Topography Control of Micro-Nanosized Anatase Coating on Magnesium Alloy
Abstract
:1. Introduction
1.1. Background
1.2. The Purpose of the Research
2. Materials and Methods
2.1. Coating Preparation
2.2. Coating Characterization
2.3. Corrosion Resistance Tests
2.4. Biocompatibility Evaluation
3. Results and Discussion
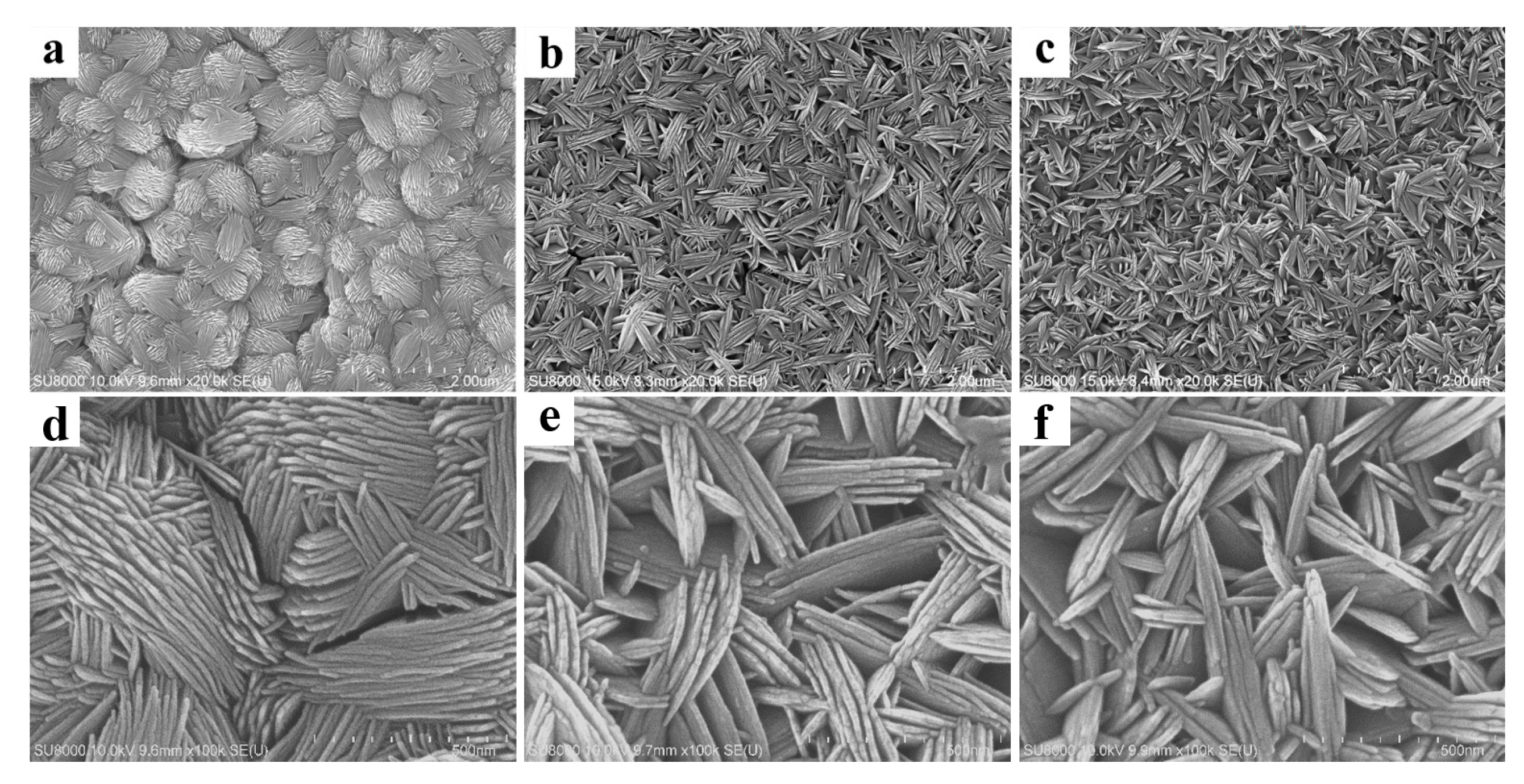
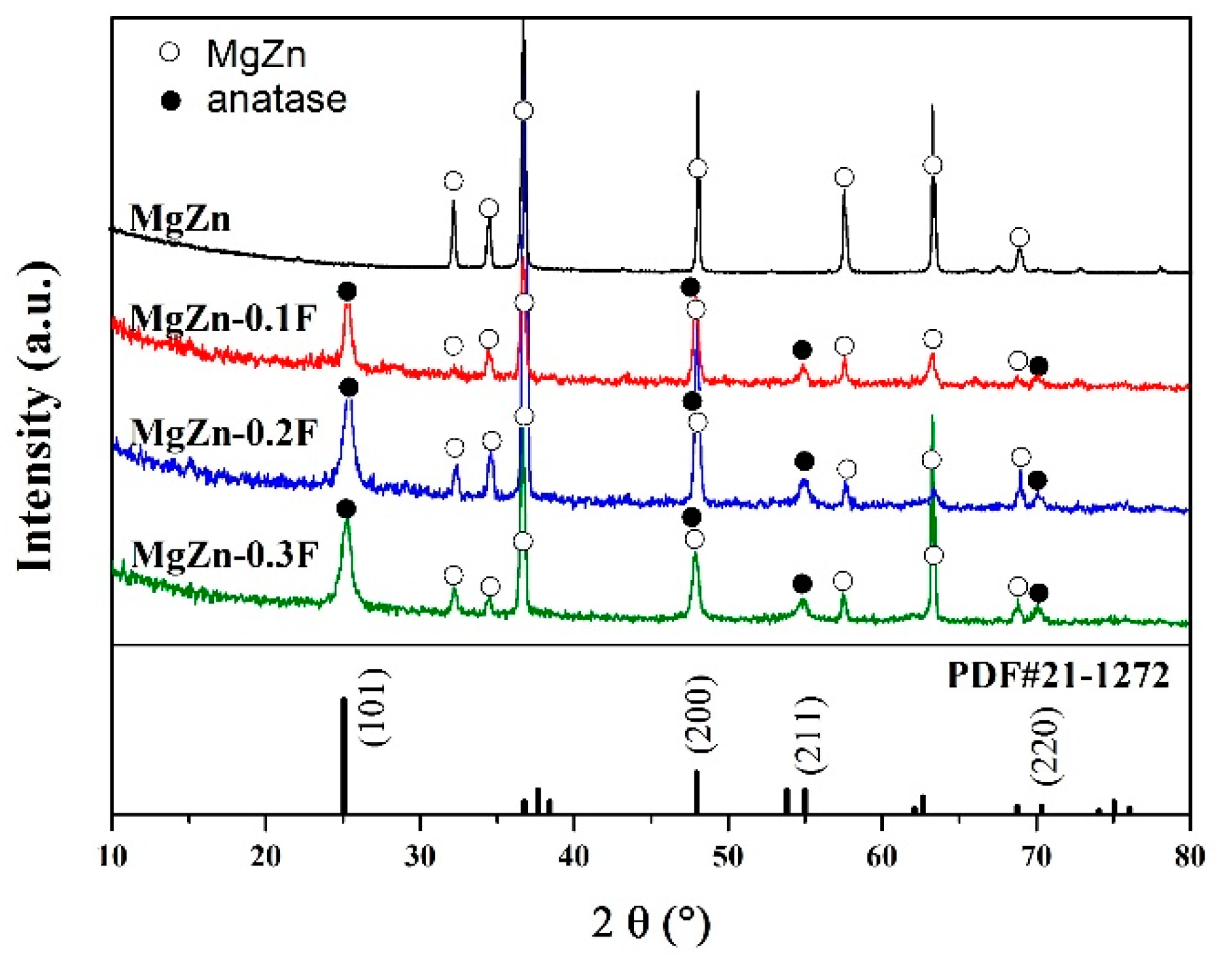
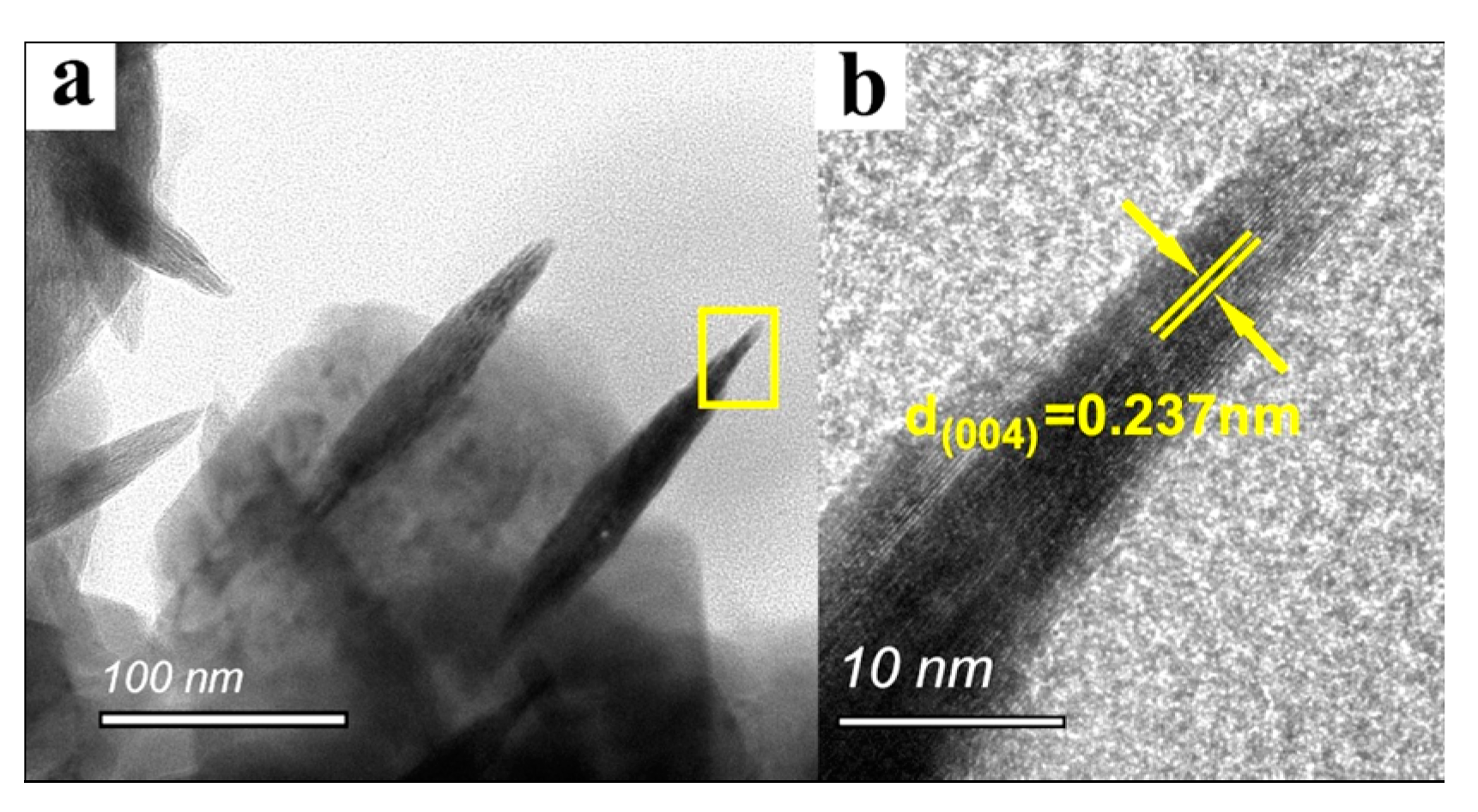
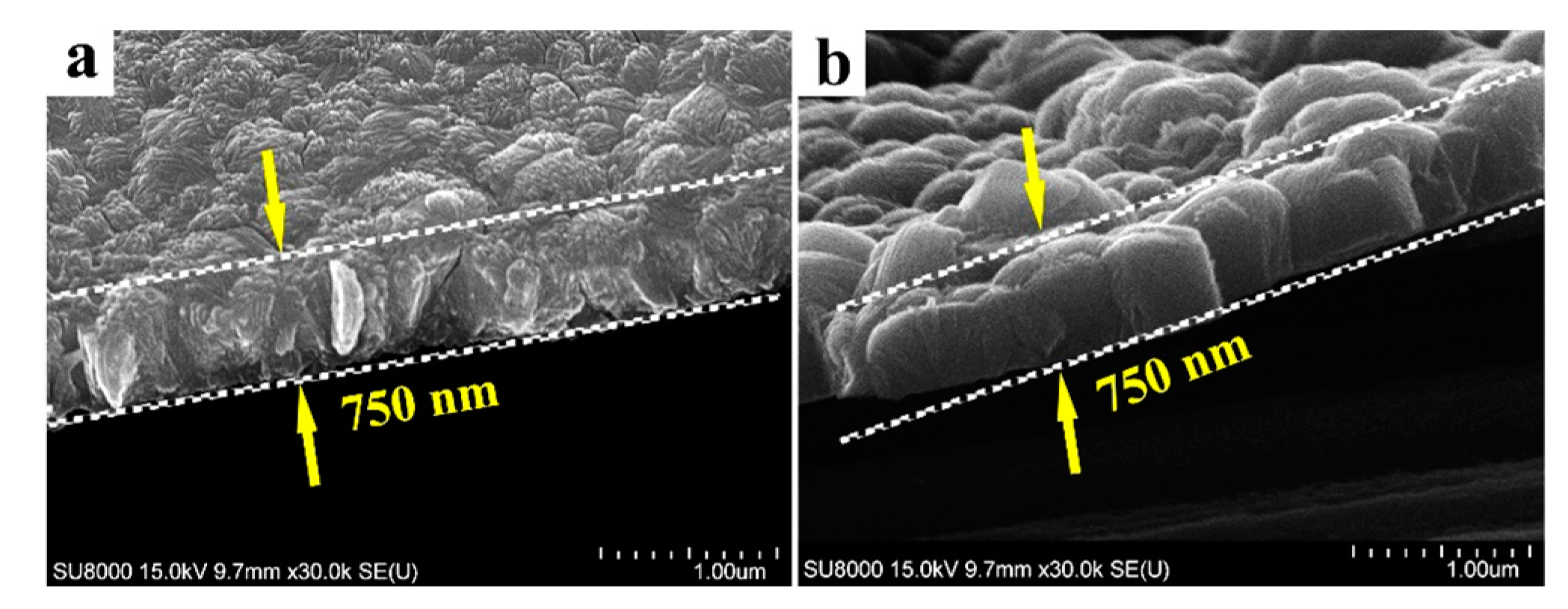
3.1. Coating Characteristics
3.2. Formation Mechanism of the Coatings
3.3. Properties of the Coatings
4. Conclusions
- (1)
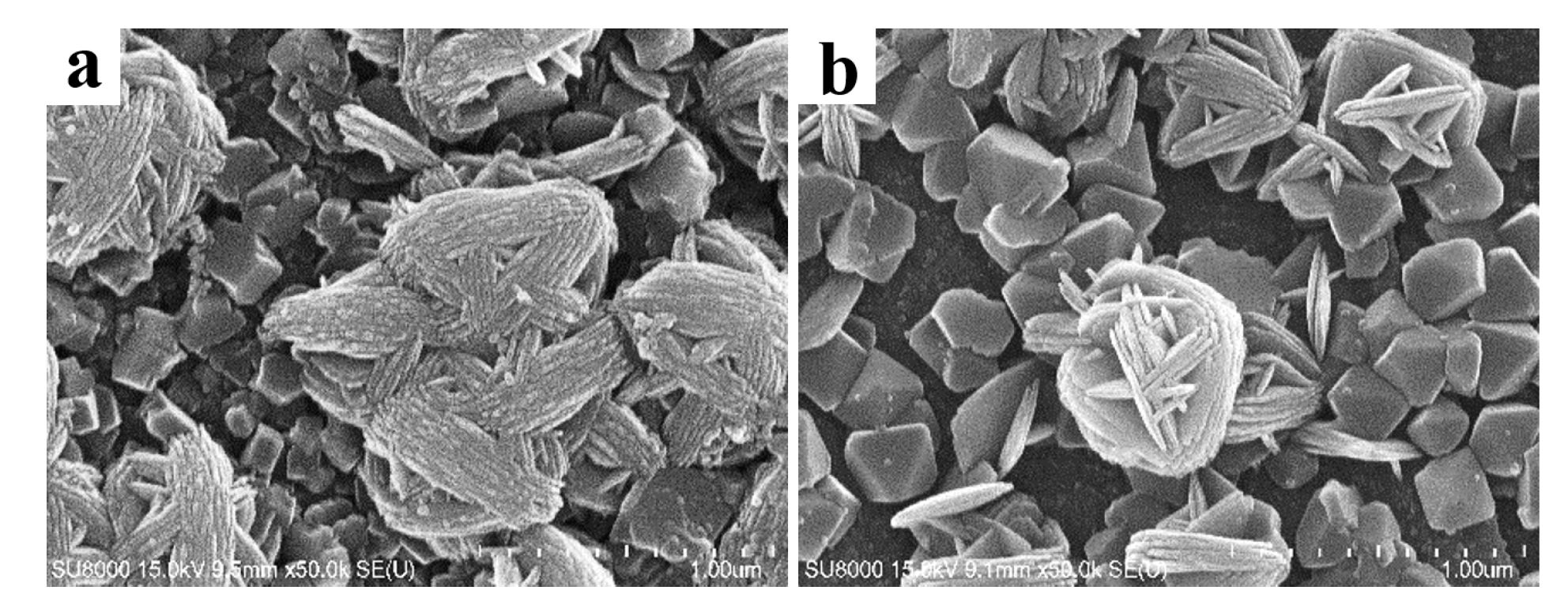
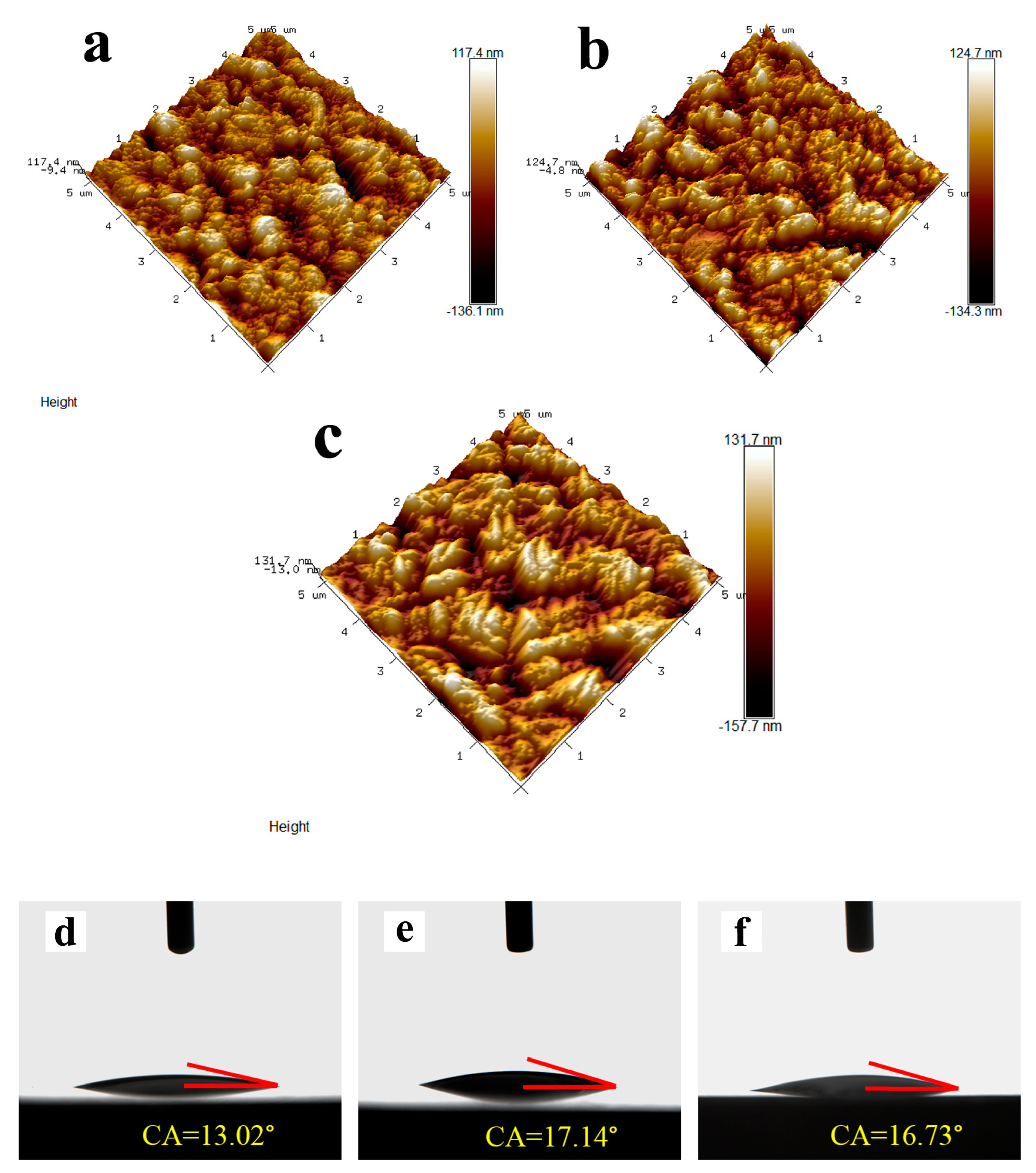
- The surface topography of anatase coating could be controlled in the micro- and nanometer range by the solvothermal method. The anatase coatings were composed of anatase sheets with a thickness of 10–20 nm, and the anatase nanosheets were agglomerated with varying degrees of density. With the increase of NH4F concentration in the solvothermal solution, the anatase nanosheets were more dispersed, and the surface roughness (Ra) was increased from 49.7 to 90.1 nm. The contact angles of MgZn–0.1 F, MgZn–0.2 F, and MgZn–0.3 F samples were 13.02°, 17.14°, and 16.73°, indicating good hydrophilicity of the anatase coatings.
- (2)
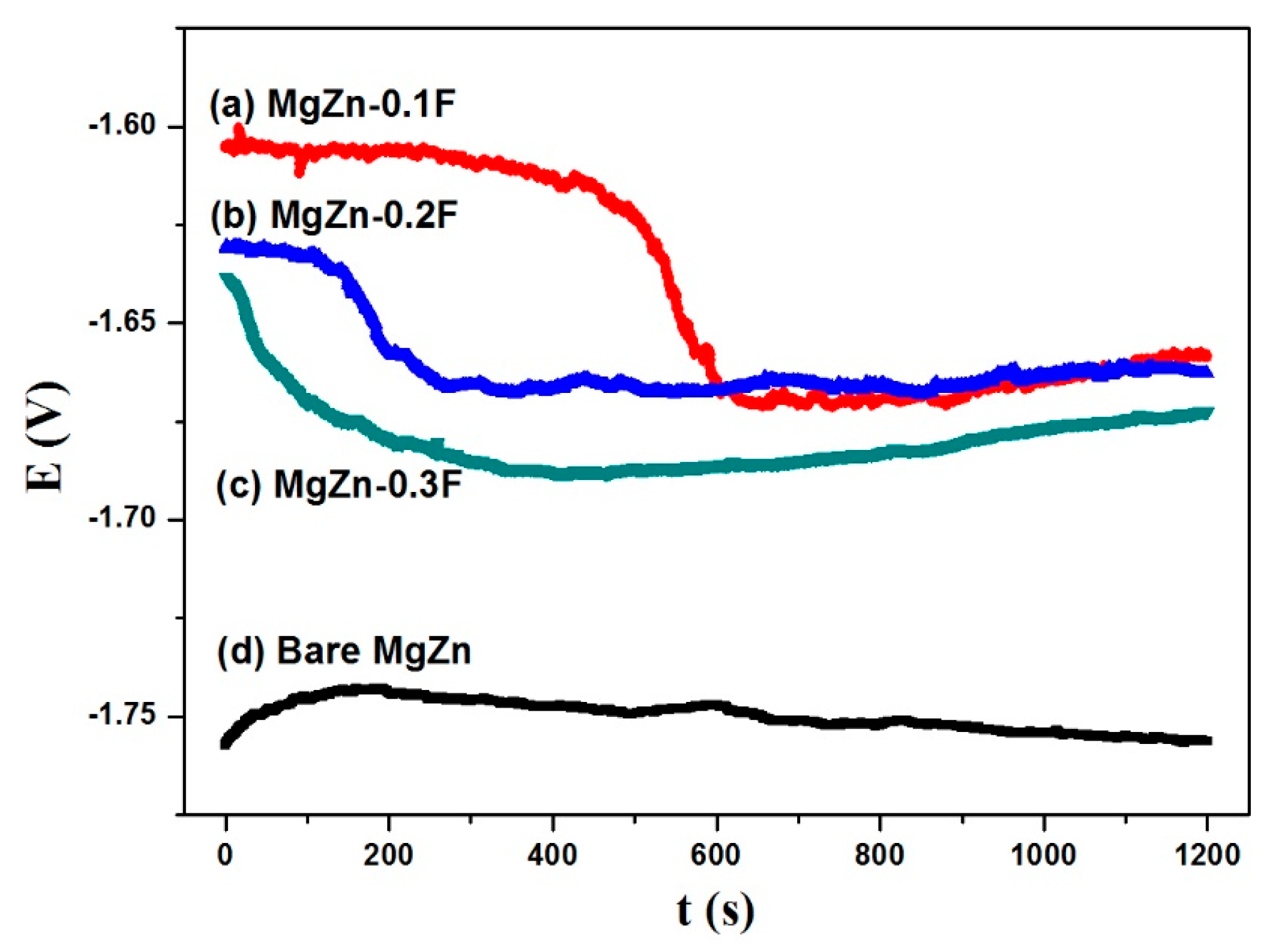
- After being coated with the anatase coatings, the corrosion resistance of the bare MgZn alloy substrate was improved. Additionally, the corrosion tendency was decreased with the increasing agglomeration density of the anatase nanosheets in the coatings.
- (3)
- The bare MgZn alloys caused serious platelet aggregation, yet the anatase-coated sample adsorbed a smaller number of platelets, and did not lead to the agglomeration of the adhered platelets. After being coated with anatase, the samples can adsorb endothelial cells on the surface, which maybe promote the re-endothelialization of stents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, Y.C.; Hung, W.C.; Lan, W.C.; Saito, T.; Huang, B.H.; Lee, C.H.; Tsai, H.Y.; Huang, M.S.; Ou, K.L. Anodized Biomedical Stainless-Steel Mini-Implant for Rapid Recovery in a Rabbit Model. Metals 2021, 11, 1575. [Google Scholar] [CrossRef]
- Ho, M.Y.; Chen, C.C.; Wang, C.Y.; Chang, S.H.; Hsieh, M.J.; Lee, C.H.; Wu, V.; Hsieh, I.C. The Development of Coronary Artery Stents: From Bare-Metal to Bio-Resorbable Types. Metals 2016, 6, 168. [Google Scholar] [CrossRef] [Green Version]
- Rout, P.K.; Roy, S.; Ganguly, S.; Rathore, D.K. A review on properties of magnesium-based alloys for biomedical applications. Biomed. Phys. Eng. Express. 2022, 8, 042002. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, M.; Guy, L.G.; Jackson, N. Evaluation of Magnesium-based Medical Devices in Preclinical Studies: Challenges and Points to Consider. Toxicol. Pathol. 2019, 47, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Liu, Z.; Wang, Q. Advances in Antibacterial Functionalized Coatings on Mg and Its Alloys for Medical Use—A Review. Coatings 2020, 10, 828. [Google Scholar] [CrossRef]
- Soleymani, F.; Emadi, R.; Sadeghzade, S.; Tavangarian, F. Bioactivity Behavior Evaluation of PCL-Chitosan-Nanobaghdadite Coating on AZ91 Magnesium Alloy in Simulated Body Fluid. Coatings 2020, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.N.; Guo, H.M.; Wang, F.; Lu, Y.; Lin, W.T.; Li, J.; Zheng, Y.F.; Fan, Y.B. Degradation, hemolysis, and cytotoxicity of silane coatings on biodegradable magnesium alloy. Mater. Lett. 2017, 193, 266–269. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Allain, J.P.; Robledo, S.M.; Echeverria, F.; Harmsen, M.C. Coatings for biodegradable magnesium-based supports for therapy of vascular disease: A general view. Mater. Sci. Eng. C Mater. 2019, 102, 150–163. [Google Scholar] [CrossRef]
- Rourke, A.S.; Beard, M.C.; Jones, S.E.; Priddy, M.W.; Priddy, L.B. Hydroxyapatite coating promotes stable physicochemical properties of pure magnesium in a longitudinal degradation study. J. Mater. Res. 2022, 37, 1231–1245. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Yang, M.; Hong, Q.; Yang, Z.; Liu, S.; Xiong, Q.; Pan, C. Construction of Chi(Zn/BMP2)/HA composite coating on AZ31B magnesium alloy surface to improve the corrosion resistance and biocompatibility. Nanotechnol. Rev. 2021, 10, 870–882. [Google Scholar] [CrossRef]
- Ben-Yehuda, O. Long-Term Outcomes with Drug-Eluting Stents: Beyond Stent Choice. J. Am. Coll. Cardiol. 2020, 76, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, D.; Mao, D.; Bai, N.; Chen, Y.; Li, Q. Enhanced performance of magnesium alloy for drug-eluting vascular scaffold application. Appl. Surf. Sci. 2018, 435, 320–328. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, B.; Wang, P.; Wang, X.; Zhang, B.; Shi, Q.; Xi, T.; Chen, M.; Guan, S. Enhanced in Vitro and in Vivo Performance of Mg-Zn-Y-Nd Alloy Achieved with APTES Pretreatment for Drug-Eluting Vascular Stent Application. ACS Appl. Mater. Interfaces 2016, 8, 17842–17858. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Yu, W.; Yang, Z.; Li, Y.; Yang, L.; Lang, S. Properties of Titanium Oxide Coating on MgZn Alloy by Magnetron Sputtering for Stent Application. Coatings 2020, 10, 999. [Google Scholar] [CrossRef]
- Ramos-Corella, K.J.; Sotelo-Lerma, M.; Gil-Salido, A.A.; Rubio-Pino, J.L.; Auciello, O.; Quevedo-López, M.A. Controlling crystalline phase of TiO2 thin films to evaluate its biocompatibility. Mater. Technol. 2019, 34, 455–462. [Google Scholar] [CrossRef]
- Hou, S.S.; Zhang, R.R.; Guan, S.K.; Ren, C.X.; Gao, J.H.; Lu, Q.B.; Cui, X.Z. In vitro corrosion behavior of Ti-O film deposited on fluoride-treated Mg–Zn–Y–Nd alloy. Appl. Surf. Sci. 2012, 258, 3571–3577. [Google Scholar] [CrossRef]
- Liang, C.; Hu, Y.; Wang, H.; Xia, D.; Li, Q.; Zhang, J.; Yang, J.; Li, B.; Li, H.; Han, D.; et al. Biomimetic cardiovascular stents for in vivo re-endothelialization. Biomaterials 2016, 103, 170–182. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Zhang, K.; Chen, J.; Huang, N. Endothelialization of implanted cardiovascular biomaterial surfaces: The development from in vitro to in vivo. J. Biomed. Mater. Res. A 2014, 102, 3754–3772. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.; Li, X.; Jiang, Y.C.; Han, S.; Ma, L.; Guo, H.; Wang, Z.; Li, Q. Endothelial Cell Migration on Poly(epsilon-caprolactone) Nanofibers Coated with a Nanohybrid Shish-Kebab Structure Mimicking Collagen Fibrils. Biomacromolecules 2020, 21, 1202–1213. [Google Scholar] [CrossRef]
- Cutiongco, M.F.A.; Goh, S.H.; Aid-Launais, R.; Le Visage, C.; Low, H.Y.; Yim, E.K.F. Planar and tubular patterning of micro and nano-topographies on poly(vinyl alcohol) hydrogel for improved endothelial cell responses. Biomaterials 2016, 84, 184–195. [Google Scholar] [CrossRef]
- Bedair, T.M.; ElNaggar, M.A.; Joung, Y.K.; Han, D.K. Recent advances to accelerate re-endothelialization for vascular stents. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, S. Endothelialization of cardiovascular devices. Acta Biomater. 2019, 99, 53–71. [Google Scholar] [CrossRef]
- Hou, S.; Mi, L.; Wang, L.; Zhu, S.; Hu, J.; Ding, Q.; Guan, S. Corrosion protection of Mg-Zn-Y-Nd alloy by flower-like nanostructured TiO2film for vascular stent application. J. Chem. Technol. Biot. 2013, 88, 2062–2066. [Google Scholar] [CrossRef]
- Hou, S. Solvothermal fabrication of TiO2 nanosheet films on degradable Mg–Zn alloys. Surf. Eng. 2016, 32, 745–749. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Guan, S.; Zhu, S.; Ren, C.; Hou, S. Microstructure and corrosion properties of as sub-rapid solidification Mg-Zn-Y-Nd alloy in dynamic simulated body fluid for vascular stent application. J. Mater. Sci. Mater. Med. 2010, 21, 2001–2008. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, G.; Zhang, H.; Zhang, Y.; Kang, S.; Zhao, H. Photoelectrochemical manifestation of intrinsic photoelectron transport properties of vertically aligned {001} faceted single crystal TiO2 nanosheet films. RSC Adv. 2015, 5, 55438–55444. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Moses, R.L.; Joshua, J.; Jacobs, J.J. Evaluation of Metallic and Polymeric Biomaterial Surface Energy and Surface Roughness Characteristics for Directed Cell Adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar] [CrossRef] [Green Version]

| No. | Abbreviation | Sample Description |
|---|---|---|
| 1 | MgZn | Bare MgZn substrate |
| 2 | MgZn–0.1 F | MgZn substrate with anatase coating, prepared in solution containing 140 μL of 0.1 M–NH4F aqueous solution |
| 3 | MgZn–0.2 F | MgZn substrate with anatase coating, prepared in solution containing 140 μL of 0.2 M–NH4F aqueous solution |
| 4 | MgZn–0.3 F | MgZn substrate with anatase coating, prepared in solution containing 140 μL of 0.3 M–NH4F aqueous solution |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Yang, T.; Li, Y.; Lian, L.; Yu, W.; Yang, L. Topography Control of Micro-Nanosized Anatase Coating on Magnesium Alloy. Coatings 2022, 12, 1063. https://doi.org/10.3390/coatings12081063
Hou S, Yang T, Li Y, Lian L, Yu W, Yang L. Topography Control of Micro-Nanosized Anatase Coating on Magnesium Alloy. Coatings. 2022; 12(8):1063. https://doi.org/10.3390/coatings12081063
Chicago/Turabian StyleHou, Shusen, Tingting Yang, Yue Li, Liming Lian, Weixin Yu, and Lin Yang. 2022. "Topography Control of Micro-Nanosized Anatase Coating on Magnesium Alloy" Coatings 12, no. 8: 1063. https://doi.org/10.3390/coatings12081063
APA StyleHou, S., Yang, T., Li, Y., Lian, L., Yu, W., & Yang, L. (2022). Topography Control of Micro-Nanosized Anatase Coating on Magnesium Alloy. Coatings, 12(8), 1063. https://doi.org/10.3390/coatings12081063





