Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation Method of Microcapsules
- (1)
- Synthesis of the prepolymer
- (2)
- Emulsion of core materials
- (3)
- Encapsulation of microcapsules
2.3. Preparation Method of Films
2.4. Testing and Characterization
3. Results and Discussion
3.1. Micromorphology, Particle Size Distribution, Coating Rate, and Chemical Composition Analysis of Transparent, Purple, and Yellow Shellac Microcapsules
3.2. Chromatic Aberration Analysis of Waterborne Coatings Containing Different Microcapsules
3.2.1. Effect of Shellac Microcapsule Content with Different Core Materials on the Chromatic Aberration of Waterborne Coatings
3.2.2. Effect of Core Material Color on the Chromatic Aberration of Waterborne Coatings before and after Self-Repairing
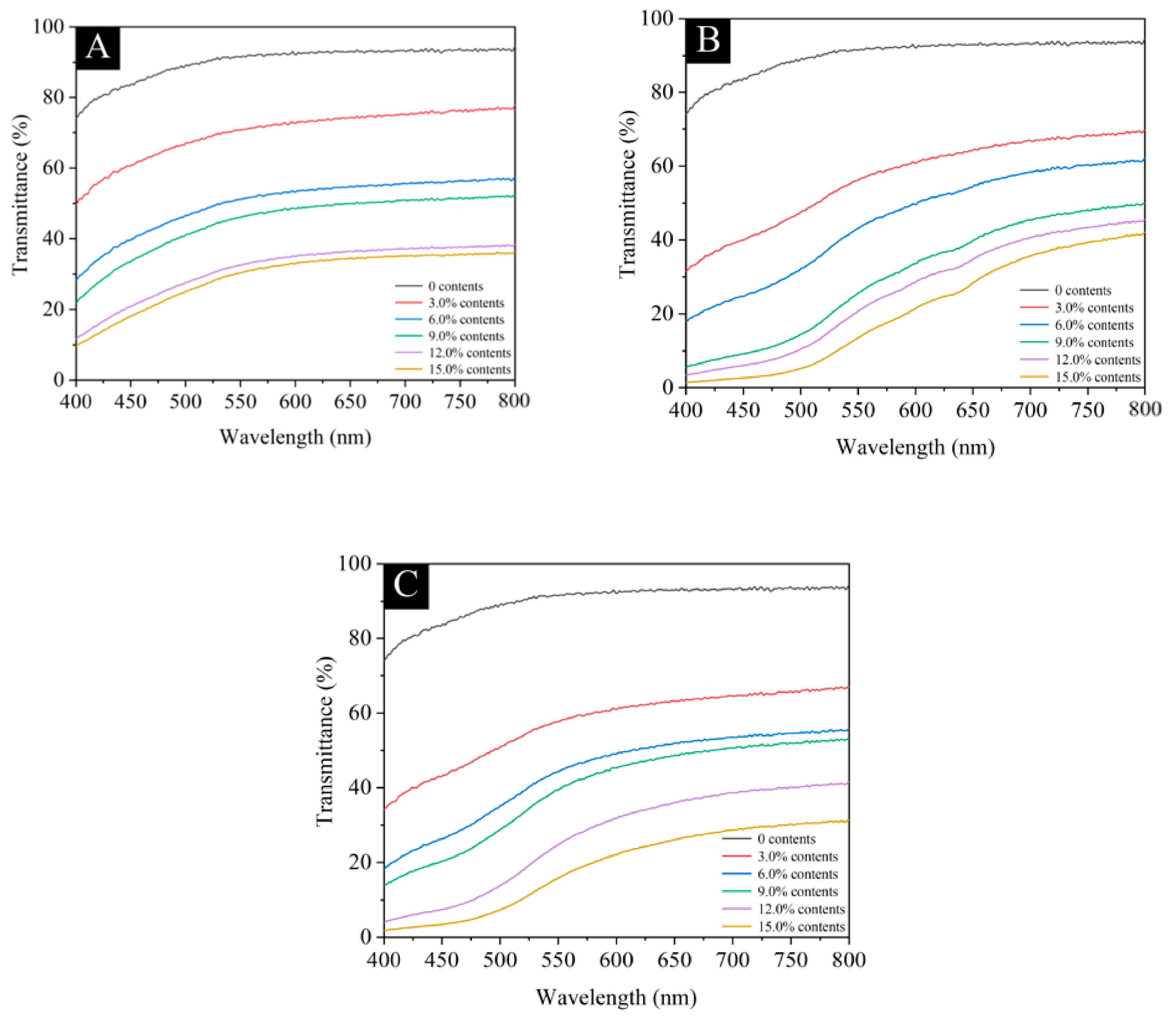
3.2.3. Analysis of Light Transmittance of Waterborne Coatings with Different Microcapsules
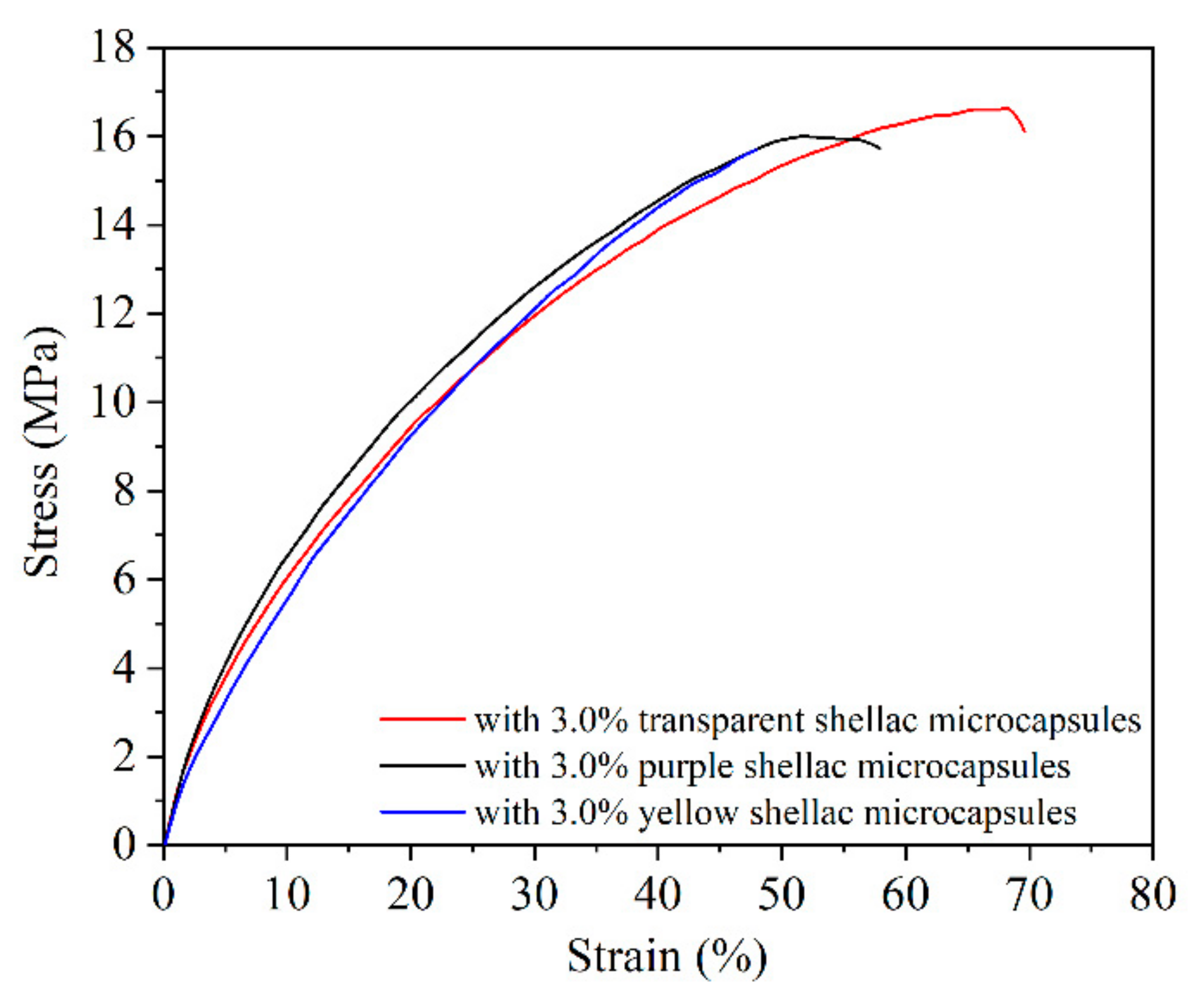
3.3. Tensile Repair Effect Analysis of Waterborne Films Containing Different Shellac Microcapsules
3.3.1. Analysis of Tensile Properties of Waterborne Films
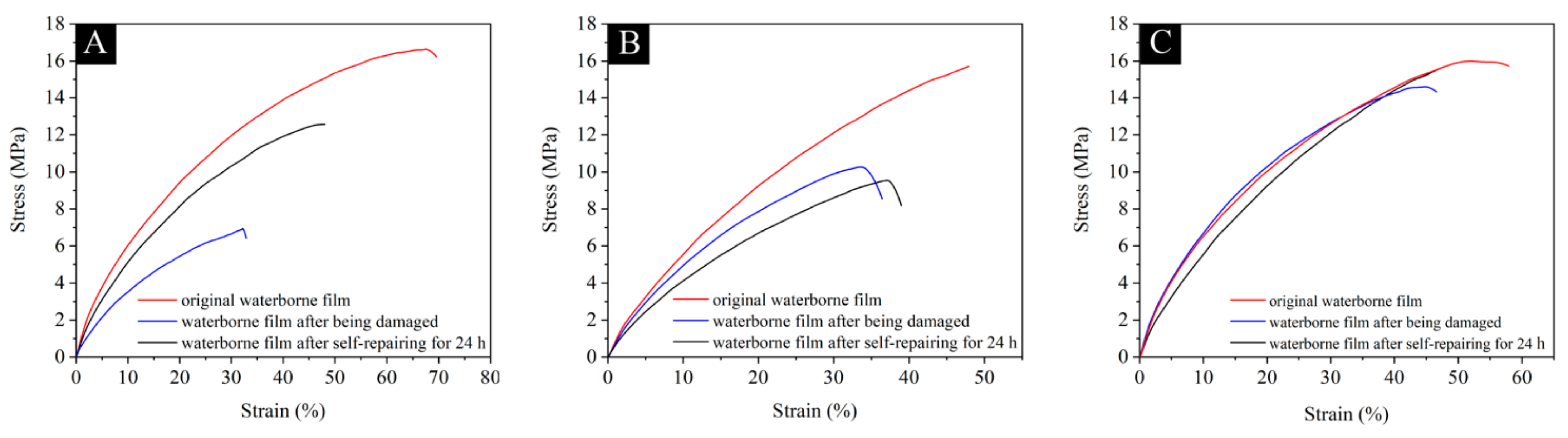
3.3.2. Analysis of Repair Rate of Waterborne Films
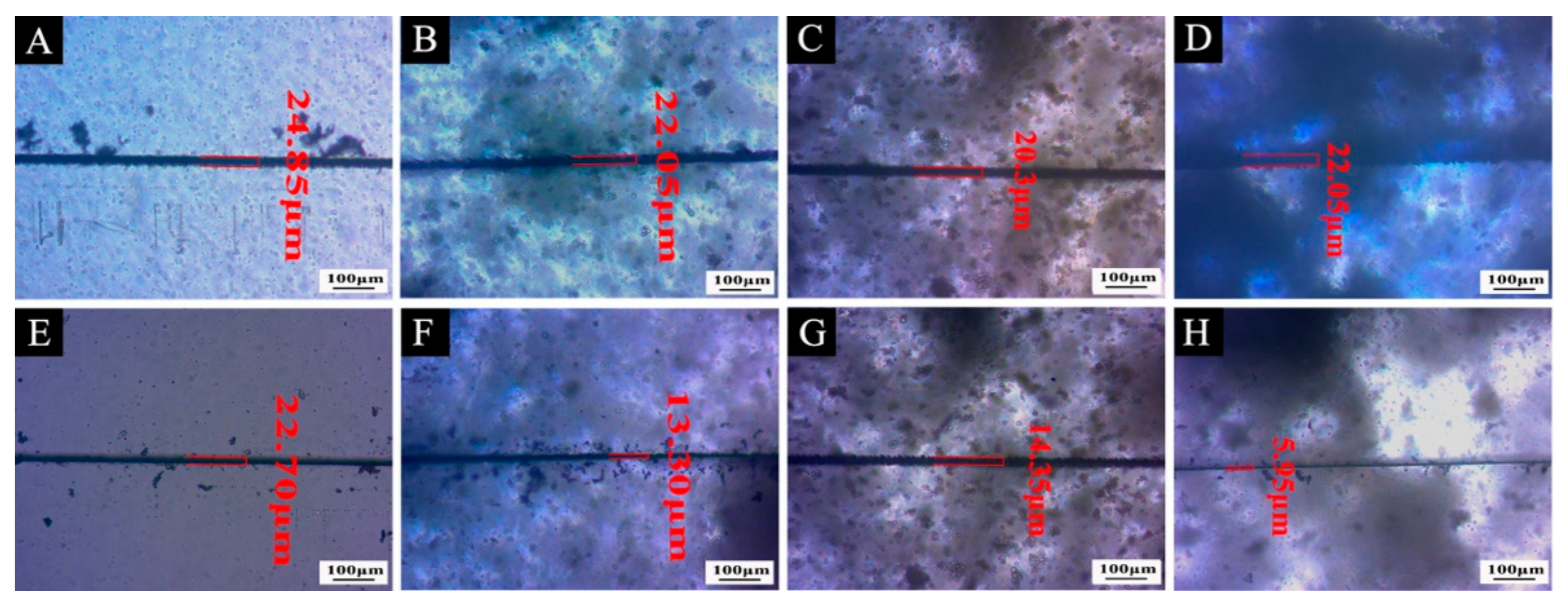
3.4. Self-Repairing Effect Analysis of the Waterborne Film Containing Microcapsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Gao, D.; Xu, W. Effect of paint process on the performance of modified poplar wood antique. Coatings 2021, 11, 1174. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Influence of the bottom color modification and material color modification process on the performance of modified poplar. Coatings 2021, 11, 660. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, W.; Li, D.; Zhang, H. Analyses on chemical composition of ancient wood structural component by using near infrared spectroscopy. J. For. Eng. 2021, 6, 114–119. [Google Scholar]
- Xu, W.; Fang, X.; Han, J.; Wu, Z. Effect of coating thickness on sound absorption property of four wood species commonly used for piano soundboards. Wood Fiber Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ren, J.; Zheng, X.; Pan, B.; Leng, W. Effects of three kinds of fungi on color, chemical composition and route of in-fection of Picea sitchensis. J. For. Eng. 2021, 6, 88–93. [Google Scholar]
- Feng, X.; Chen, J.; Wu, Z.; Wu, Y.; Gan, J. The formation mechanism of “skin-tactile” coating and its application and trends in furniture. J. For. Eng. 2021, 6, 167–175. [Google Scholar]
- Liu, Q.; Gao, D.; Xu, W. Effect of sanding processes on the surface properties of modified poplar coated by primer compared with mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, J.; Zhang, M.; Chang, S.; Liu, Y.; Zheng, L.; Li, X. Research on wettability of wood surface with waterborne-resin priming paint. J. For. Eng. 2021, 6, 178–183. [Google Scholar]
- Hu, J.; Liu, Y.; Wu, Z.; Pang, X. Construction of bionic structural color coating on wood surface based on polystyrene micro-spheres. J. For. Eng. 2021, 6, 35–42. [Google Scholar]
- Liu, Y. Self-assembly of Poly (Styrene-methyl Methacrylate-acrylic Acid) (P(St-MMA-AA)) Colloidal Microspheres on Wood Surface by Thermal-assisted Gravity Deposition. Wood Sci. Technol. 2021, 55, 403–417. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.R.; Zhou, Q.X. Preparation and characterization of microcapsules based self-healing coatings containing epoxy ester as healing agent. Prog. Org. Coat. 2018, 125, 403–410. [Google Scholar] [CrossRef]
- Thakur, T.; Gaur, B.; Singha, A.S. Bio-based epoxy/imidoamine encapsulated microcapsules and their application for high performance self-healing coatings. Prog. Org. Coat. 2021, 159, 106436. [Google Scholar] [CrossRef]
- Zotiadis, C.; Patrikalos, I.; Loukaidou, V.; Korres, D.M.; Karantonis, A.; Vouyiouka, S. Self-healing coatings based on poly(urea-formaldehyde) microcapsules: In situ polymerization, capsule properties and application. Prog. Org. Coat. 2021, 161, 106475. [Google Scholar] [CrossRef]
- Li, J.Y.; Shi, H.W.; Liu, F.C.; Han, E.H. Self-healing of an epoxy resin using scandium (III) triflate as a catalytic curing agent. Prog. Org. Coat. 2021, 156, 106236. [Google Scholar] [CrossRef]
- Naikwadi, A.T.; Samui, A.B.; Mahanwar, P.A. Melamine-formaldehyde microencapsulated n-Tetracosane phase change material for solar thermal energy storage in coating. Sol. Energy Mater. Sol. C. 2020, 215, 110676. [Google Scholar] [CrossRef]
- Zhang, B.L.; Li, S.S.; Fei, X.N.; Zhao, H.B.; Lou, X.Y. Enhanced mechanical properties and thermal conductivity of paraffin microcapsules shelled by hydrophobic-silicon carbide modified melamine-formaldehyde resin. Colloids Surf. A 2020, 603, 125219. [Google Scholar] [CrossRef]
- Guo, M.L.; Li, W.; Han, N.; Wang, J.P.; Su, J.F.; Li, J.J.; Zhang, X.X. Novel dual-component microencapsulated hydrophobic amine and microencapsulated isocyanate used for self-healing anti-corrosion coating. Polymers 2018, 10, 319. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.D.; Oilier, R.; Alvarez, V.; Vallo, C. Effect of the preparation method on the structure of linseed oil-filled poly(urea-formaldehyde) microcapsules. Prog. Org. Coat. 2016, 97, 194–202. [Google Scholar] [CrossRef]
- Revuelta, M.V.; Bogdan, S.; Gamez-Espinosa, E.; Deya, M.C.; Romagnoli, R. Green antifungal waterborne coating based on essential oil microcapsules. Prog. Org. Coat. 2021, 151, 106101. [Google Scholar] [CrossRef]
- Bar, H.; Bianco-Peled, H. The unique nanostructure of shellac films. Prog. Org. Coat. 2021, 157, 106328. [Google Scholar] [CrossRef]
- Prawatborisut, M.; Seidi, F.; Yiamsawas, D.; Crespy, D. PEGylation of shellac-based nanocarriers for enhanced colloidal stability. Colloids Surf. B 2019, 183, 110434. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yan, X.X. Preparation and Self-Repairing Properties of MF-Coated Shellac Water-Based Microcapsules. Coatings 2020, 10, 778. [Google Scholar] [CrossRef]
- Tao, Y.; Yan, X.X. Influence of HLB Value of Emulsifier on the Properties of Microcapsules and Self-Healing Properties of Waterborne Coatings. Polymers 2022, 14, 1304. [Google Scholar] [CrossRef] [PubMed]
- Panchapornpon, D.; Limmatvapirat, C.; Luangtana-anan, M.; Nunthanid, J.; Sriamornsak, P.; Limmatvapirat, S. Fabrication of thermally stabilized shellac through solid state reaction with phthalic anhydride. Mater. Lett. 2011, 65, 1241–1244. [Google Scholar] [CrossRef]
- Ansari, M.F.; Sarkhel, G.; Goswami, D.N.; Baboo, B. Effect of temperature on coating properties of shellac-novolac blends. Pigment Resin Technol. 2013, 42, 326–334. [Google Scholar] [CrossRef]
- Ansari, M.F.; Sarkhel, G.; Goswami, D.N.; Baboo, B. Changes in the properties of shellac on blending with rosin, effect on storage. Pigment Resin Technol. 2013, 42, 256–263. [Google Scholar] [CrossRef]
- Yuan, Y.; He, N.; Xue, Q.R.; Guo, Q.Y.; Dong, L.Y.; Haruna, M.H.; Zhang, X.; Li, B.; Li, L. Shellac: A promising natural polymer in the food industry. Trends Food Sci. Technol. 2021, 109, 139–153. [Google Scholar] [CrossRef]
- Buch, K.; Penning, M.; Wachtersbach, E.; Maskos, M.; Langguth, P. Investigation of various shellac grades: Additional analysis for identity. Drug Dev. Ind. Pharm. 2009, 35, 694–703. [Google Scholar] [CrossRef]
- Yan, X.X.; Chang, Y.J.; Qian, X.Y. Preparation and Self-Repairing Properties of Urea Formaldehyde-Coated Epoxy Resin Microcapsules. Int. J. Polym. Sci. 2019, 2019, 7215783. [Google Scholar] [CrossRef] [Green Version]
- Li, R.R.; He, C.J.; Wang, X.D. Evaluation and modeling of processability of laser removal technique for bamboo outer layer. JOM 2021, 73, 2423–2430. [Google Scholar] [CrossRef]
- Novak, I.; Krupa, I.; Chodak, I. Investigation of the correlation between electrical conductivity and elongation at break in polyurethane-based adhesives. Synth. Met. 2002, 131, 93–98. [Google Scholar] [CrossRef]
- Yan, X.X.; Zhao, W.T.; Wang, L. Mechanism of Thermochromic and Self-Repairing of Waterborne Wood Coatings by Synergistic Action of Waterborne Acrylic Microcapsules and Fluorane Microcapsules. Polymers 2022, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Satdive, A.; Mestry, S.; Patil, D.; Mhaske, S.T. Synthesis of melamine formaldehyde cured castor oil based hydroxyl functional alkyd for coating application. Prog. Org. Coat. 2019, 131, 165–175. [Google Scholar] [CrossRef]
- Bhattacharyya, S.K.; Chakraborty, S.; Sharma, G.; Kumawat, P.; Dasgupta, S.; Bandyopadhyay, S.; Mukhopadhyay, R.; Bandyopadhyay, A. Shellac as a Multifunctional Additive (MFA) in a Typical Truck Tyre Sidewall Compound. Prog. Rubber Plast. Recycl. Technol. 2012, 28, 173–188. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J. Investigation of polystyrene-based microspheres from different copolymers and their structural color coatings on wood surface. Coatings 2021, 11, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, Z. Fabrication of coatings with structural color on a wood surface. Coatings 2020, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.X.; Han, Y.; Yin, T.Y. Coating Process Optimization and Self-Healing Performance Evaluation of Shellac Microcapsules Coated with Melamine/Rice Husk Powder. Appl. Sci. 2021, 11, 8373. [Google Scholar] [CrossRef]


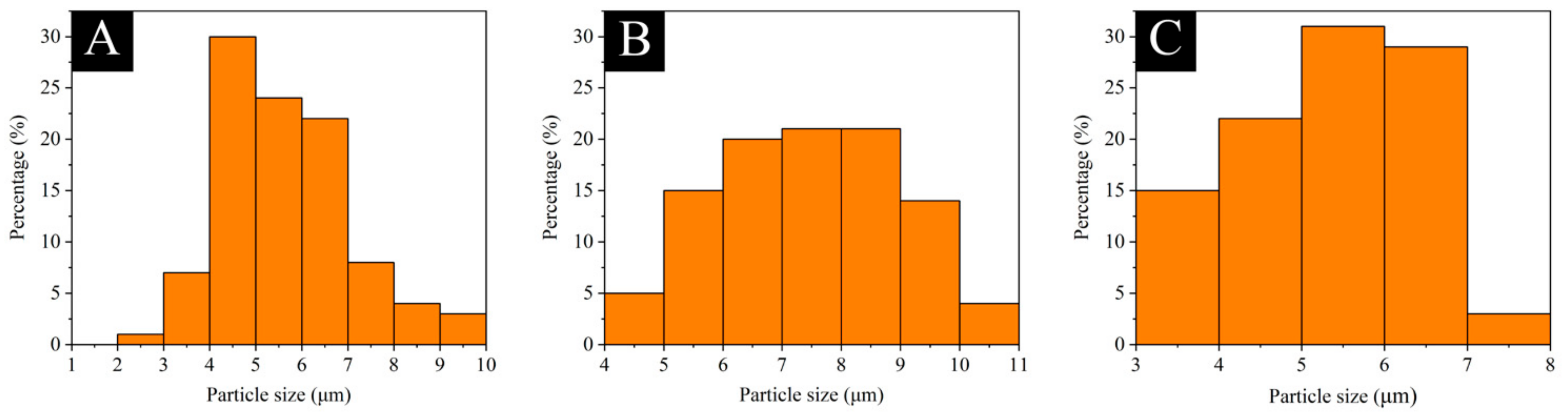
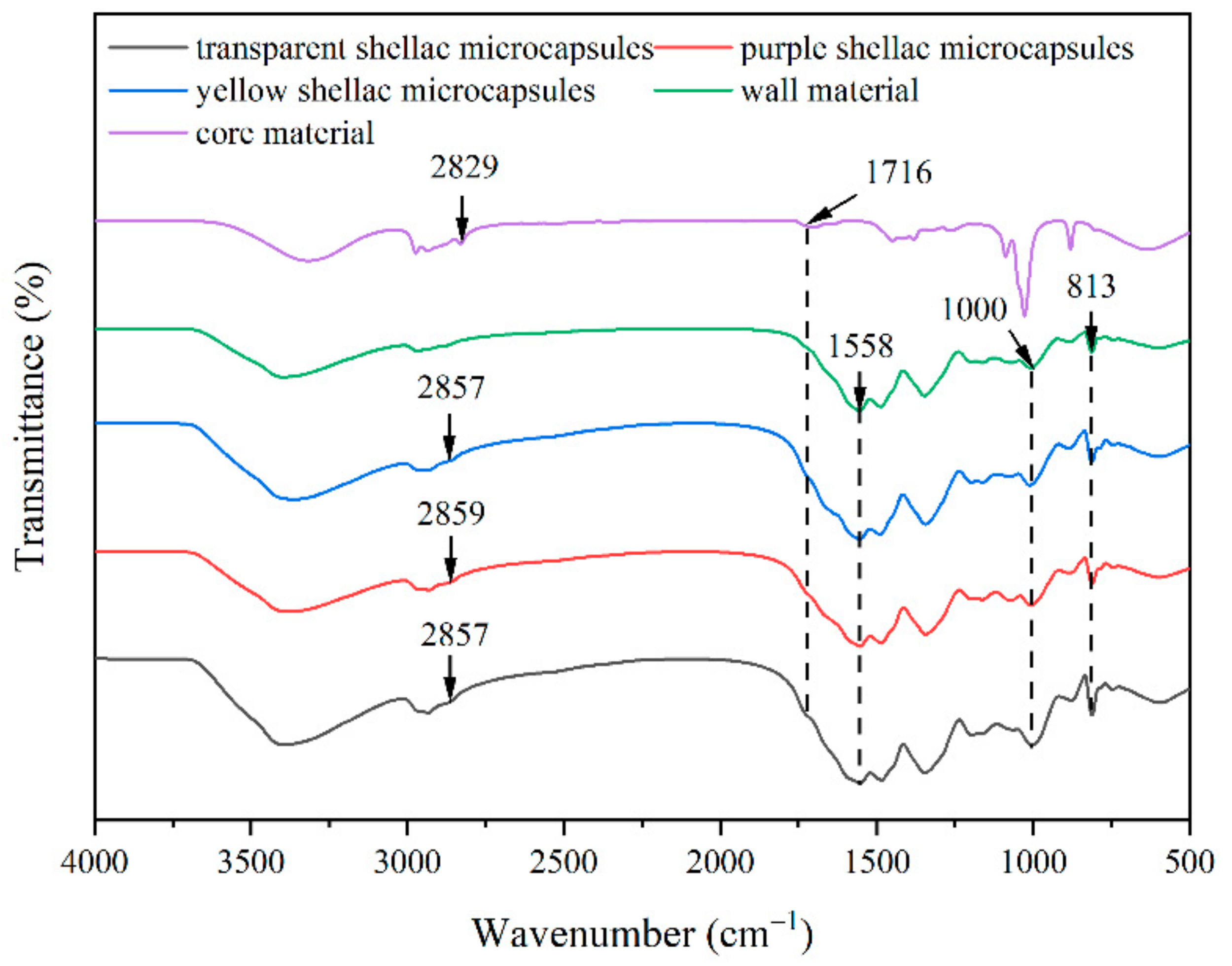
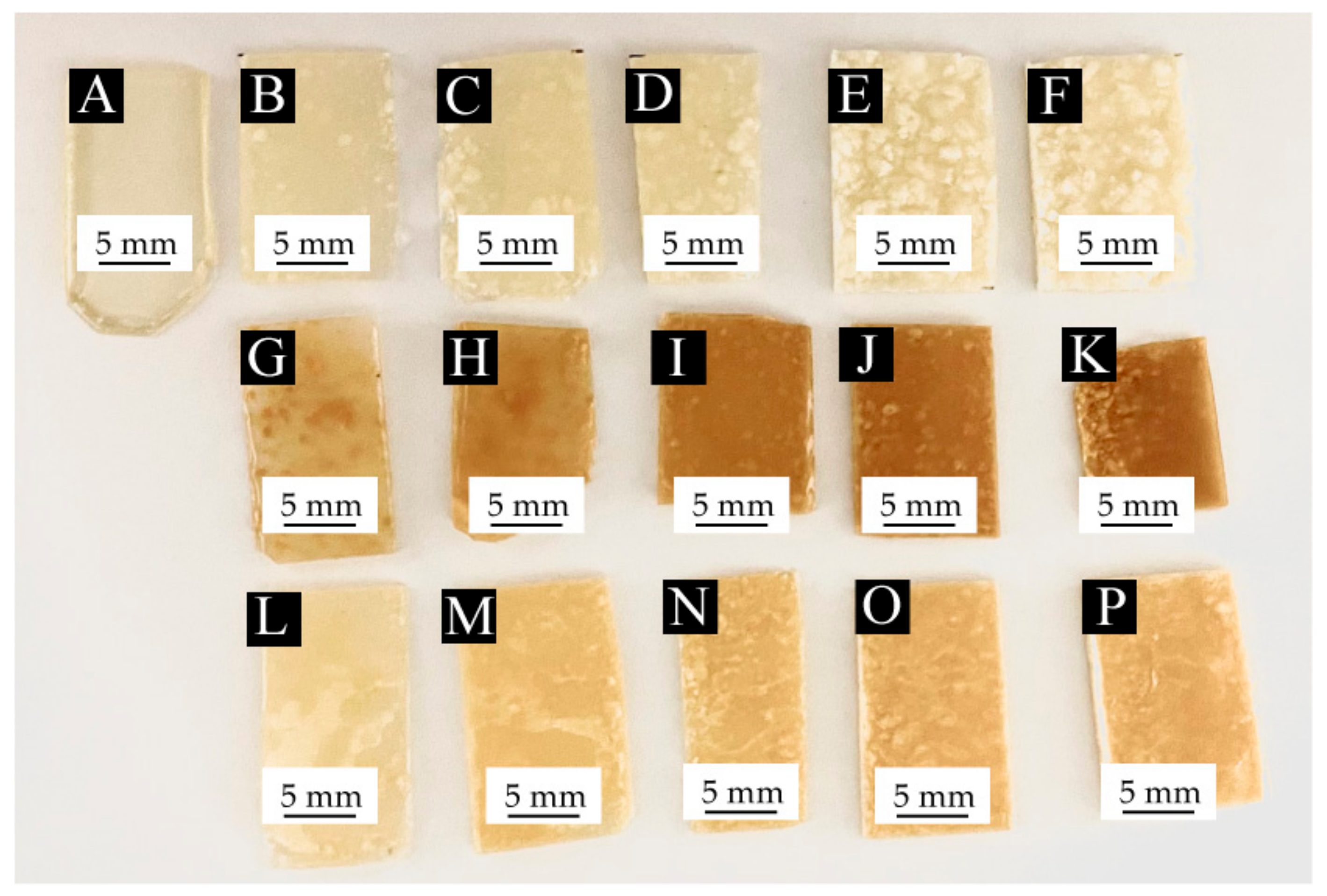
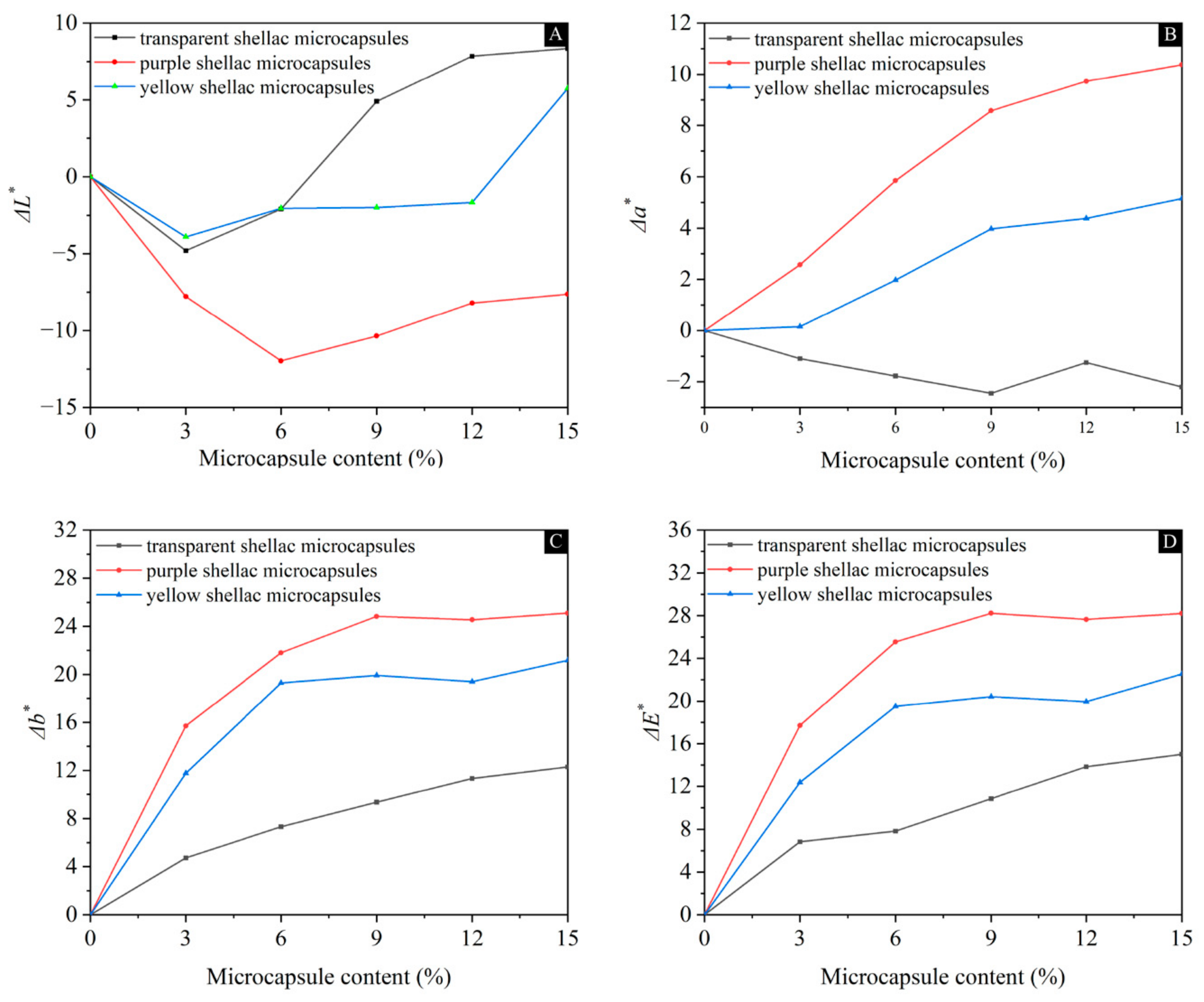
| Microcapsule Content (%) | Mass of Microcapsules (g) | Mass of Waterborne Coatings (g) | Total Mass (g) |
|---|---|---|---|
| 0 | 0 | 4.00 | 4.0 |
| 3.0 | 0.12 | 3.88 | 4.0 |
| 6.0 | 0.24 | 3.76 | 4.0 |
| 9.0 | 0.36 | 3.64 | 4.0 |
| 12.0 | 0.48 | 3.52 | 4.0 |
| 15.0 | 0.60 | 3.40 | 4.0 |
| Core Material | m1 (g) | m2 (g) | Coating Rate (%) |
|---|---|---|---|
| Transparent shellac | 1.00 | 0.64 | 38.0 |
| Puple shellac | 1.00 | 0.62 | 36.0 |
| Yellow shellac | 1.00 | 0.58 | 42.0 |
| Microcapsule Content (%) | Transparent Shellac Microcapsules | Purple Shellac Microcapsules | Yellow Shellac Microcapsules | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔL* | Δa* | Δb* | ΔE* | ΔL* | Δa* | Δb* | ΔE* | ΔL* | Δa* | Δb* | ΔE* | |
| 0 | −2.15 | 0.60 | −2.25 | 3.17 | −2.15 | 0.60 | −2.25 | 3.17 | −2.15 | 0.60 | −2.25 | 3.17 |
| 3.0 | −0.75 | 0.85 | −0.03 | 1.12 | −0.33 | 0.13 | 0.65 | 0.74 | −0.50 | −0.08 | −0.35 | 0.61 |
| 6.0 | −0.45 | 0.40 | 0.60 | 0.85 | −0.05 | 0.33 | −0.43 | 0.54 | −1.15 | 0.35 | −1.50 | 1.92 |
| 9.0 | 0.15 | −0.60 | 0.33 | 0.70 | −0.55 | −0.38 | −0.15 | 0.68 | 0.53 | 0.50 | 0.45 | 0.85 |
| 12.0 | −1.27 | −0.75 | −1.20 | 1.90 | 0.70 | −0.05 | −0.08 | 0.71 | −0.03 | −0.45 | −0.78 | 0.90 |
| 15.0 | −1.08 | −1.15 | −1.03 | 1.88 | 0.38 | −0.30 | −0.68 | 0.83 | 0.45 | 0.33 | −0.35 | 0.67 |
| Microcapsule Types | Microcapsule Content (%) | Maximum Tensile Strength (MPa) | ||
|---|---|---|---|---|
| Original Waterborne Film | Waterborne Film after Being Damaged | Waterborne Film after Self-Repairing for 24 h | ||
| Transparent shellac microcapsules | 0 | 15.86 ± 0.33 | 8.89 ± 0.12 | 8.78 ± 0.19 |
| 3.0 | 16.67 ± 0.60 | 6.93 ± 0.14 | 12.65 ± 0.21 | |
| 6.0 | 13.58 ± 0.40 | 11.94 ± 0.26 | 13.92 ± 0.35 | |
| 9.0 | 12.21 ± 0.39 | 13.83 ± 0.16 | 11.58 ± 0.32 | |
| 12.0 | 9.77 ± 0.23 | 9.51 ± 0.17 | 9.25 ± 0.22 | |
| 15.0 | 13.97 ± 0.17 | 11.07 ± 0.20 | 6.46 ± 0.20 | |
| Purple shellac microcapsules | 3.0 | 15.71 ± 0.42 | 10.30 ± 0.28 | 9.58 ± 0.12 |
| 6.0 | 14.67 ± 0.27 | 12.65 ± 0.29 | 10.61 ± 0.26 | |
| 9.0 | 9.70 ± 0.22 | 8.49 ± 0.21 | 11.52 ± 0.36 | |
| 12.0 | 10.45 ± 0.40 | 12.62 ± 0.44 | 5.78 ± 0.14 | |
| 15.0 | 10.30 ± 0.23 | 10.13 ± 0.16 | 10.64 ± 0.26 | |
| Yellow shellac microcapsules | 3.0 | 16.06 ± 0.23 | 14.65 ± 0.44 | 15.71 ± 0.33 |
| 6.0 | 14.22 ± 0.35 | 20.43 ± 0.48 | 9.57 ± 0.22 | |
| 9.0 | 11.95 ± 0.28 | 14.14 ± 0.33 | 11.15 ± 0.30 | |
| 12.0 | 9.33 ± 0.25 | 12.07 ± 0.26 | 18.09 ± 0.30 | |
| 15.0 | 10.51 ± 0.29 | 8.95 ± 0.14 | 18.83 ± 0.35 | |
| Microcapsule Types | Microcapsule Content (%) | Elongation at Break (%) | ||
|---|---|---|---|---|
| Original Waterborne Film | Waterborne Film after Being Damaged | Waterborne Film after Self-Repairing for 24 h | ||
| Transparent shellac mi-crocapsules | 0 | 58.14 ± 0.79 | 40.07 ± 0.72 | 43.77 ± 0.68 |
| 3.0 | 65.58 ± 0.71 | 32.58 ± 0.53 | 47.03 ± 0.98 | |
| 6.0 | 44.60 ± 0.94 | 21.59 ± 0.36 | 31.47 ± 0.99 | |
| 9.0 | 27.86 ± 0.64 | 16.28 ± 0.46 | 24.64 ± 0.36 | |
| 12.0 | 21.57 ± 0.29 | 13.67 ± 0.29 | 20.08 ± 0.42 | |
| 15.0 | 18.08 ± 0.46 | 12.22 ± 0.34 | 15.32 ± 0.34 | |
| Purple shellac microcapsules | 3.0 | 47.84 ± 0.75 | 33.99 ± 0.93 | 36.97 ± 0.88 |
| 6.0 | 36.35 ± 0.72 | 16.79 ± 0.43 | 22.16 ± 0.62 | |
| 9.0 | 20.56 ± 0.43 | 14.44 ± 0.44 | 18.70 ± 0.57 | |
| 12.0 | 18.52 ± 0.52 | 10.99 ± 0.32 | 13.71 ± 0.40 | |
| 15.0 | 18.16 ± 0.11 | 9.71 ± 0.13 | 11.70 ± 0.31 | |
| Yellow shellac microcapsules | 3.0 | 94.71 ± 0.57 | 19.85 ± 0.63 | 82.26 ± 2.06 |
| 6.0 | 26.35 ± 0.71 | 9.06 ± 0.18 | 22.14 ± 0.58 | |
| 9.0 | 21.13 ± 0.55 | 5.87 ± 0.13 | 14.65 ± 0.45 | |
| 12.0 | 20.52 ± 0.35 | 3.74 ± 0.11 | 6.12 ± 0.16 | |
| 15.0 | 14.84 ± 0.18 | 0.92 ± 0.02 | 4.40 ± 0.13 | |
| Microcapsule Content (%) | Repair Rate of Waterborne Coatings (%) | ||
|---|---|---|---|
| Transparent Shellac Microcapsules | Purple Shellac Microcapsules | Yellow Shellac Microcapsules | |
| 0 | 20.49 | 20.49 | 20.49 |
| 3.0 | 43.79 | 21.47 | 36.48 |
| 6.0 | 42.94 | 27.46 | 47.19 |
| 9.0 | 72.22 | 69.60 | 72.66 |
| 12.0 | 81.17 | 36.08 | 25.32 |
| 15.0 | 52.96 | 23.50 | 70.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Yan, X.; Tao, Y. Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings. Coatings 2022, 12, 1056. https://doi.org/10.3390/coatings12081056
Han Y, Yan X, Tao Y. Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings. Coatings. 2022; 12(8):1056. https://doi.org/10.3390/coatings12081056
Chicago/Turabian StyleHan, Yan, Xiaoxing Yan, and Yu Tao. 2022. "Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings" Coatings 12, no. 8: 1056. https://doi.org/10.3390/coatings12081056
APA StyleHan, Y., Yan, X., & Tao, Y. (2022). Effect of Transparent, Purple, and Yellow Shellac Microcapsules on the Optical Properties and Self-Healing Performance of Waterborne Coatings. Coatings, 12(8), 1056. https://doi.org/10.3390/coatings12081056





