Sustainable Use of Cruciferous Wastes in Nanotechnological Applications
Abstract
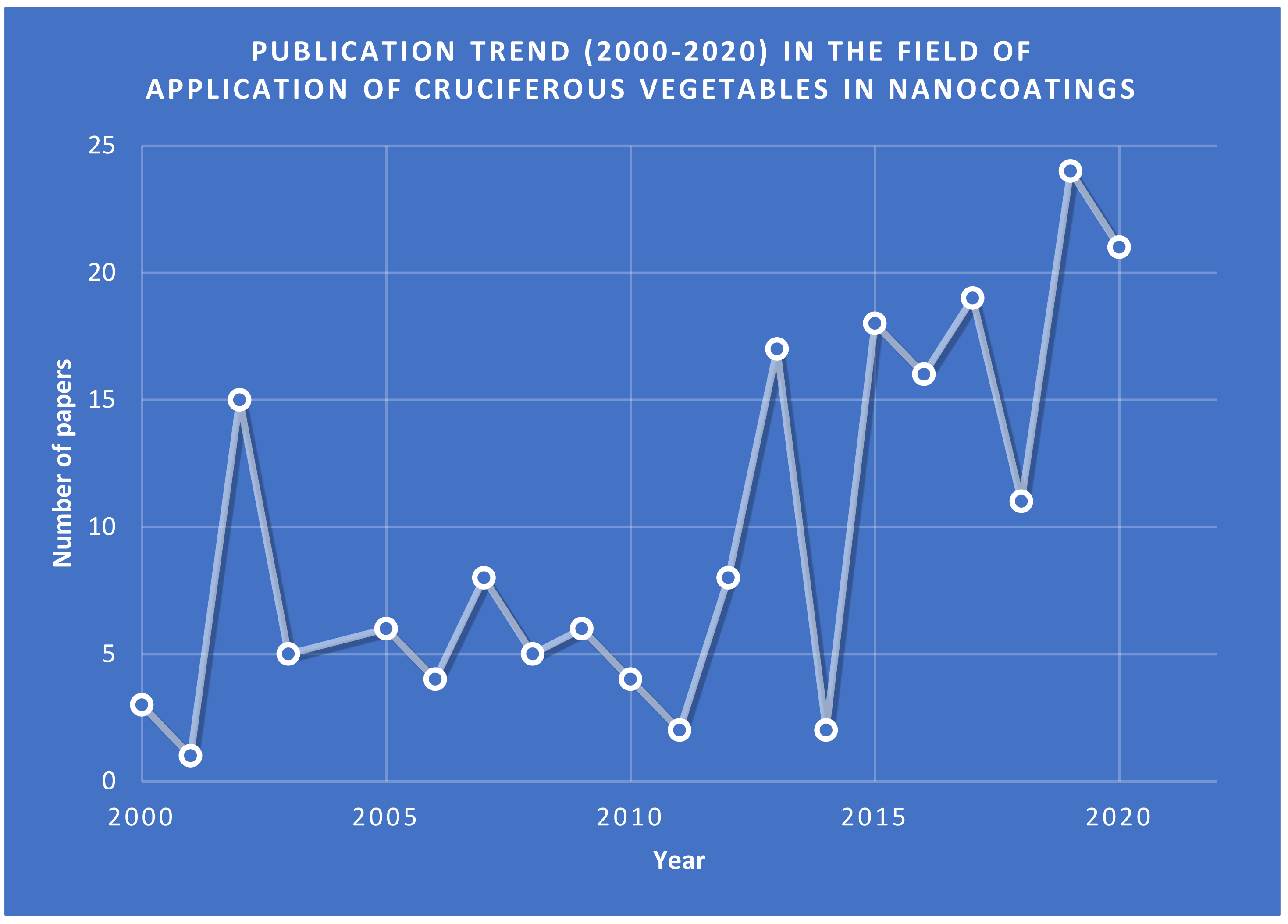
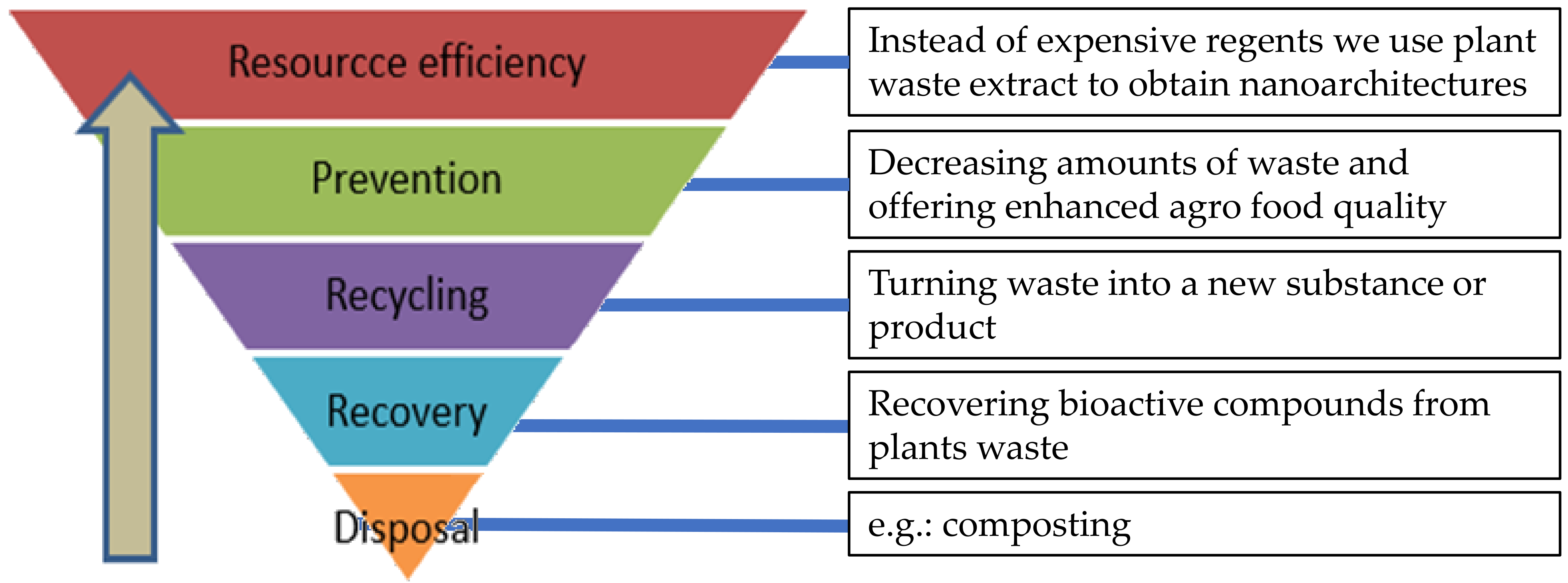
:1. Introduction
2. General Composition of Brassica Genus
Mustard Leaves
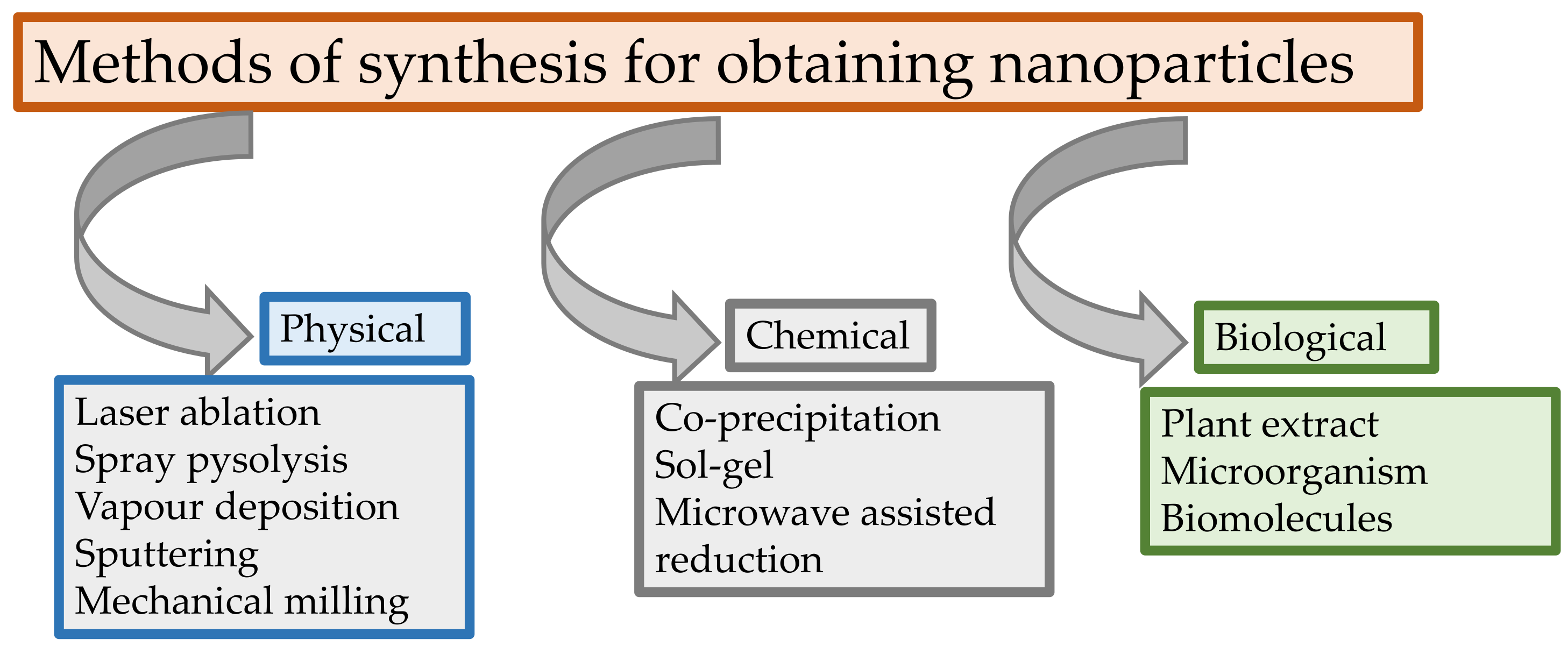
3. Application of Brassica Genus in Green Nanotechnology
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| spp. | subspecies |
| var. | varieties (in Latin: varietas) |
| LDL | low-density lipoprotein |
| COVID 19 | Coronavirus disease 2019 |
| AgNPs | silver nanoparticles |
| ZnO | zinc oxide |
| MTCC | Microbial Type Culture Collection |
| IZD | inhibition zone diameter |
| IC50 | the half maximal inhibitory concentration |
| A549 | cell lines |
| CuI | copper iodide |
| XRD | X-ray diffraction analysis |
| nm | nanometer |
| TEM | Transmission electron microscopy |
| HR- TEM | High-resolution transmission electron microscopy |
| AFM | Atomic force microscopy |
| DLS | Dynamic light scattering |
| Z potential | zeta potential |
| °C | degrees Celsius |
| h | hours |
| mL | milliliter |
| mV | millivolt |
| MDR | multidrug-resistant |
| w/v | mass concentration of solution is expressed as % w/v for weight per volume |
| rpm | rotation per minute |
| min. | minutes |
| eV | electron Volt |
| Au | gold |
| Ag | silver |
| NaBH4 | Sodium borohydride |
| W | Watt |
| E. coli | Escherichia coli |
| S. aureus | Staphylococcus aureus |
| AuNP | gold nanoparticles |
| μg | microgram |
References
- Niculescu, A.G.; Chircov, C.; Bîrca, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Sutan, N.A.; Manolescu, D.S.; Fierascu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fierascu, R.C. Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. rhizomes alcoholic extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 746. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Anuta, V.; Velescu, B.S.; Jinga, M.; Jinga, V. A Short Overview of Recent Developments on Antimicrobial Coatings Based on Phytosynthesized Metal Nanoparticles. Coatings 2019, 9, 787. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, I.; Fierascu, R.C.; Ungureanu, C.; Draghiceanu, O.A.; Soare, L.C. Application of Polypodiopsida Class in Nanotechnology–Potential towards Development of More Effective Bioactive Solutions. Antioxidants 2021, 10, 748. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Organization of the United Nations. Top exports of Cabbages and Other Brassicas. In FAOSTAT Database; FAOSTAT: Rome, Italy, 2009. [Google Scholar]
- Hayek, A. Entwurf eines Cruciferensystems auf phylogenetischer Grundlage. Beih. Zum Bot. Cent. 1911, 27, 127. [Google Scholar]
- Schulz, O.E. Cruciferae. In Die Naturlichen Pflanzenfamilien; Engler, A., Harms, H., Eds.; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1936; Volume 17B, pp. 227–658. [Google Scholar]
- Goel, U.; Kawatra, B.L. Nutrition evaluation of a cauliflower leaf protein. J. Sci. Food Agr. 1997, 28, 786. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Organization of the United Nations. Top exports of Cabbages and Other Brassicas. In FAOSTAT Database; FAOSTAT: Rome, Italy, 2011. [Google Scholar]
- Francisco, M.; Tortosa, M.; Martinez-Ballesta, M.; Velasco, P.; Garcia-Viguera, C.; Moreno, D.A. Nutritional, and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2017, 170, 273. [Google Scholar] [CrossRef]
- Fahey, J.W. Brassicas. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 606–615. ISBN 9780122270550. [Google Scholar]
- Van Popple, G.; Verhoeven, D.T.; Verhagen, H.; Goldbohm, R.A. Brassica vegetables and cancer prevention. Adv. Exp. Med. Biol. 1999, 472, 159. [Google Scholar]
- Bjorkman, M.; Klingen, I.; Birch, A.; Bones, A.; Bruce, T.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phyto-chemicals of Brassicaceae in plant protection and human health. Influences of climate; environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Bortoloti, G.A.; Baron, D. Phytoremediation of toxic heavy metals by brassica plants: A biochemical and physiological approach. Environ. Adv. 2022, 8, 100204. [Google Scholar] [CrossRef]
- Jo, J.S.; Bhandari, S.R.; Kang, G.H.; Shin, Y.K.; Lee, J.G. Selection of broccoli (brassica oleracea var. italica) on composition and content of glucosinolates and hydrolysates. Sci. Hortic. 2022, 298, 110984. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Wu, C.; Rao, Z.; Du, L.; Zhou, Y. Cruciferous vegetable and isothiocyanate intake and multiple health outcomes. Food Chem. 2022, 375, 131816. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Traditional uses of wild and tended plants in maintaining ecosystem services in agricultural landscapes of the eastern cape province in South Africa. J. Ethnobiol. Ethnomed. 2022, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Q.; Fu, J.; Wang, Y.; Zhang, Y.; Sun, Y.; Tong, H.; Dhankher, O.P. Co-exposure of sulfur nanoparticles and cu alleviate cu stress and toxicity to oilseed rape brassica napus L. J. Environ. Sci. 2023, 124, 319. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and Glucosinolates as Strong Determinants of the Nutritional Quality in Rocket Leafy Salads. Nutrients 2014, 6, 1519. [Google Scholar] [CrossRef] [Green Version]
- Argento, S.; Melilli, M.G.; Branca, F. Enhancing greenhouse tomato-crop productivity by using Brassica macrocarpa guss. Leaves forcontrolling root-knot nematodes. Agronomy 2019, 9, 820. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, H.; Shad, M.A.; Muzaffar, S. Phytochemical composition and antioxidant potential of Brassica. In Brassica Germplasm—Characterization, Breeding, and Utilization; El-Esawi, M.A., Ed.; Intech Open: London, UK, 2018; pp. 7–26. [Google Scholar]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Salopek-Sondi, B. Cruciferous (Brassicaceae) vegetables. In Nonvitaminand Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 195–202. [Google Scholar]
- Chang, C.T.; Hseueh, Y.L.; Shung, H.Y. Purification and properties of chitinase from cabbage stems with roots. Biochem. Mol. Biol. Int. 1996, 40, 417. [Google Scholar]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367. [Google Scholar] [CrossRef] [Green Version]
- Nestle, M. Broccoli sprouts in cancer prevention. Nutr. Rev. 1998, 56, 127. [Google Scholar] [CrossRef]
- Datta, A.; Gopal, M. Safety evaluation of the fungicide iprodioneon cauliflower. Bull. Environ. Contamination. Toxicol. 1999, 62, 496. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roller, S.; Covil, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67. [Google Scholar] [CrossRef]
- Wang, G.H. Inhibition, and inactivation of five species of food borne pathogens by chitosan. J. Food Prot. 1992, 55, 916. [Google Scholar] [CrossRef]
- Lucca, A.J.D.; Walsh, T.J. Antifungal peptides: Novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 1999, 43, 1. [Google Scholar] [CrossRef] [Green Version]
- Wright, C. Mediterranean Vegetables; Harvard Common Press: Boston, MA, USA, 2012; p. 27. ISBN 9781558321960. [Google Scholar]
- Can, H.; Sari, B.; Kavuşan, H.S.; Serdaroǧlu, M. Arugula and pre-converted arugula extract as natural Nitrate/Nitrite sources for heat-treated sausages. In Proceedings of the 61st International Meat Industry Conference, Zlatibor, Serbia, 26–29 September 2021. [Google Scholar]
- Maggioni, L.; Bothmer, R.; Poulsen, G.; Branca, F. Origin and Domestication of Cole Crops (Brassica oleracea L.): Linguistic and Literary Considerations. Econ. Bot. 2010, 64, 109. [Google Scholar] [CrossRef]
- López-Hernández, A.A.; Ortega-Villarreal, A.S.; Vázquez Rodríguez, J.A.; López-Cabanillas Lomelí, M.; González-Martínez, B.E. Application of different cooking methods to improve nutritional quality of broccoli (brassica oleracea var. italica) regarding its compounds content with antioxidant activity. Int. J. Gastron. Food Sci. 2022, 28, 100510. [Google Scholar] [CrossRef]
- Ortner, E.; Granvogl, M. Thermally induced generation of desirable aroma-active compounds from the glucosinolate sinigrin. J. Agric. Food Chem. 2018, 66, 2485. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Dunkel, A.; Szwengiel, A.; Czaczyk, K.; Drożdżyńska, A.; Zawirska-Wojtasiak, R.; Jeleń, H.H. The relation between phytochemical composition and sensory traits of selected brassica vegetables. LWT 2022, 156, 113028. [Google Scholar] [CrossRef]
- USDA Database Table for Raw Cabbage per 100 g, US Department of Agriculture, National Nutrient Database for Standard Reference, Version SR-27. 1 April 2019. Available online: http://www.ars.usda.gov/nea/bhnrc/mafcl (accessed on 5 January 2020).
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A Mechanistic Perspective on Process-Induced Changes in Glucosinolate Content in Brassica Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; An, R.; Zhou, H.; Zhang, Y.; Ling, J.; Hu, H.; Li, P. The glucosinolate profiles of brassicaceae vegetables responded differently to quick-freezing and drying methods. Food Chem. 2022, 383, 132624. [Google Scholar] [CrossRef] [PubMed]
- Armesto, J.; Gómez-Limia, L.; Carballo, J.; Martínez, S. Effects of different cooking methods on the antioxidant capacity and flavonoid, organic acid and mineral contents of Galega kale (Brassica oleracea var. acephala cv. Galega). Int. J. Food Sci. Nutr. 2018, 70, 136. [Google Scholar] [CrossRef] [PubMed]
- Soengas, P.; Velasco, P.; Fernández, J.C.; Cartea, M.E. New Vegetable Brassica Foods: A Promising Source of Bioactive Compounds. Foods 2021, 10, 2911. [Google Scholar] [CrossRef]
- Hien, T.T.; Truc, T.T.; Muoi, N.V. Effect of salt concentration and pH value on the lactic fermentation process of kohlrabi (brassica oleracea L.). J. Food Sci. Technol. 2022, 10, 399. [Google Scholar]
- Popović-Djordjević, J.B.; Kostić, A.Ž.; Rajković, M.B.; Miljković, I.; Krstić, Đ.; Caruso, G.; Moghaddam, S.S.; Brčeski, I. Organically vs. conventionally grown vegetables: Multi-elemental analysis and nutritional evaluation. Biol. Trace Elem. Res. 2022, 200, 426. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Kim, J.; Moon, B. Effect of main vegetable ingredient on the glucosinolate, carotenoids, capsaicinoids, chlorophylls, and ascorbic acid content of kimchis. J. Food Compos. Anal. 2022, 110, 104523. [Google Scholar] [CrossRef]
- McAlvay, A.C.; Ragsdale, A.P.; Mabry, M.E.; Qi, X.; Bird, K.A.; Velasco, P.; An, H.; Pires, J.C.; Emshwiller, E. Brassica rapa Domestication: Untangling Wild and Feral Forms and Convergence of Crop Morphotypes. Mol. Biol. Evol. 2021, 38, 3358. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Perez, R. Raphanus sativus (radish): Their chemistry and biology. Sci. World J. 2004, 4, 811. [Google Scholar] [CrossRef]
- Ungureanu, C.; Fierascu, I.; Fierascu, R.C.; Costea, T.; Avramescu, S.M.; Călinescu, M.F.; Somoghi, R.; Pirvu, C. In Vitro and In Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts. Materials 2021, 14, 1845. [Google Scholar] [CrossRef]
- Hilliard, S.B. Hog Meat and Hoecake: Food Supply in the Old South, 1840-1860. In Southern Foodways Alliance Studies in Culture, People, and Place; Southern Illinois University Press: Carbondale, IL, USA, 1972; ISBN 978-08203-4702-8. [Google Scholar]
- Khadivi, A.; Mirheidari, F.; Moradi, Y. Selection of superior accessions of turnip (Brassica rapa var. rapa L.) based on tuber quality-related characters. Food Sci. Nutr. 2022, online first. [Google Scholar] [CrossRef]
- Bladh, K.W.; Olssonb, K.M. Introduction and use of horseradish (Armoracia rusticana) as food and medicine from antiquity to the present: Emphasis on the nordic countries. J. Herbs Spices Med. Plants. 2011, 17, 197. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Tsau, G.J.; Brown, A.; Howard, L.; Klein, B.P.; Jeffery, E.H.; Kushad, M.; Walling, M.A.; Juvik, J.A. Carotene; tocopherol; and ascorbate contents in subspecies of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1576. [Google Scholar] [CrossRef] [PubMed]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1. [Google Scholar] [CrossRef]
- Muller, H. Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection. Food Res. Technol. 1997, 204, 88. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Rimm, E.B. Epidemiologic evidence for vitamin E in prevention of cardiovascular disease. Am. J. Clin. Nutr. 1995, 62, 1365. [Google Scholar] [CrossRef]
- Piironen, V.; Syvaoja, E.L.; Varo, P.; Salminen, K.; Koivistoinen, P. Tocopherols and tocotrienols in Finnish foods: Vegetables; fruits; and berries. J. Agric. Food Chem. 1986, 34, 742. [Google Scholar] [CrossRef]
- Moreno, D.A.; Carvajal, M.; Lopez-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251. [Google Scholar] [CrossRef]
- Sousa, C.; Lopes, G.; Pereira, D.M.; Taveira, M.; Valentao, P.; Seabra, R.M.; Pereira, J.A. Screening of antioxidant compounds during sprouting of Brassica oleracea L. var. costata DC. Comb. Chem. High Throughput Screen. 2007, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob Agents. 2005, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Biondi, F.; Balducci, F.; Capocasa, F.; Visciglio, M.; Mei, E.; Vagnoni, M.; Mezzetti, B.; Mazzoni, L. Environmental Conditions and Agronomical Factors Influencing the Levels of Phytochemicals in Brassica Vegetables Responsible for Nutritional and Sensorial Properties. Appl. Sci. 2021, 11, 1927. [Google Scholar] [CrossRef]
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, A.; García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins Contents, Profiles, and Color Characteristics of Red Cabbage Extracts from Different Cultivars and Maturity Stages. J. Agric. Food Chem. 2014, 62, 7524. [Google Scholar] [CrossRef]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model system. Food Chem. 2001, 72, 431. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol; flavonoid; proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553. [Google Scholar] [CrossRef]
- Ackland, M.L.; Van De Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavanols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69. [Google Scholar]
- Kim, D.O.; Padilla-Zakour, O.I.; Griffiths, P.D. Flavonoids and antioxidant capacity of various cabbage genotypes at juvenile stage. J. Food Sci. 2004, 69, C685. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Pérez-Guerrero, C.; Lòpez-Lazaro, M. A review on the dietary flavonoid kaempferol. Med. Chem. 2011, 11, 298. [Google Scholar] [CrossRef]
- Sanlier, N.; Guler Saban, M. The Benefits of Brassica Vegetables on Human Health. J. Hum. Health Res. 2018, 1, 104. [Google Scholar]
- Blaževic, I.; Montaut, S.; Burcul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Abdel-Farida, I.B.; Kima, H.K.; Choia, Y.H.; Verpoortea, R. Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 2009, 67, 23. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337. [Google Scholar] [CrossRef] [PubMed]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordàs, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and Functionalized Films/Coatings—Performances and Perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Islam, S.S.; Midya, S.; Sinha, S.; Saadi, S.M.A.I. Natural medicinal plant products as an immune-boosters: A possible role to lessen the impact of Covid-19. Chem. Environ. Eng. 2021, 4, 100105. [Google Scholar] [CrossRef]
- De Carvalho Lima, E.N.; Octaviano, A.L.M.; Piqueira, J.R.C.; Diaz, R.S.; Justo, J.F. Coronavirus and Carbon Nanotubes: Seeking Immunological Relationships to Discover Immunotherapeutic Possibilities. Int. J. Nanomed. 2022, 17, 751. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Bhatia, A.K. Azadirachta Indica and Brassica Oleracea Mediated Green Synthesis vs. Chemical Synthesis of Silver Nanoparticles and their Antibacterial Properties. Nanosci. Nanotechnol. Asia 2019, 9, 393. [Google Scholar] [CrossRef]
- Ansar, S.; Tabassum, H.; Aladwan, N.S.M.; Naiman, A.M.; Almaarik, B.; AlMahrouqi, S.; Abudawood, M.; Banu, N.; Alsubki, R. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci Rep. 2020, 10, 18564. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R. Green synthesis of silver nanoparticles from seed extract of Brassica nigra and its antibacterial activity. Nusant. Biosci. 2015, 7, 1. [Google Scholar] [CrossRef]
- Bali, R.; Harris, A.T. Biogenic synthesis of Au nanoparticles using vascular plants. Ind. Eng. Chem. Res. 2010, 49, 12762. [Google Scholar] [CrossRef]
- Haq, S.; Dildar, S.; Ben Ali, M.; Mezni, A.; Hedfi, A.; Shahzad, M.I.; Shahzad, N.; Shah, A. Antimicrobial and antioxidant properties of biosynthesized of NiO nanoparticles using Raphanus sativus (R. sativus) extract. Mater. Res. Express 2021, 8, 055006. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Fierăscu, R.C.; Fierăscu, I.; Lungulescu, E.M.; Nicula, N.; Șomoghi, R.; Dițu, L.; Ungureanu, C.; Șuțan, A.N.; Drăghiceanu, O.A.; Păunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1. [Google Scholar] [CrossRef]
- Gong, H.; Liu, M.; Li, H. In situ green preparation of silver nanoparticles/chemical pulp fiber composites with excellent catalytic performance. J. Mater. Sci. 2019, 54, 6895. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Annamalai, S.K.; Arunachalam, A.M.; Kennedy, S. Green synthesis of crystalline silver nanoparticles using Indigofera aspalathoides-medicinal plant extract for wound healing applications. Asian J. Chem. 2013, 25, S311. [Google Scholar]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf. B Biointerfaces 2010, 80, 94. [Google Scholar] [CrossRef]
- Umamaheswari, A.; Lakshmana Prabu, S.; AdharshJohn, S.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef]
- Kiran Kumar, A.B.V.; Sre Saila, E.; Narang, P.; Aishwarya, M.; Raina, R.; Gautam, M.; Shankar, E.G. Biofunctionalization and biological synthesis of the ZnO nanoparticles: The effect of Raphanus sativus (white radish) root extract on antimicrobial activity against MDR strain for wound healing applications. Inorg. Chem. Commun. 2019, 21, 2079. [Google Scholar] [CrossRef]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S.S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. 2017, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.C.; Archana, K.M.; Rajagopal, R. Green synthesis, characterization, catalytic and antibacterial studies of copper iodide nanoparticles synthesized using Brassica oleracea var. capitata f. rubra extract. Chem. Data Collect 2020, 29, 100538. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Ri, H.; Khan, A.U.; Khan, U.A.; Yuan, Q. Green synthesis of catalytic zinc oxide nano-flowers and their bacterial infection therapy. Appl. Organomet. Chem. 2019, 34, e5298. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of dopamine in plants: A review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef]
- Perveen, R.; Shujaat, S.; Qureshi, Z.; Nawaz, S.; Khand, M.I.; Iqbal, M. Green versus sol-gel synthesis of ZnO nanoparticles and antimicrobial activity evaluation against panel of pathogens. J. Mater. Res. Technol. 2020, 9, 7817. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Ichwan, S.J.; Parine, N.R.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. 2015, 29, 151. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Phytosynthesis of Silver Nanoparticles using Andean Cabbage: Structural Characterization and its Application. Mater. Today Proc. 2020, 21, 2079. [Google Scholar] [CrossRef]
- Unal, I.S.; Demirbas, A.; Onal, Y.; Ildiz, N.; Ocsoy, I. One step preparation of stable gold nanoparticle using red cabbage extracts under UV light and its catalytic activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111800. [Google Scholar] [CrossRef]
- Lokhande, A.C.; Babar, P.T.; Karade, V.C.; Jang, J.S.; Lokhande, V.C.; Lee, D.J.; Kim, I.-C.; Patole, S.P.; Qattan, I.A.; Lokhande, C.D.; et al. A viable green route to produce Ag nanoparticles for antibacterial and electrochemical supercapacitor applications. Mater. Today Bio. 2019, 14, 100181. [Google Scholar] [CrossRef]
- Jain, A.; Sinilal, B.; Starnes, D.L.; Sanagala, R.; Krishnamurthy, S.; Sahi, S.V. Role of Fe-responsive genes in bioreduction and transport of ionic gold to roots of Arabidopsis thaliana during synthesis of gold nanoparticles. Plant. Physiol. Biochem. 2014, 84, 189. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M.; Ganjloo, A.; Bimakr, M.; Moradi, N.; Nikoomanesh, N. Effect of active coating containing radish leaf extract with or without vacuum packaging on the postharvest changes of sweet lemon during cold storage . J. Food Process. Preserv. 2021, 45, e15252. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. A green Brassica oleracea L extract as a novel corrosion inhibitor for Q235 steel in two typical acid media. Colloids Surf. A Physicochem. Eng. 2021, 616, 126077. [Google Scholar] [CrossRef]
- Ngobiri, N.C.; Oguzie, E.E.; Li, Y.; Liu, L.; Oforka, N.C.; Akaranta, O. Eco-friendly corrosion inhibition of pipeline steel using brassica oleracea. Int. J. Corros. 2015, 2015, 404139. [Google Scholar] [CrossRef] [Green Version]
- Chinweuba, A.J. Corrosion inhibition potentials of Brassica oleracea capitata extract on mild steel and zinc in acidic media. Chem. Mater. Res. 2014, 6, 62. [Google Scholar]
- Casaletto, M.P.; Figà, V.; Privitera, A.; Bruno, M.; Napolitano, A.; Piacente, S. Inhibition of Cor-Ten steel corrosion by “green” extracts of Brassica campestris. Corros. Sci. 2018, 136, 91. [Google Scholar] [CrossRef]
- Ren, J.; Kong, R.; Gao, Y.; Zhang, L.; Zhu, J. Bioinspired adhesive coatings from polyethylenimine and tannic acid complexes exhibiting antifogging, self-cleaning. J. Colloid Interface Sci. 2021, 602, 406. [Google Scholar] [CrossRef]
- Bojanowska, M. Changes in chemical composition of rapeseed meal during storage, influencing nutritional value of its protein and lipid fractions. J. Anim. Feed. Sci. 2017, 26, 157. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Li, S.; Lei, W.; Liu, Y.; Sommer, T.; Friederich, P.; Sobek, C.; Messersmith, P.B.; Levkin, P.A. High-throughput screening of multifunctional nanocoatings based on combinations of polyphenols and catecholamines. Mater. Today Bio. 2021, 10, 100108. [Google Scholar] [CrossRef]
- Nikoo, A.H.; Malayeri, M.R. Incorporation of surface energy properties into general crystallization fouling model for heat transfer surfaces. Chem. Eng. Sci. 2020, 215, 115461. [Google Scholar] [CrossRef]
- DeFlorio, W.; Liu, S.; White, A.R.; Taylor, T.M.; Cisneros-Zevallos, L.; Min, Y.; Scholar, E.M.A. Recent developments in antimicrobial and antifouling coatings to reduce or prevent contamination and cross-contamination of food contact surfaces by bacteria. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3093. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, M.F.R.; Radzi, N.M.; Jusoh, S.M.; Saidin, J.; Nik, W.M.N.W. Potential of cabbage extract (Brassica oleracea) as anti-fouling agent in alkyd undercoat for mild steel in seawater. Malays. J. Anal. Sci. 2019, 23, 451. [Google Scholar]

| Species | ssp./var. | Common Name |
|---|---|---|
| japonica | Curled mustard | |
| Brassica rapa L. | rapa | Turnip broccoli |
| rapifera | Turnip | |
| chinensis | Pak-choi, Chinese mustard | |
| peckinensis | Chinese cabbage | |
| Brassica napus L. | napus | Rapeseed |
| napobrassica | rutabaga | |
| Brassica oleracea L. | italica | Broccoli |
| gemmifera | Brussels sprouts | |
| capitata capitata f. rubra capitata f. alba | Cabbage Red cabbage White cabbage | |
| botrytis | Cauliflower | |
| alboglabra | Chinese kale | |
| Raphanus sativus L. | Radish | |
| Brassica alba/Sinapis alba, Brassica hirta | White or yellow mustard | |
| Brassica nigra/Sinapis nigra | Black mustard | |
| Brassica juncea | Brown mustards | |
| Raphanus raphanistrum L. | Wild radish | |
| Eruca sativa Mill. | Rucola | |
| Diplotaxis muralis L. | Wall rocket |
| Vegetal Material | Extraction Method | NP | NPs Characteristics | NPs Applications | Ref. |
|---|---|---|---|---|---|
| Medical Applications of Metallic Nanoparticles | |||||
| Raphanus sativus var. Longipinnatus leaves | Boiling; solvents: water, dichloromethane and ethanol; t-15 min; 10 g fresh vegetal material | ZnO | The average size −209 nm (DLS); Z potential—−13.7 mV NP—spherical, hexagonal ZnO NPs—66.43 nm (XRD) | Evaluation of anticancer property in A549 cell lines; IC50—40 μg mL−1 | [89] |
| R. sativus root extract | Fresh juice | ZnO | 40 nm, Spherical shape, | Activity against Escherichia fergusonii and Escherichia coli (15–23 mm inhibition zone) | [90] |
| R. sativus leaves | Fungal fermentation of Alternaria sp. isolated from fresh leaves; Inoculation of Alternaria sp.: conical flasks containing 200 mL of fermentation medium (Potato dextrose broth); incubation: T = 28 °C, t = 72 h; Percolation treatment: 10 g of washed and filtered fungal biomass, 100 mL double distilled water, t = 2 h; | AgNP | The average size: 4–30 nm (TEM and AFM), ~80 nm (DLS); Z potential: −16.8 mV NP—spherical shape | Evaluation of antimicrobial effects against Bacillus subtilis (MTCC 441) (IZD = 23 mm), Staphylococcus aureus (MTCC 740) (IZD = 12 mm); E. coli (MTCC 443) (IZD = 34 mm); Serratia marcescens (MTCC 97) (IZD = 36 mm); | [91] |
| R. sativus roots | Boiling; Solvent: 100 mL water; Vegetal material: dried and powdered; | ZnO | The average size: 15–25 nm (XRD); 25–40 nm (HR-TEM); Z potential: −33 mV; NP—mixed morphology (TEM) | Notable antimicrobial activity against toward microbes isolated from diabetic foot ulcers: Pseudomonas aeruginosa ATCC 27853, MDR–E. coli, S. aureus ATCC 29213, MDR–MRSA, E. coli 36 ATCC 25922, Enterococus faecalis ATCC 29212, MDR—P. aeruginosa, and MDR—Acinetobacter baumannii | [94] |
| Brassica oleracea var. botrytis (Cauliflower head and Raphanus sativus tuber | 20% (w/v) aqueous extract: vegetal material grounded; solvent: deionized water; Agitating: t = 1h; centrifugation: 10,000 rpm, t = 15 min, T = 4 °C | AgNP | The average size: 4–18 nm (TEM);NP—monodisperse and spherical shape; | Strong antimicrobial activity against Gram-positive and negative bacteria; | [91] |
| R. sativus var. longipinnatus Bailey (red radish, GC.Herb.Bot.3296) R. sativus (white radish, GC.Herb. Bot. 3297) B. rapa (turnip, GC.Herb.Bot.3298) B. campestris var. sarson Prain (Saag, GC.Herb.Bot.3299) B. oleracea var. botrytis (Cauliflower, GC.Herb.Bot.3300) | Maceration: 10 g of seeds; solvent: 100 mL of deionized water; T = room temperature; | ZnO-NP | The average size: 50-150 nm (XRD) NP: spherical shape (XRD) | Evaluation of antimicrobial activity against bacterial strains: B. subtilis, S. aureus, Salmonella typhimurim and fungal strains: Aspergillus niger, Fusarium oxysporum and Penicillium digita-tum; Range of IZD: 10-20 mm | [95] |
| B. oleracea var. capitata f. rubra | 5 g of cabbage flakes boiled in 100 mL distilled water. | CuI | Spherical shape, 77.5 nm, band gap energy—2.75 eV | antibacterial activity against E. coli (ATCC 443) and S. aureus (ATCC 96) > 50 µg/mL catalytic activity in Hantzsch reaction- higher yield of 0.9 g of 1,4— dihydropyridine derivatives | [92] |
| B. oleracea | 1 g of vegetal material powder in 90% ethanol (200 mL) at 37 °C, 12 h | Au NPs Ag NPs | Triangular, rod, spherical shaped morphology 24–38 nm (Au), 30–45 nm (Ag) | Inhibition the growth of the tested bacterial and fungal pathogens at the concentration of 50 μg/mL (Salmonella typhi, B. subtilis, E. coli, S. aureus, Pneumocystis sp.) | [96] |
| Environmental Application for Metallic Nanoparticles | |||||
| Brassica oleracea var. capitata | 500 mg vegetal material; heated at 60–65 °C for 30 min in 25 mL water | AgNPs | Spherical, polydisperse, and size range between 15 and 80 nm | Catalytic performance of AgNPs by the reduction of 4-Nitrophenol (4-NP) into 4- Aminophenol (4-AP) in the presence of NaBH4 in 60 s | [97] |
| Brassica oleracea var. Capitata F. rubra | 100 g vegetal material, microwave extraction, P-900 W; time-2 min. | AuNP | The average size 25 nm; Narrow size distribution and spherical shape | Conversion of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) | [98] |
| Multiple Application of Metallic Nanoparticles | |||||
| Thlaspi arvense L. | 20 g vegetal material; 220 mL de-ionized water; 60 °C; 45 min. | Zinc Oxide nanoflowers | 70–90 nm (DLS) | Antibacterial properties against E. coli (Gram-negative bacteria)—inhibition zone of 20 ± 0.6 mm Photocatalytic by reducing 4-nitrophenol and methylene blue. | [93] |
| Kimchi cabbage | 10 g vegetal material in 100 mL double distilled water | AgNP | The average size 10 to 30 n; spherical size | Antibacterial activity against E. coli and S. aureus Electrochemical supercapacitor applications: specific capacitance of 424 F/g with an energy density of 14.04 Wh/kg and a power density of 6.41 kW/kg | [99] |
| In Situ Formation of Metallic Nanoparticles | |||||
| B. juncea root | In situ formation | AuNP | 5–10 nm Spherical, hexagonal,Triangular | [82] | |
| A. thaliana root | In situ formation | AuNP | 20–50 Spherical, triangular | [100] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, C.; Fierascu, I.; Fierascu, R.C. Sustainable Use of Cruciferous Wastes in Nanotechnological Applications. Coatings 2022, 12, 769. https://doi.org/10.3390/coatings12060769
Ungureanu C, Fierascu I, Fierascu RC. Sustainable Use of Cruciferous Wastes in Nanotechnological Applications. Coatings. 2022; 12(6):769. https://doi.org/10.3390/coatings12060769
Chicago/Turabian StyleUngureanu, Camelia, Irina Fierascu, and Radu Claudiu Fierascu. 2022. "Sustainable Use of Cruciferous Wastes in Nanotechnological Applications" Coatings 12, no. 6: 769. https://doi.org/10.3390/coatings12060769
APA StyleUngureanu, C., Fierascu, I., & Fierascu, R. C. (2022). Sustainable Use of Cruciferous Wastes in Nanotechnological Applications. Coatings, 12(6), 769. https://doi.org/10.3390/coatings12060769








