 12(8):1066. https://doi.org/10.3390/coatings12081066
Chicago/Turabian StyleZhidkov, Ivan S., Azat F. Akbulatov, Marina I. Ustinova, Andrey I. Kukharenko, Lyubov A. Frolova, Seif O. Cholakh, Chu-Chen Chueh, Pavel A. Troshin, and Ernst Z. Kurmaev. 2022. "Temperature Dependence of Photochemical Degradation of MAPbBr3 Perovskite" Coatings 12, no. 8: 1066. https://doi.org/10.3390/coatings12081066
APA StyleZhidkov, I. S., Akbulatov, A. F., Ustinova, M. I., Kukharenko, A. I., Frolova, L. A., Cholakh, S. O., Chueh, C.-C., Troshin, P. A., & Kurmaev, E. Z. (2022). Temperature Dependence of Photochemical Degradation of MAPbBr3 Perovskite. Coatings, 12(8), 1066. https://doi.org/10.3390/coatings12081066









