Cost-Effective Nanoporous Gold Obtained by Dealloying Metastable Precursor, Au33Fe67, Reveals Excellent Methanol Electro-Oxidation Performance
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
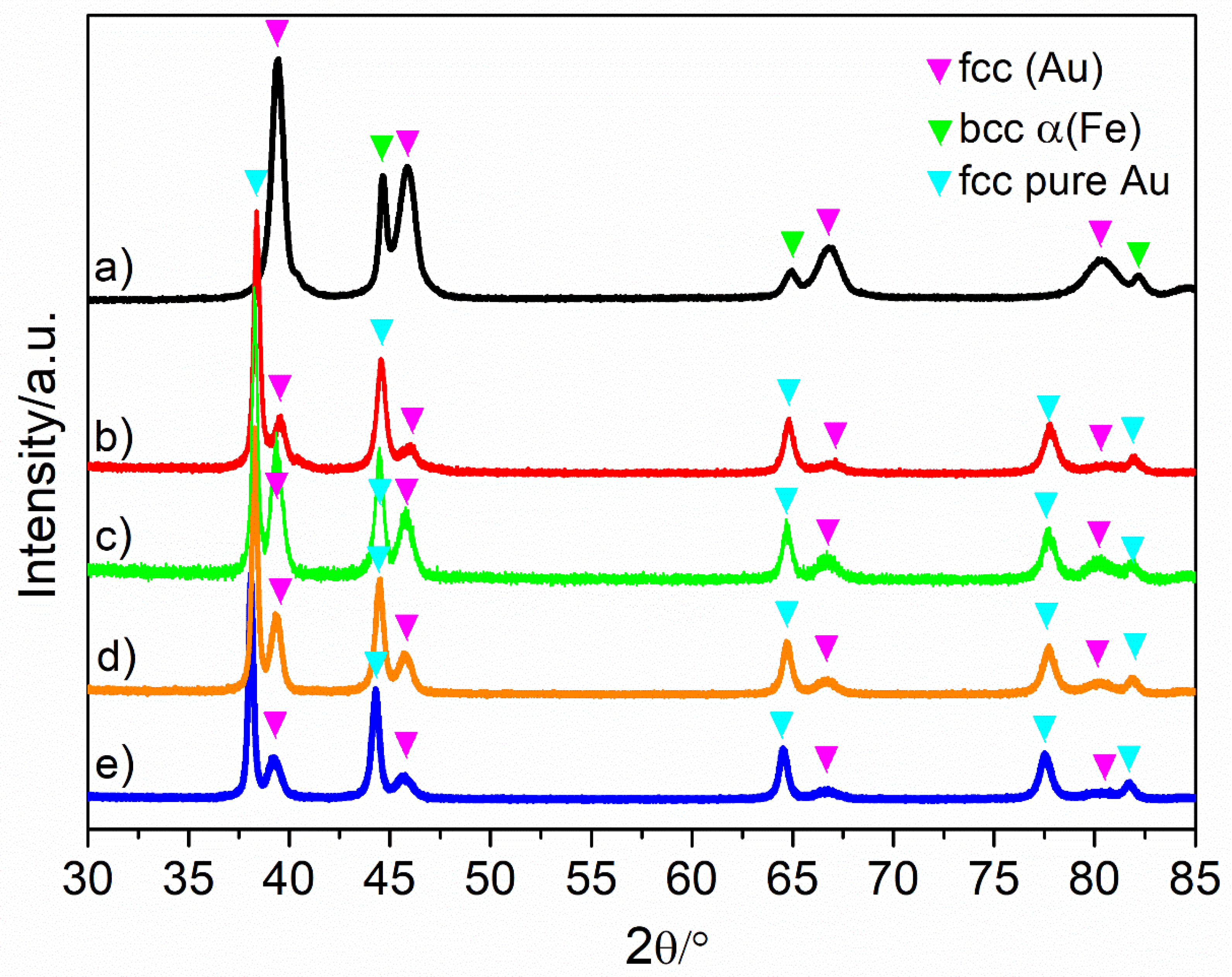
3.1. As-Quenched Ribbon Characterization
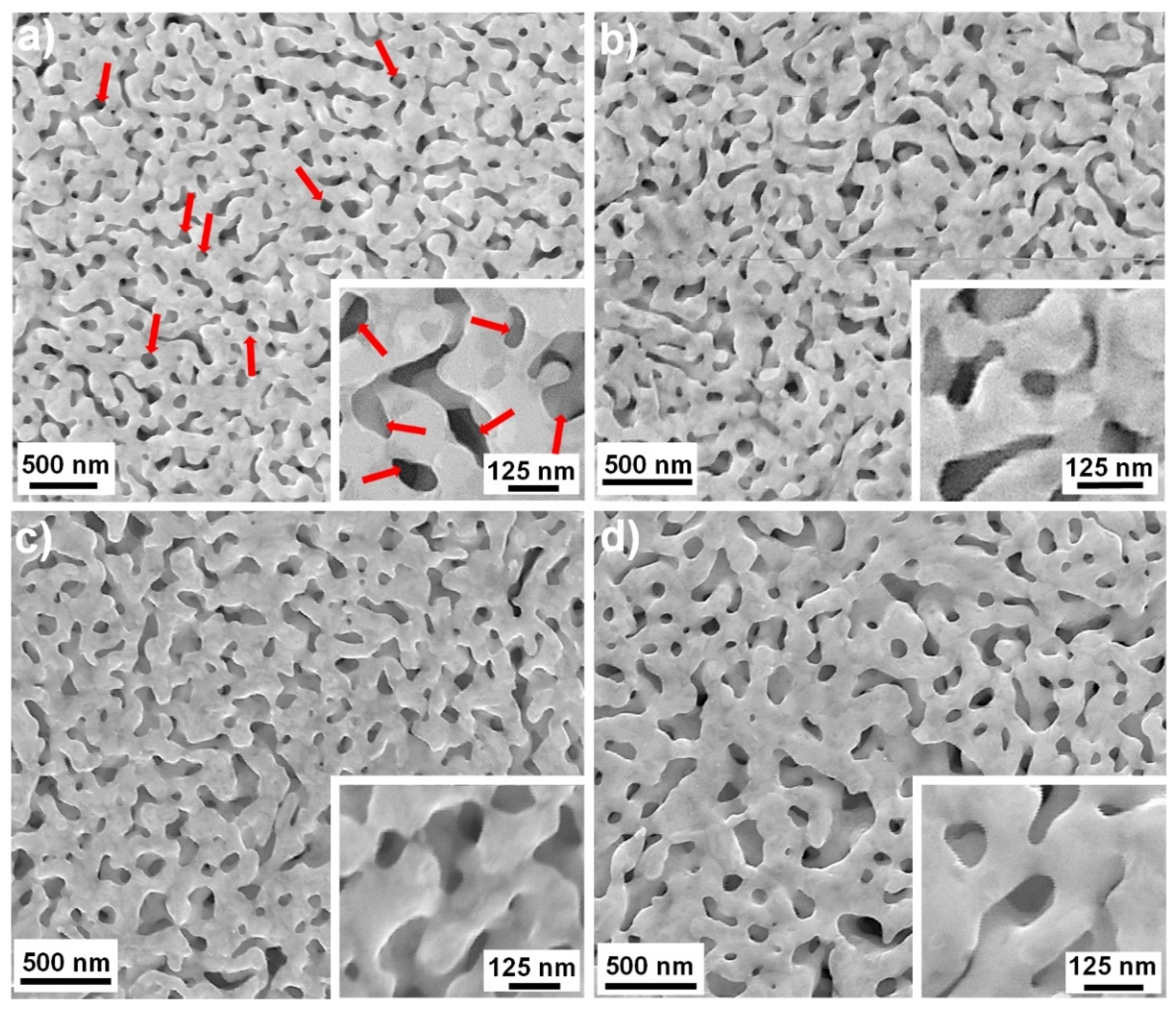
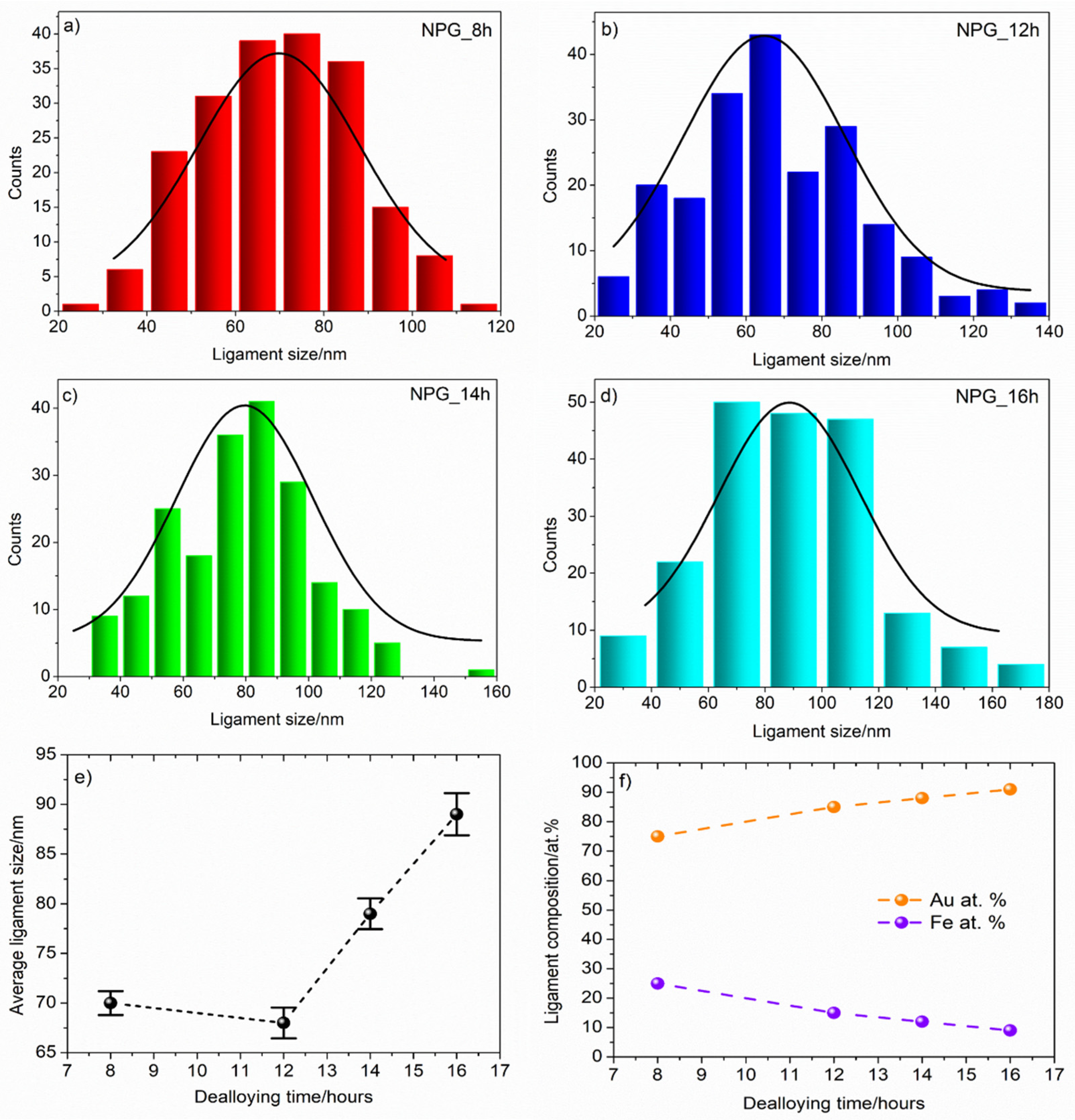
3.2. Chemical Dealloying and Nanoporous Gold
3.3. Electrochemical Behavior in Basic Solution
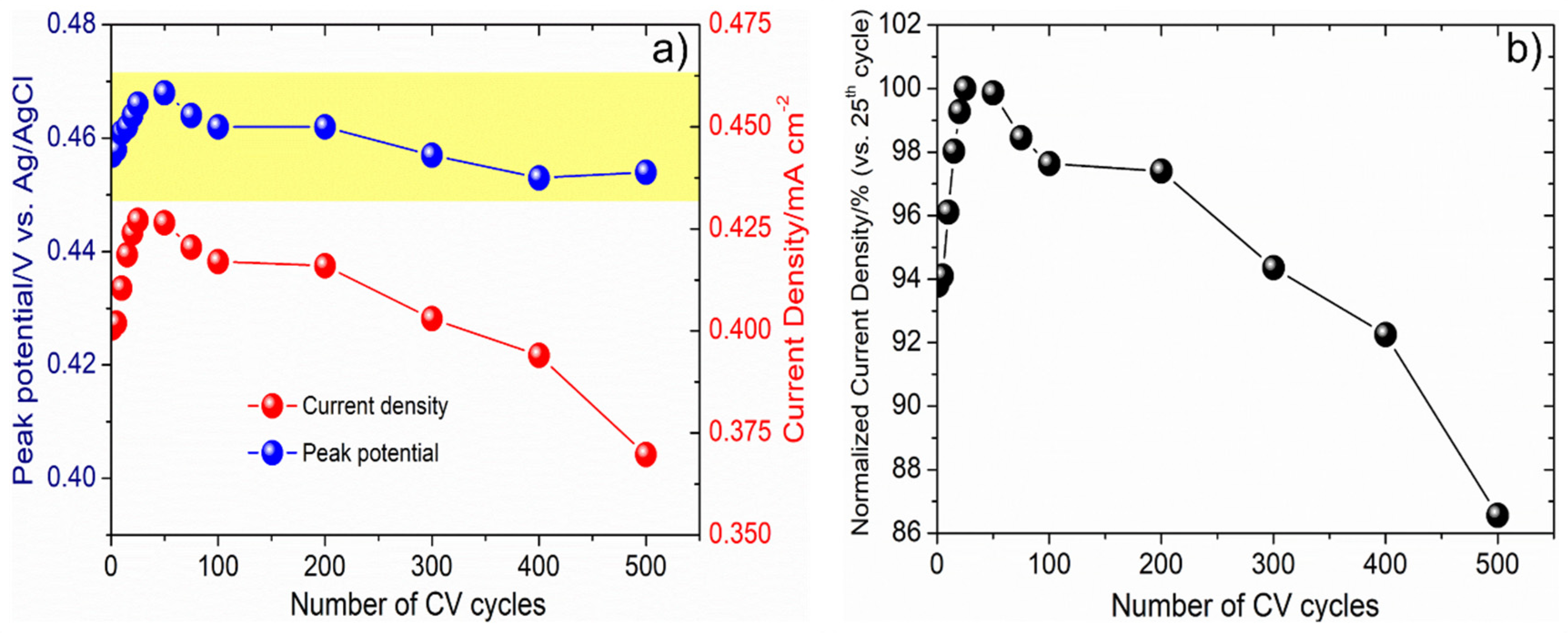
3.4. Methanol Electro-Oxidation Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.-J. Electrodeposited platinum on de-alloyed nanoporous gold with enhanced electro-catalytic performance. Appl. Surf. Sci. 2019, 476, 412–417. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Shi, P.; Huang, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.J. Nanoporous gold chemically de-alloyed from Au-based amorphous thin film for electrochemical nonenzymatic H2O2 sensing. Chem. Phys. Lett. 2019, 723, 22–27. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, S.; Wang, X.; Xu, P. Nanoporous gold: A review and potentials in biotechnological and biomedical applications. Nano Sel. 2021, 2, 1437–1458. [Google Scholar] [CrossRef]
- Mohan, K.; Shahane, N.; Liu, R.; Smet, V.; Antoniou, A. A Review of Nanoporous Metals in Interconnects. JOM 2018, 70, 2192–2204. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous Gold for Energy Applications. Chem. Rec. 2021, 21, 1199–1215. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous gold: Preparation and applications to catalysis and sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Scaglione, F.; Gebert, A.; Battezzati, L. Dealloying of an Au-based amorphous alloy. Intermetallics 2010, 18, 2338–2342. [Google Scholar] [CrossRef] [Green Version]
- Scaglione, F.; Xue, Y.; Celegato, F.; Rizzi, P.; Battezzati, L. Amorphous molybdenum sulphide @ nanoporous gold as catalyst for hydrogen evolution reaction in acidic environment. J. Mater. Sci. 2018, 53, 12388–12398. [Google Scholar] [CrossRef] [Green Version]
- Scaglione, F.; Celegato, F.; Rizzi, P.; Battezzati, L. A comparison of de-alloying crystalline and amorphous multicomponent Au alloys. Intermetallics 2015, 66, 82–87. [Google Scholar] [CrossRef]
- Scaglione, F.; Rizzi, P.; Battezzati, L. De-alloying kinetics of an Au-based amorphous alloys. J. Alloys Compd. 2012, 536, S60–S64. [Google Scholar] [CrossRef]
- Rizzi, P.; Scaglione, F.; Battezzati, L. Nanoporous gold by dealloying of an amorphous precursor. J. Alloys Compd. 2014, 586, S117–S120. [Google Scholar] [CrossRef]
- Van Petegem, S.; Brandstetter, S.; Maass, R.; Hodge, A.M.; El-Dasher, B.S.; Biener, J.; Schmitt, B.; Borca, C.; Swygenhoven, H. Van On the Microstructure of Nanoporous Gold: An X-ray Diffraction Study. Nano Lett. 2009, 9, 1158–1163. [Google Scholar] [CrossRef]
- He, Z.; Huang, Y.; He, F. Preparation of nanoporous molybdenum film by dealloying an immiscible Mo–Zn system for hydrogen evolution reaction. RSC Adv. 2016, 6, 15390–15393. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; McCue, I.; Snyder, J.; Crozier, P.; Erlebacher, J.; Sieradzki, K. Dealloying of Noble-Metal Alloy Nanoparticles. Nano Lett. 2014, 14, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Paschalidou, E.M.; Fiore, G.; Xue, Y.; Scaglione, F.; Celegato, F.; Gebert, A.; Oswald, S.; Wolff, U.; Rizzi, P.; Battezzati, L. Comparing selective corrosion of Au-based amorphous, partially amorphous, and devitrified alloys. J. Alloys Compd. 2018, 745, 212–216. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L. Improving the chemical de-alloying of amorphous Au alloys. Corros. Sci. 2017, 127, 141–146. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Celegato, F.; Denis, P.; Fecht, H.-J.; Rizzi, P.; Battezzati, L. Shape controlled gold nanostructures on de-alloyed nanoporous gold with excellent SERS performance. Chem. Phys. Lett. 2018, 709, 46–51. [Google Scholar] [CrossRef]
- The Materials Information Society. ASM Handbook Volume 3—Alloy Phase Diagrams; The Materials Information Society: Materials Park, OH, USA, 1992. [Google Scholar] [CrossRef]
- Alaba, P.A.; Lee, C.S.; Abnisa, F.; Aroua, M.K.; Cognet, P.; Pérès, Y.; Wan Daud, W.M.A. A review of recent progress on electrocatalysts toward efficient glycerol electrooxidation. Rev. Chem. Eng. 2020, 36, 779–811. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Cossar, E.; Ntais, S.; Baranova, E.A. Electrochemical modification of nickel surfaces for efficient glycerol electrooxidation. J. Power Sources 2018, 375, 310–319. [Google Scholar] [CrossRef]
- Vaishnavi, B.J.; Sujith, S.; Kulal, N.; Manjunathan, P.; Shanbhag, G.V. Utilization of renewable resources: Investigation on role of active sites in zeolite catalyst for transformation of furfuryl alcohol into alkyl levulinate. Mol. Catal. 2021, 502, 111361. [Google Scholar] [CrossRef]
- Venkateswarlu, K. Ashes from Organic Waste as Reagents in Synthetic Chemistry: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Cruz-Navarro, J.A.; Mendoza-Huizar, L.H.; Salazar-Pereda, V.; Cobos-Murcia, J.Á.; Colorado-Peralta, R.; Álvarez-Romero, G.A. Progress in the use of electrodes modified with coordination compounds for methanol electro-oxidation. Inorg. Chim. Acta 2021, 520, 120293. [Google Scholar] [CrossRef]
- Valter, M.; Wickman, B.; Hellman, A. Solvent Effects for Methanol Electrooxidation on Gold. J. Phys. Chem. C 2021, 125, 1355–1360. [Google Scholar] [CrossRef]
- Kang, Y.; Xue, Q.; Jin, P.; Jiang, J.; Zeng, J.; Chen, Y. Rhodium Nanosheets–Reduced Graphene Oxide Hybrids: A Highly Active Platinum-Alternative Electrocatalyst for the Methanol Oxidation Reaction in Alkaline Media. ACS Sustain. Chem. Eng. 2017, 5, 10156–10162. [Google Scholar] [CrossRef]
- Krawczyk, P.; Rozmanowski, T.; Frankowski, M. Methanol Electrooxidation at Electrodes Made of Exfoliated Graphite/Nickel/Palladium Composite. Catal. Lett. 2019, 149, 2307–2316. [Google Scholar] [CrossRef] [Green Version]
- Tritsaris, G.A.; Rossmeisl, J. Methanol oxidation on model elemental and bimetallic transition metal surfaces. J. Phys. Chem. C 2012, 116, 11980–11986. [Google Scholar] [CrossRef]
- Peng, F.; Zhou, C.; Wang, H.; Yu, H.; Liang, J.; Yang, J. The role of RuO2 in the electrocatalytic oxidation of methanol for direct methanol fuel cell. Catal. Commun. 2009, 10, 533–537. [Google Scholar] [CrossRef]
- Nagavolu, C.; Susmitha, K.; Raghavender, M.; Giribabu, L.; Bhanu Sankara Rao, K.; Smith, C.T.G.; Mills, C.A.; Silva, S.R.P.; Srikanth, V.V.S.S. Pt-free spray coated reduced graphene oxide counter electrodes for dye sensitized solar cells. Sol. Energy 2016, 137, 143–147. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Meenakshamma, A.; Susmitha, K.; Charanadhar, N.; Srikanth, V.V.S.S.; Narendra Babu, S.; Venkata Subbaiah, Y.P.; Venkateswarlu, K.; Raghavender, M. A transparent and Pt-free all-carbon nanocomposite counter electrode catalyst for efficient dye sensitized solar cells. Sol. Energy 2019, 193, 568–575. [Google Scholar] [CrossRef]
- Dong, B.; Li, W.; Huang, X.; Ali, Z.; Zhang, T.; Yang, Z.; Hou, Y. Fabrication of hierarchical hollow Mn doped Ni(OH)2 nanostructures with enhanced catalytic activity towards electrochemical oxidation of methanol. Nano Energy 2019, 55, 37–41. [Google Scholar] [CrossRef]
- Remmel, A.L.; Ratso, S.; Divitini, G.; Danilson, M.; Mikli, V.; Uibu, M.; Aruväli, J.; Kruusenberg, I. Nickel and Nitrogen-Doped Bifunctional ORR and HER Electrocatalysts Derived from CO2. ACS Sustain. Chem. Eng. 2022, 10, 134–145. [Google Scholar] [CrossRef]
- Rezaee, S.; Shahrokhian, S. Facile synthesis of petal-like NiCo/NiO-CoO/nanoporous carbon composite based on mixed-metallic MOFs and their application for electrocatalytic oxidation of methanol. Appl. Catal. B Environ. 2019, 244, 802–813. [Google Scholar] [CrossRef]
- Raj, D.; Scaglione, F.; Fiore, G.; Celegato, F.; Rizzi, P. Nanostructured molybdenum oxides from aluminium-based intermetallic compound: Synthesis and application in hydrogen evolution reaction. Nanomaterials 2021, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Wang, Y.; Wang, X.; Qi, Z.; Ji, H.; Zhao, C. Formation of ultrafine nanoporous gold related to surface diffusion of gold adatoms during dealloying of Al2Au in an alkaline solution. Scr. Mater. 2010, 62, 137–140. [Google Scholar] [CrossRef]
- Machado, S.A.S.; Avaca, L.A. The hydrogen evolution reaction on nickel surfaces stabilized by H-absorption. Electrochim. Acta 1994, 39, 1385–1391. [Google Scholar] [CrossRef]
- Ahn, S.H.; Hwang, S.J.; Yoo, S.J.; Choi, I.; Kim, H.-J.; Jang, J.H.; Nam, S.W.; Lim, T.-H.; Lim, T.; Kim, S.-K.; et al. Electrodeposited Ni dendrites with high activity and durability for hydrogen evolution reaction in alkaline water electrolysis. J. Mater. Chem. 2012, 22, 15153–15159. [Google Scholar] [CrossRef]
- Lukaszewski, M.; Soszko, M.; Czerwiński, A. Electrochemical methods of real surface area determination of noble metal electrodes—An overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Ogihara, H.; Fujii, M.; Saji, T. Hydrogen evolution reaction (HER) over electroless-deposited nickel nanospike arrays. RSC Adv. 2014, 4, 58660–58663. [Google Scholar] [CrossRef] [Green Version]
- Favez, D.; Wagnière, J.D.; Rappaz, M. Au-Fe alloy solidification and solid-state transformations. Acta Mater. 2010, 58, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.M.; Sivakumar, T.; Fujita, T.; Jayavel, R.; Abe, H. Synthesis of metastable Au-Fe alloy using ordered nanoporous silica as a hard template. Metals 2018, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Detsi, E.; De Jong, E.; Zinchenko, A.; Vuković, Z.; Vuković, I.; Punzhin, S.; Loos, K.; Ten Brinke, G.; De Raedt, H.A.; Onck, P.R.; et al. On the specific surface area of nanoporous materials. Acta Mater. 2011, 59, 7488–7497. [Google Scholar] [CrossRef]
- Ji, C.; Searson, P.C. Synthesis and characterization of nanoporous gold nanowires. J. Phys. Chem. B 2003, 107, 4494–4499. [Google Scholar] [CrossRef]
- Tan, Y.H.; Davis, J.A.; Fujikawa, K.; Ganesh, N.V.; Demchenko, A.V.; Stine, K.J. Surface area and pore size characteristics of nanoporous gold subjected to thermal, mechanical, or surface modification studied using gas adsorption isotherms, cyclic voltammetry, thermogravimetric analysis, and scanning electron microscopy. J. Mater. Chem. 2012, 22, 6733–6745. [Google Scholar] [CrossRef]
- Wittstock, A.; Zielasek, V.; Biener, J.; Friend, C.M.; Bäumer, M. Nanoporous Gold Catalysts for Selective Gas-Phase Oxidative Coupling of Methanol at Low Temperature. Science 2010, 327, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.Y.; Yuan, H.T.; Iwasa, Y.; Chen, M.W. Three-dimensional nanoporous gold for electrochemical supercapacitors. Scr. Mater. 2011, 64, 923–926. [Google Scholar] [CrossRef]
- Weissmüller, J.; Viswanath, R.N.; Kramer, D.; Zimmer, P.; Würschum, R.; Gleiter, H. Charge-induced reversible strain in a metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef]
- Jin, H.J.; Wang, X.L.; Parida, S.; Wang, K.; Seo, M.; Weissmüller, J. Nanoporous au-pt alloys as large strain electrochemical actuators. Nano Lett. 2010, 10, 187–194. [Google Scholar] [CrossRef]
- Biener, J.; Wittstock, A.; Zepeda-Ruiz, L.A.; Biener, M.M.; Zielasek, V.; Kramer, D.; Viswanath, R.N.; Weissmüller, J.; Bäumer, M.; Hamza, A.V. Surface-chemistry-driven actuation in nanoporous gold. Nat. Mater. 2009, 8, 47–51. [Google Scholar] [CrossRef]
- Detsi, E.; van de Schootbrugge, M.; Punzhin, S.; Onck, P.R.; De Hosson, J.T.M. On tuning the morphology of nanoporous gold. Scr. Mater. 2011, 64, 319–322. [Google Scholar] [CrossRef]
- Paschalidou, E.M.; Scaglione, F.; Gebert, A.; Oswald, S.; Rizzi, P.; Battezzati, L. Partially and fully de-alloyed glassy ribbons based on Au: Application in methanol electro-oxidation studies. J. Alloys Compd. 2016, 667, 302–309. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Kumar, A.; da Silva, M.I.; Toma, H.E.; Martins, P.R.; Araki, K.; Bertotti, M.; Angnes, L. Nanoporous Gold-Based Materials for Electrochemical Energy Storage and Conversion. Energy Technol. 2021, 9, 2000927. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Y.; Zhang, N.; Yin, C.; Zhang, X.; Liu, X. Carbon riveted Pt-MnO2/reduced graphene oxide anode catalyst for DMFC. Catal. Commun. 2017, 100, 66–70. [Google Scholar] [CrossRef]
- Graf, M.; Haensch, M.; Carstens, J.; Wittstock, G.; Weissmüller, J. Electrocatalytic methanol oxidation with nanoporous gold: Microstructure and selectivity. Nanoscale 2017, 9, 17839–17848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blengini, G.A.; Latunussa, C.E.L.; Eynard, U.; Torres de Matos, C.; Wittmer, D.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials (2020) Final Report; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- de Oliveira, J.P.J.; de Sá, A.C.; Paim, L.L. Electrocatalysis of Ethanol and Methanol Electrooxidation by Composite Electrodes with NiOOH/FeOOH Supported on Reduced Graphene Oxide onto Composite Electrodes. Chem. Proc. 2020, 2, 2. [Google Scholar] [CrossRef]
- Abdullah Mirzaie, R.; Eshghi, A. Study of methanol electro-oxidation on Ni and Ni–Pt/carbon paper electrodes for direct methanol fuel cell applications. Surf. Eng. 2014, 30, 263–267. [Google Scholar] [CrossRef]
- Chen, S.; Wu, H.; Tao, J.; Xin, H.; Zhu, Y.; Chen, J. Pt–Ni Seed-Core-Frame Hierarchical Nanostructures and Their Conversion to Nanoframes for Enhanced Methanol Electro-Oxidation. Catalysts 2019, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhou, Z.; Zheng, T.; Gu, C.; Gong, X.; Zhang, Y.; Xie, Y.; Yang, N.; Fei, J. A novel strategy to synthesize Pt/CNTs nanocatalyst with highly improved activity for methanol electrooxidation. J. Electroanal. Chem. 2021, 897, 115557. [Google Scholar] [CrossRef]
- Shu, J.; Li, R.; Lian, Z.; Zhang, W.; Jin, R.; Yang, H.; Li, S. In-situ oxidation of Palladium–Iridium nanoalloy anchored on Nitrogen-doped graphene as an efficient catalyst for methanol electrooxidation. J. Colloid Interface Sci. 2022, 605, 44–53. [Google Scholar] [CrossRef]
- Thorat, G.M.; Jadhav, H.S.; Seo, J.G. Bi-functionality of mesostructured MnCo2O4 microspheres for supercapacitor and methanol electro-oxidation. Ceram. Int. 2017, 43, 2670–2679. [Google Scholar] [CrossRef]
- Rebekah, A.; Anantharaj, S.; Viswanthan, C.; Ponpandian, N. Zn-substituted MnCo2O4 nanostructure anchored over rGO for boosting the electrocatalytic performance towards methanol oxidation and oxygen evolution reaction (OER). Int. J. Hydrogen Energy 2020, 45, 14713–14727. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, K.; Hou, G.; Zhang, H.; Cao, H. A highly active Pt nanocatalysts supported on RuO2 modified TiO2-NTs for methanol electrooxidation with excellent CO tolerance. Int. J. Hydrogen Energy 2019, 44, 31506–31514. [Google Scholar] [CrossRef]
- Şahin, E.A.; Solmaz, R. Methanol electrooxidation activity of binary CoAg electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 35013–35022. [Google Scholar] [CrossRef]
- Askari, M.B.; Rozati, S.M.; Salarizadeh, P.; Saeidfirozeh, H.; Di Bartolomeo, A. A remarkable three-component RuO2-MnCo2O4/rGO nanocatalyst towards methanol electrooxidation. Int. J. Hydrogen Energy 2021, 46, 36792–36800. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Ebrahimi Qaratapeh, K.; Jadali, S.; Moharramnezhad, M. Decorating the carbon felt electrode with polymeric platinize nanocomposite: Characterization and electrocatalytic activity towards methanol oxidation reaction. J. Chem. Sci. 2019, 131, 61. [Google Scholar] [CrossRef] [Green Version]
- Raoof, J.B.; Azizi, N.; Ojani, R.; Ghodrati, S.; Abrishamkar, M.; Chekin, F. Synthesis of ZSM-5 zeolite: Electrochemical behavior of carbon paste electrode modified with Ni (II)-zeolite and its application for electrocatalytic oxidation of methanol. Int. J. Hydrogen Energy 2011, 36, 13295–13300. [Google Scholar] [CrossRef]
- Mojović, Z.; Mudrinić, T.; Rabi-Stankovic, A.; Ivanovic-Sasic, A.; Marinovic, S.; Žunić, M.; Jovanović, D. Methanol Electrooxidation on PtRu Modified Zeolite X. Sci. Sinter. 2013, 45, 89–96. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Yusof, N.; Salleh, W.N.W.; Aziz, F.; Abdul Ajid, A.Z. Advanced ternary RGO/bimetallic Pt-Pd alloy/CeO2 nanocomposite electrocatalyst by one-step hydrothermal-assisted formic acid reduction reaction for methanol electrooxidation. J. Environ. Chem. Eng. 2021, 9, 104991. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, J.; Pan, Z.; Kong, X.; Cui, Z.; Wu, D.; Hu, G. Pt supported on boron, nitrogen co-doped carbon nanotubes (BNC NTs) for effective methanol electrooxidation. Int. J. Hydrogen Energy 2020, 45, 33634–33640. [Google Scholar] [CrossRef]


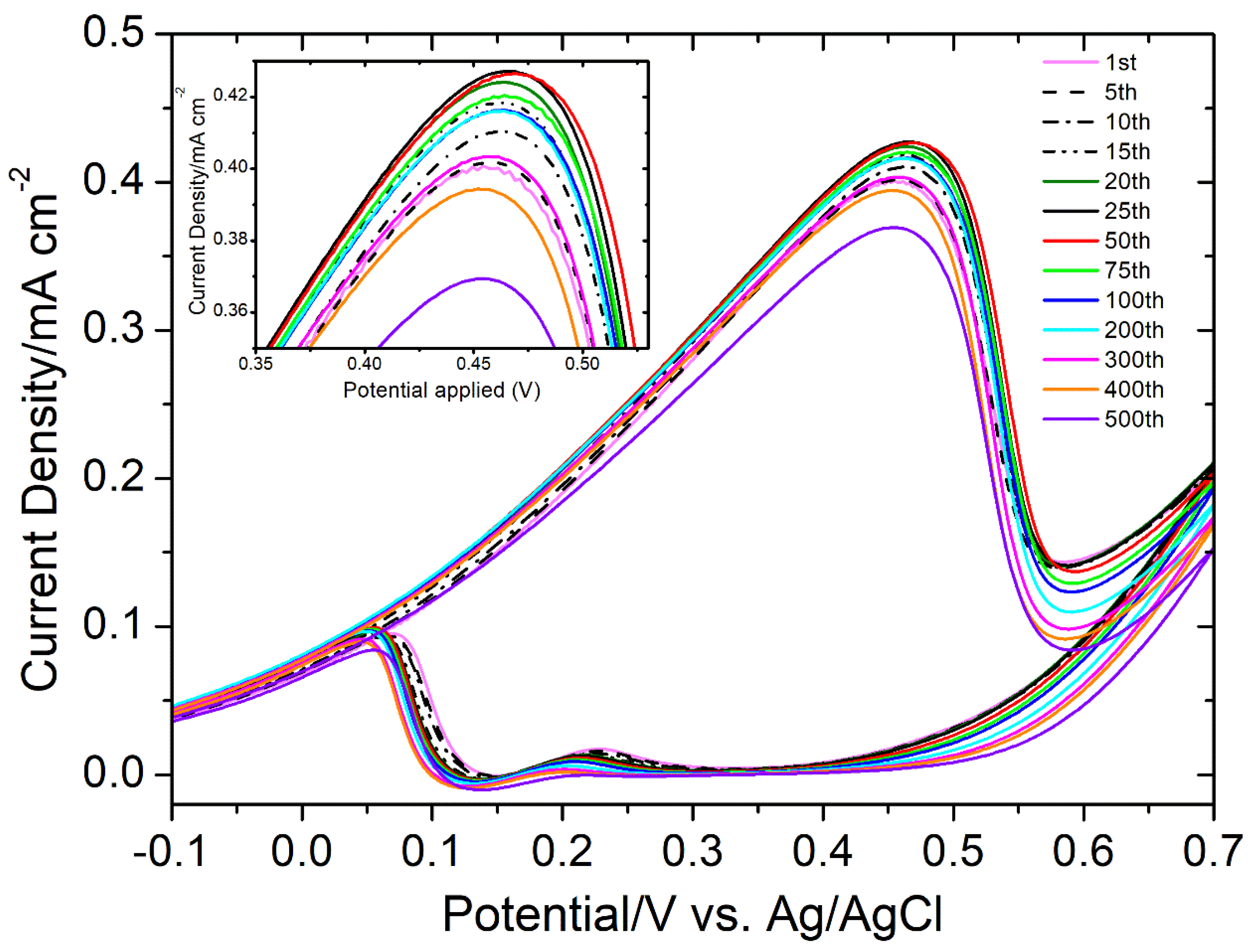
| Sample | Electrolyte | Scan Rate (mV/s) | Peak Oxidation Voltage | Peak Oxidation Current/Current Density | Ref. |
|---|---|---|---|---|---|
| NPG_16h | 5 M CH3OH in 0.5 M KOH | 20 | 0.47 V Ag/AgCl | 0.43 mA/cm2 | This work |
| NPG-A from Au–Ag (data) | 1 M KOH + 1 M CH3OH | 10 | 0.550 V Hg/Hg2SO4 0.491 Ag/AgCl | 42.0 µA/cm2 | [55] |
| NPG-B from Au–Ag | 1 M KOH + 1 M CH3OH | 10 | 0.532 V Hg/Hg2SO4 0.473 Ag/AgCl | 101.1 µA/cm2 | [55] |
| NPG-C from Au–Ag | 1 M KOH + 1 M CH3OH | 10 | 0.420 V Hg/Hg2SO4 0.361 Ag/AgCl | 119.4 µA/cm2 | [55] |
| NPG_6h | 5 M CH3OH in 0.5 M KOH | 20 | 0.22 V Ag/AgCl | 0.68 mA/cm2 | [33] |
| Rh-NSs/RGO hybrids | 1 M CH3OH in 1 M KOH | 50 | 0.61 V Ag/AgCl | 0.52 mA/cm2 | [26] |
| EC/rGO/NiOOH | 0.01 M CH3OH in 0.1 M NaOH | 50 | 0.66 V vs. Ag/AgCl | 0.75 mA | [57] |
| EC/rGO/NiOOH-FeOOH | 0.01 M CH3OH in 0.1 M NaOH | 50 | 0.57 V vs. Ag/AgCl | 0.8 mA | [57] |
| Ni–Pt/carbon paper electrode | 1 M CH3OH in 0.5 M KOH | 20 | −0.2 V vs. Ag/AgCl | 1.8 mA | [58] |
| Pt3Ni nanoparticles | 1 M CH3OH in 0.1 M HClO4 | 50 | 1.04 V vs. RHE | 0.5 mA/cm2 | [59] |
| Pt/CNTs-80 | 0.5 M CH3OH In 0.5 M H2SO4 | 50 | 0.69 V vs. Ag/AgCl | 1.83 mA/cm2 | [60] |
| Pd-Ir-O/NGS | 1 M CH3OH in 1 M KOH | 50 | 0.81 V vs. RHE | 3.26 mA cm−2 | [61] |
| MnCo2O4 | 0.5 M CH3OH in 1 M KOH | 10 | 0.7 V vs. Ag/AgCl | 95 A/g | [62] |
| Mn0.6Zn0.4Co2O4/rGO | 0.5 M CH3OH in 1 M KOH | 50 | 0.8 V vs. Ag/AgCl | 142.3 mA/cm2 | [63] |
| Pt/MnO2/Rgo | 0.5 M CH3OH in 0.5 M H2SO4 | 50 | 0.9 V vs. RHE | 23 mA/cm2 | [54] |
| Pt/RuO2/CNT | 1 M CH3OH in 1 M HClO4 | - | 0.74 V vs. Ag/AgCl | 609 A/g Pt | [29] |
| Pt/RuO2/TiO2-NTs | 0.5 M CH3OH in 1 M H2SO4 | 0.5 | 0.8 V | 100 mA/cm2 | [64] |
| C/CoAg | 1 M CH3OH in 0.1 M KOH | 50 | 0.48 V | 2.30 A/g C | [65] |
| C/Co | 1 M CH3OH in 0.1 M KOH | 50 | 0.58 V | 0.35 A/g C | [65] |
| RuO2/MnCo2O4/rGO | 0.5 M CH3OH in 1 M KOH | 20 | 0.5 V vs Ag/AgCl | 20.44 mA/cm2 | [66] |
| Pt/p-MDAB/CF | 1 M CH3OH in 0.5 M H2SO4 | 50 | 0.71 V vs Ag/AgCl | 405 mA mg−1 Pt | [67] |
| Ni/ZMCPE | 0.1 M CH3OH in 0.1 M NaOH | 20 | 0.68 V vs. Ag/AgCl | 0.85 mA | [68] |
| 13XPtRu | 0.5 M CH3OH in 0.5 M NaOH | 20 | −0.23 V vs. Ag/AgCl | 0.56 mA | [69] |
| RGO/bimetallic Pt–Pd alloy/CeO2 | 1 M CH3OH in 0.5 M H2SO4 | 50 | 0.8 V vs. Ag/AgCl | 69.82 mA cm2 | [70] |
| Pt/BNC NTs | 1 M CH3OH in 0.5 M H2SO4 | 50 | 0.73 V vs. Ag/AgCl | 0.73 A mg−1 Pt | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, D.; Scaglione, F.; Fiore, G.; Rizzi, P. Cost-Effective Nanoporous Gold Obtained by Dealloying Metastable Precursor, Au33Fe67, Reveals Excellent Methanol Electro-Oxidation Performance. Coatings 2022, 12, 831. https://doi.org/10.3390/coatings12060831
Raj D, Scaglione F, Fiore G, Rizzi P. Cost-Effective Nanoporous Gold Obtained by Dealloying Metastable Precursor, Au33Fe67, Reveals Excellent Methanol Electro-Oxidation Performance. Coatings. 2022; 12(6):831. https://doi.org/10.3390/coatings12060831
Chicago/Turabian StyleRaj, Deepti, Federico Scaglione, Gianluca Fiore, and Paola Rizzi. 2022. "Cost-Effective Nanoporous Gold Obtained by Dealloying Metastable Precursor, Au33Fe67, Reveals Excellent Methanol Electro-Oxidation Performance" Coatings 12, no. 6: 831. https://doi.org/10.3390/coatings12060831
APA StyleRaj, D., Scaglione, F., Fiore, G., & Rizzi, P. (2022). Cost-Effective Nanoporous Gold Obtained by Dealloying Metastable Precursor, Au33Fe67, Reveals Excellent Methanol Electro-Oxidation Performance. Coatings, 12(6), 831. https://doi.org/10.3390/coatings12060831







