Abstract
Over the last few years, new nanoparticle preparation methods have emerged by replacing the usual reagents with plant extracts obtained in different conditions. An example of a natural plant extract is those of cruciferous vegetables, to obtain the new bio-nano-coatings. Given the composition of cruciferous extracts and large amounts of wastes produced all over the world, they can be successful substitutes to replace conventional coatings and extend the possibility of “smart coatings“. The present review aims to be a critical discussion regarding the application of cruciferous waste in nanotechnological applications. This review paper can be a starting report for different researchers who intend to use this sustainable approach “from green to nanotechnology” to transpose manufacturing from laboratory to industry. Applying this approach to obtain nanostructures with plant waste highlights the importance of minimizing and re-utilizing residues from primary and secondary processing via chemical and social intervention, in order to contribute to the sustainability needs of the planet and its inhabitants.
1. Introduction
In the last decade, nanomaterials have been considered a pivotal tool for different domains such as healthcare, food and agriculture, and the environment. To use nanoparticles in chemical reactions, adequate preparation methods are needed. As these materials are diversified in morphology and chemical composition, various methods have been developed, such as sol–gel, spinning, chemical vapor deposition, and microemulsion or microfluidic methods [1]. These methods require expensive equipment and environmentally hazardous reagents. Mostly, metal salts are reduced using sodium borohydride, citrate, ascorbates, and glucose, and the type of the reducing agent determines the size of the nanoparticles [2]. The bigger particles are produced by the strong reducing agents, while nanoparticles with smaller dimensions are produced by the mild reducing agents; by utilizing appropriate surfactants, particle morphology control is achieved [3,4]. Lately, new nanoparticle preparation methods have developed by replacing the classical reagents with plant extracts obtained in different conditions. An example of a natural plant extract is those of cruciferous vegetables, to obtain the new bio-nano-coatings. As observed from Figure 1, there has been an annual increase in the rate of papers from 2011 to 2019, with two exceptions in 2014 and 2018. Compared to other publications, their number is not large and, for this reason, this review is important to bring scientists’ attention to the usage of cruciferous vegetables in nano-coatings considering their properties.
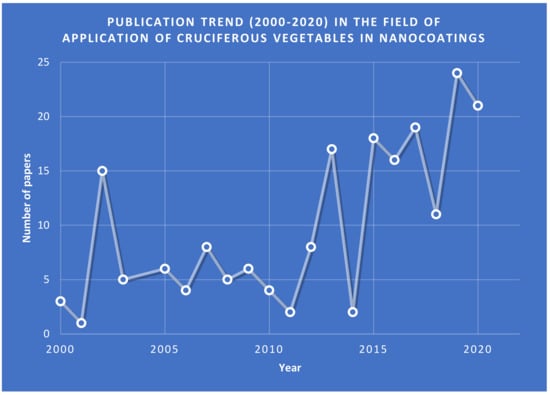
Figure 1.
Publication trend (2000–2020) in the field of application of cruciferous vegetables in nanocoating (source of data: https://www.sciencedirect.com, accessed on 28 April 2022).
At the international level, only during 2019, the production of cabbage and other related brassicas was 97 million tons with a proportional amount of waste (leaves, roots, some stems, a.o.) [5]. In the last ten years, bioeconomy strategies and policy-related bioeconomy initiatives have been developed all over the world (Figure 2). Europe’s strategy addresses the sustainable production of renewable biomass, value-added products, bioproducts, and bioenergy from different type of wastes through an efficient transformation.
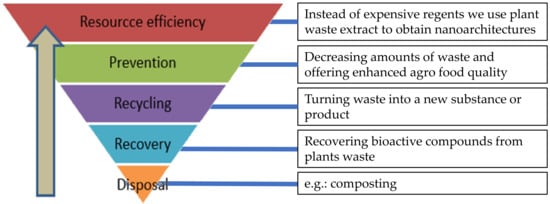
Figure 2.
Current bioeconomy approaches of the cruciferous vegetables waste in nanocoating.
Hayek and Schultz [6,7] were the first researchers who published information about the origin of Brassicaceae. Brassica is a genus of plants in the Brassicaceae family (the cabbage and mustard family). The members of the Brassica genus are known as cruciferous vegetables and Brassica spp. (Table 1) is generally classified into three groups: mustards, coles, and rapeseeds [8]. The name comes from the shape of the flowers that are in the shape of a cross. They have been cultivated for centuries, mainly in Europe and Asia, and there are several varieties that belong to this large family of crucifers.

Table 1.
Classification of Brassicaceae family (main species, subspecies (ssp.), and varieties (var.)) [5,8,9,10,11].
The present review aims to be a critical discussion regarding the application of cruciferous waste in nanotechnological applications. It contains general information regarding the composition of the Brassica genus, the application of the Brassica genus in green nanotechnology, and conclusions and future perspectives.
2. General Composition of Brassica Genus
Crucifers contain a lot of nutrients and are beneficial to the body, from vitamins, minerals, and proteins to carbohydrates and fats. They are rich in vitamin A, vitamin C, folic acid, and fiber, with a low caloric content. Ideally, a person should eat at least three servings of cruciferous vegetables a week, and to retain as much of the beneficial nutrients of these vegetables as possible. These should be eaten as fresh as possible. A diet rich in cruciferous vegetables is also good for detoxifying the body and strengthening the immune system, with some studies suggesting that these vegetables also contain certain anticancer substances [8,12].
The genus Brassica is very important from an agricultural and horticultural point of view. Cruciferous vegetables including cabbage, broccoli, turnip, and radish are among the agricultural crops produced in the largest quantities, and their residues (leaves) have become a burgeoning environmental problem. Nonedible cruciferous vegetable residues are bio-convertible into other valuable products, providing a way to reduce pollution and to obtain value-added products, eliminating other problems such as handling and storing Cruciferous vegetables such as radish, cauliflower, cabbage, mustard, carrot, and turnip are edible plants that are high in fiber, rich in vitamins and minerals, and have physiologic effects in humans [8,9,10,11,12,13]. Most of the plants listed above are seasonal plants (annuals or biennials), while some are small shrubs.
There are over 30 wild species and their hybrids plus many controlled cultivated varieties. Due to their high importance in agriculture, Brassica plants have been the subject of numerous publications [14,15,16,17,18].
Plants in the Brassica genus contain several phytochemical compounds such as glucosinolates, glucosides, phenolic acids, erucic acid, polyphenols and tocopherols, carotenoids, flavonoids, alkaloids, terpenoids and terpenes, phytoalexins, and Phyto steroids with diverse biological activities, mainly antimicrobial and antioxidant effects, but also act against some herbivores [19,20,21,22,23].
Some important enzymes such as chitinase, glutathione transferase, and epoxide hydrolase are also found in cruciferous vegetables [24,25]. Indole and isothiocyanate, enzymatic products of myrosinase from glucosinolate found in cauliflower and cabbage, lower the incidence of tumor formation, and have an antioxidative effect [26]. There are also antimicrobial components such as iprodione [27], chitosan [28,29,30], and a peptide [31] in cruciferous vegetables.
Nowadays, as noted in the references mentioned above, the cruciferous vegetables are becoming more and more present in restaurants because they are very healthy (Figure 3). The following is a summary of the healthiest crucifers in terms of their chemical composition and the benefits they bring to human health [8,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31].
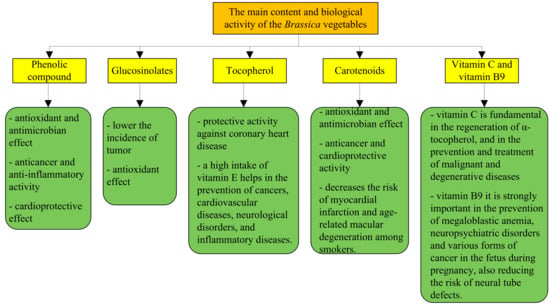
Figure 3.
Main biological activities of Brassica vegetables.
Arugula is a plant native to the Mediterranean area, related to kale and cauliflower. The arugula leaves have a specific, peppery, slightly bitter, and intense taste, which fits perfectly in any salad, in combination with other green leaves. Arugula has antimicrobial properties and stimulates immunity, supporting the body in the transition periods between the cold and hot seasons. Being an excellent source of vitamin A, together with the flavonoids in the composition, it supports eye health and helps maintain healthy skin. Fresh arugula contains a significant amount of vitamin C, which has an anti-inflammatory effect and protects against the harmful effects of free radicals [32,33].
Broccoli is a vegetable with an exceptional nutritional value, which can help maintain a long and healthy life. Broccoli contains a significant amount of glucosinolate, which is converted in the body into sulforaphane, a substance that has a proven effect in stopping the growth of cancer cells [34]. Sulforaphane also has the effect of removing toxins from lung cells, eliminating harmful compounds from pollutants and cigarette smoke. Both the stem and the fluorescence of this vegetable are rich in nutrients and are edible. When cooked, certain substances such as carotenoids become more concentrated, but the number of glucosinolates decreases. These losses can be avoided if it is steamed instead of boiled [35].
Brussels sprouts, the relative of ordinary cabbage, looks like a miniature cabbage. It is very rich in nutrients, being primarily a good source of vitamins C and K. It is composed of mineral salts (especially manganese, potassium, calcium, iron), folate, vitamin A, and B vitamins, all accompanying a large amount of fiber [36]. Brussels sprouts can help prevent or alleviate many ailments: rheumatoid arthritis, cardiovascular disease, digestive disorders. As with other cruciferous vegetables, they contain sulforaphane [37].
Cabbage is a vegetable with a very low caloric content. It is one of the vegetables with the highest vitamin C content (the richest are the leaves on the outside). Cabbage is also very rich in vitamin E. Provitamin A (or carotene, which is converted to vitamin A in the body) is also very well represented [37]. Cabbage also contains B vitamins. Dietary fiber is abundant in cabbage, being composed mostly of cellulose and hemicellulose, which explains some intestinal intolerances. Dietary fiber can stimulate the gut and prevent constipation. The most popular varieties of cabbage are white cabbage and red cabbage. All these varieties of cabbage are eaten raw, cooked, or added to pickles [38].
The cauliflower head consists of hundreds of white bouquets that are attached to a single stem. In addition to the well-known white cauliflower, on the market you can find dark green varieties (which are obtained by crossing with broccoli), purple or orange varieties, and cauliflower of the variety “Chartreuse Romanesco” (Roman cauliflower) [39]. Cauliflower contains significant amounts of vitamin C, B-complex vitamins such as folate, pantothenic acid (vitamin B5), pyridoxine (vitamin B6), and thiamine (vitamin B1), niacin (B3), and vitamin K. These vitamins are essential as the body needs external input to restore the reserves needed for proper functioning. It can be a reliable ally in the body’s fight against toxic substances, which helps reduce the risk of various forms of malignancy. It also helps prevent cardiovascular disease by lowering triglyceride and cholesterol levels [40].
Kale cabbage, also known as “borecole” or “farmer’s cabbage”, has been grown for more than 2000 years. Over time, it has been widely consumed, especially in Europe, due to its resistance to frost and the fact that these crops can be harvested late at the end of the year. Kale cabbage has a sweet and slightly pungent aroma, with an astringent flavor, due to its high iron content. The different types of kale cabbage also have different tastes: red kale is suitable for salads, for a fine taste, and has a pungent taste, while kale palm has a sweet taste [41]. The smaller kale leaves are generally thinner and sweeter than the larger ones. Kale can act as an anti-inflammatory due to its high content of omega-3 and fatty acids, which are essential in the fight against rheumatoid arthritis, asthma, or autoimmune diseases. Kale also contains a significant amount of vitamin K [42].
Kohlrabi is a vegetable with a sweet and delicate aroma, between radish and cabbage, whose origins are in northwestern Europe. Although it appears to be a root that grows in the ground, Kohlrabi grows on the surface of the soil. These vegetables are an excellent source of vitamins C, A, E, B, and K, minerals such as phosphorus, iron, zinc, potassium, magnesium, calcium, and copper, folic acid, lutein, but also fiber. Kohlrabi contains over 90% water. Glucosinolate determines the characteristic aroma and taste, known to have anticarcinogenic activity [43,44].
Mustard Leaves
Research has shown that mustard originated in ancient Egypt, and the population here combined meat with mustard seeds. In the Greeks, mustard was also used for meat, while the Romans used it as an antidote to snake bites, muscle fever, and even plague. Not only are the berries from this vegetable used, but also the leaves. They are an excellent source of vitamins A, C, and K. With a strong taste, mustard leaves are preferably eaten cooked and not raw [45,46].
Radishes have been consumed since ancient times and have been mentioned in Chinese history since 2700 BC. The Egyptians cultivated these vegetables before the period when they built the pyramids. Radishes are distinguished by their antibacterial and antifungal action, abundance of vitamins and minerals (vitamin C, vitamin B complex, magnesium, potassium, sodium, etc.), and low cholesterol and calories. Significant amounts of dietary fiber and folic acid found in radishes are recommended for daily consumption in a balanced diet [47]. Although most of us are familiar with red radishes, white radishes from Asia and black radishes grown in Spain are part of the same family. Radishes are eaten raw, in salads, in garnishes, or can be added to soups and other dishes [48].
Turnip began to be widely cultivated during Greco-Roman Antiquity, after the 15th century BC, and seems to have been originally discovered by the Asian civilization. The root of this plant is used primarily, but its leaves are not left out either, being highly valued in traditional medicine or as a substitute for spinach leaves in culinary preparations [49]. Small varieties are intended for human consumption, while larger ones are used as feed. Fresh turnip root is an excellent source of vitamin C, but the leaves of the plant have a higher content of antioxidants and nutrients. An excellent source of vitamin K, Omega-3, and vitamin A, turnip leaves have an anti-inflammatory role and protect the body against cardiovascular disease [50].
Horseradish is appreciated for its sweet and very hot taste, being frequently used with beetroot salad (it is a combination that brings valuable nutrients to the body during the winter), but also together with meat dishes. From horseradish, the young leaves and root can be used. Horseradish root is very rich in vitamin C, mineral salts, enzymes, amino acids, moderate amounts of B-complex vitamins, and carotenoids. Horseradish has effects on the respiratory tract due to volatile compounds, is very rich in vitamin C, has antibiotic and stimulating properties on the metabolism of the adrenal glands, lowers blood pressure, and has a deworming and diuretic effect. Due to its effects, horseradish is recommended in respiratory infections, asthma and allergies, urinary tract infections, to regulate metabolism and digestion, in hypertension, asthenia, and fatigue [51].
As noted in the references mentioned above, in the case of Brassica spp., nutritional quality is given by the presence of molecules such as phenols, vitamin C (antioxidant compounds), or nonantioxidant compounds such as glucosinolates and minerals.
Antioxidant compounds that are found in Brassica spp. are of two types: lipo-soluble antioxidants and water-soluble antioxidants. Lipo-soluble antioxidants (carotenoids such as β-carotene and xanthophylls) are responsible for the yellow, orange, and red pigmentation of some cruciferous plants. Through the metabolic processes that take place in the body, β carotene is transformed into lutein, zeaxanthin, and vitamin A. Many epidemiological studies show that the use of lutein and zeaxanthin reduce the risk of cataracts and maculodystrophy, two of the leading causes of vision loss and blindness. In addition to the many benefits it brings to the human body, beta-carotene helps prevent cardiovascular disease and reduces the risk of heart attack [50,51,52,53]. Beta carotene is converted to vitamin A in the body and is the most important source of provitamin A in food. It acts as an antioxidant together with vitamins E and C and can reduce the risk of chronic diseases. Beta carotene also plays an important role in protecting the skin from the harmful effects of ultraviolet radiation [52]. According to Podsedek, kale is the cruciferous with the highest content of lutein and zeaxanthin (3.04–39.55 mg/100 g) [53]. Muller [54] reported the decrease in the total carotenoid content of some cruciferous species as follows: Brussels sprouts (6.1 mg/100 g), broccoli (1.6 mg/100 g), red cabbage (0.43 mg/100 g), and white cabbage (0.26 mg/100 g).
In addition to carotenoids in the category of lipo-soluble antioxidants, vitamin E plays an important role in the composition of cruciferous plants. As with beta carotene and vitamin E, it prevents cardiovascular disease (inhibition of LDL oxidation), cancer, and inflammatory and neurological diseases. Vitamin E is a major lipo-soluble antioxidant responsible for protecting membranes from lipid peroxidation [52]. The antioxidant properties of vitamin E that act on free radicals could slow down the biological aging process. In humans, vitamin E deficiency can cause neurological and cardiovascular dysfunction [55]. Vitamin E is formed by tocopherols and tocotrienols; in cruciferous plants, α-tocopherol is the main compound except for the cauliflower where γ- tocopherol is found in its composition [56].
In addition to the lipo-soluble antioxidants in Brassica vegetables, we also find water-soluble antioxidants. This category includes phenolic compounds (flavonols, flavones, anthocyanins), vitamin C (ascorbic acid), and vitamin B9 (folic acid). Polyphenols are one of the most numerous and important substances that have their origin in the vegetable kingdom. Along with vitamins C, E, and A, they are considered compounds that give fruits and vegetables all those well-known properties, beneficial for the human body [50,51,52,53,54,55,56]. According to Moreno et. al and Heimler et al., kale and broccoli have the highest quantity of phenolic compounds [57,58]. In addition to its antioxidant properties, polyphenols also hide other secrets, fulfilling complex functions regarding the immune system, more precisely in the prevention of tumors, inflammatory processes, and cardiovascular pathologies. The main beneficial activity for the health of the human body is represented by the antioxidant action. In fact, polyphenols can neutralize free radicals, reducing inflammatory processes and the effects of cellular aging [59]. Certain types of polyphenols (procyanidin) also promote the death of irreversibly “damaged” cells, thus preventing the formation of cancer cells. Specifically, polyphenols manage to reduce the buildup of cholesterol located especially on the walls of blood vessels (the anti-atherogenic action) [60].
Flavonoids are the most important phenolic compounds found in Brassica; these phenolic compounds possess many properties such as antioxidant and antimicrobial activity [61], and a capillary protective and inhibitory effect [53,59,62].
The most common flavonoids in Brassica are: flavonols, flavones, and anthocyanins. Anthocyanins, which have very high antioxidant activity, are present only in colored vegetables such as purple broccoli and red cabbage. In herbal medicine, anthocyanins have long been used to treat many ailments (including high blood pressure, colds, and urinary tract infections). Recent research suggests that anthocyanins may also help alleviate major health problems, including heart disease and cancer [63,64,65]. As with the anthocyanins presented above, flavones also show antioxidant activity through apigenin and luteolin (the only flavones found in the cruciferous composition) [66]. Medical studies conducted in recent years on the properties of flavonoids were aimed at anti-inflammatory, antioxidant, antimicrobial, antiviral, antitumor, antihemorrhagic, and antiallergic [60]. The whole set of benefits attributed to flavonoids depends largely on their ability to block the damage caused by free radicals, associated with various chronic degenerative diseases: considered powerful antioxidants, they successfully intervene in the proper functioning of the liver, capillaries, and immune system; contribute to the prevention of many pathologies of cardiovascular origin and inflammatory diseases; with the ability to boost the immune system, these substances manage to counteract some forms of tumor; diets rich in flavonoids help prevent cardiovascular disease and reduce the risk of early death; these chemicals in plants are able to act on cholesterol and other factors that affect the health of the circulatory system. The most represented flavonols in cruciferous vegetables are quercetin, up to 23 mg/100 g of fresh product in kale, and the second most common flavonoid is found in broccoli (Kaempferol 3-O-sophoroside) [67,68,69].
Sanlier et al. and Blaževi et al. [70,71] showed that among Brassicaceae, kale is the richest in almost all the main macro and micro elements. The high capacity of Brassica to accumulate the metals present in the soil is considered a very good metal hyperaccumulator. Macronutrients (nitrogen, phosphorus, potassium) help plants in essential structural and energetic roles, and the minerals such as copper, iron, zinc, and selenium are required by plants in very small quantities and are so-called microelements.
In addition to the components mentioned above in Brassica, there are also glucosinolates and isothiocyanates [71,72]. Glucosinolates are glucosidic compounds (with sulfur) derived from amino acid biosynthesis, and the concentrations vary according to the developmental stage and environmental factor. They serve no direct function to human health, but the health effects are fulfilled by the hydrolysis breakdown products, the isothiocyanates. They are responsible for the typical and spicy aroma of cruciferous vegetables. The most used isothiocyanates is sulforaphane [73], which is found in large quantities in broccoli and Brussels sprout; sulforaphane acts as a catalyst in the stimulation of the cellular antioxidant system and some enzymes against tumoral cell proliferation [74].
3. Application of Brassica Genus in Green Nanotechnology
Considering the data presented in the previous section, cruciferous vegetables contain many substances that have antimicrobial and antioxidant activity [48], making them ideal candidates for use in the phytosynthesis of metallic nanoparticles for the nanocoating’s purpose.
In the 21st century, finding new “green” materials with potential applications in various fields is an important goal. The use of these materials and the real interest in their research is due to green synthesis methods that have considerable advantages such as avoiding toxic chemicals, high cost, and severe reaction conditions [75]. Even direct precipitation, the sol–gel method, reverse micelles, thermal decomposition, or the solvothermal method was successfully used for the obtaining of metallic nanoparticles; “green methods” (with biological sources such as bacteria, fungi, yeast, actinobacteria, and plants) can be used as well (Figure 4).
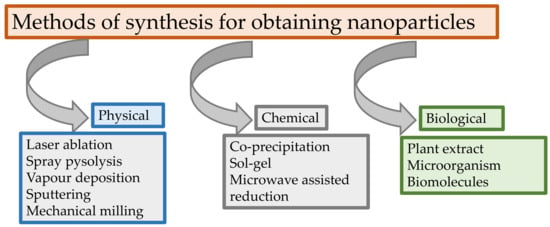
Figure 4.
Methods of synthesis for obtaining nanoparticles.
The recent pandemic of the COVID-19 virus has demonstrated that costs in both human life and economic terms can be very high if the spread of the infection is not controlled [76]. Therefore, it is very important to have measures to minimize this spread of infection.
Nano-coatings containing nanoparticles, such as metal nanoparticles, metal oxide nanoparticles, carbon nanotubes, or various heterostructures have been shown to be up to 99.99% effective against many microorganisms [77]. Inorganic nanomaterials are stable at high temperatures and have a long shelf life, and they also show anti-microbial and anti-viral activities compared to organic nanomaterials [78]. Future research needs to focus on new antiviral and antibacterial nanocoatings for a wide range of applications, especially for so-called high-traffic objects (e.g., hospitals, schools, door, and window handles in public places; public transport) and/or coatings for textiles (e.g., face masks, laboratory coats).
Sharma and coworkers [79] showed that the antibacterial activity against Escherichia coli of biologically synthesized AgNPs (silver nanoparticles, via Brassica oleracea) are more pronounced than chemically synthesized AgNPs. Ansar et al. also demonstrated that the green synthesis of AgNPs via Brassica oleracea has antimicrobial, antioxidant, and anti-tumor activities [80].
Pandit [81] reported that the green synthesis of AgNPs from seed extract of B. nigra possess antimicrobial activity; these nanoparticles can be successfully incorporated into antimicrobial coatings.
In addition, it has been observed that metallic nanoparticles can be synthesized with the vegetal material extract or can be formed in situ in different plant organs [82]. Moreover, if the nanoparticles are calcinated at enhanced temperatures (from 100 to 900 °C), they lose their beneficial properties, and the properties gradually decrease with increasing calcination, due to the increase in particle size [83]. Green synthesis is the use of bio-reduction that is 100% eco-friendly and is energy-saving and economically viable [84]. Plant extracts can offer a complex matrix of chemical compounds and secondary metabolites acting as natural reducing agents as well as a capping agent [85]. AgNP applications are numerous, and there is strong potential for continuous growth in this area (Table 2). They can be used as active compounds for medical applications (wound healing, drug carrier, and as a material for medical diagnostics), personal care products, food and feed industry, environmental applications, or sensors [86,87,88]. Following the aspects of global solid waste management and the ecofriendly green synthesis approach, Umamaheswari and collaborators evaluated the anticancer property of ZnO nanoparticles obtained from Raphanus sativus var. Longipinnatus leaves extracts using A549 cell lines. IC50 was found to be 40 μg mL−1, explaining that the higher surface area-to-volume ratio of these small particles may be responsible for the enhanced cytotoxic effect [89]. More studies have focused on the effect of metallic nanoparticles as antifungal agents. Spherical ZnO nanoparticles can be efficient against Escherichia fergusonii and Escherichia coli (approx. 15 to 23 mm inhibition zone, depending on the strain) [90]; meanwhile, spherical AgNPs are efficient as antibacterial agents against Bacillus subtilis (MTCC 441) (IZD = 23 mm); Staphylococcus aureus (MTCC 740) (IZD = 12 mm); Escherichia coli (MTCC 443) (IZD = 34 mm); Serratia marcescens (MTCC 97) (IZD = 36 mm) [91] and CuI nanoparticles can be successfully used against Escherichia coli (ATCC 443) and Staphylococcus aureus (ATCC 96) [92]. The complex matrix of the extract and plant moieties on the surface of NPs can change the charge of the particles, enhancing the potential effect, and offering multiple applications (medical and environment protection) [93].

Table 2.
Application of Brassica genus in green nanotechnology.
In addition, Zandi et al. [101] recently published a study about an active coating containing radish leaf extract and can be used as an effective strategy for sweet lemon preservation.
Other types of nano-coatings in which crucifers are used are the following functional antimicrobial and anticorrosion coatings.
Given the composition of cruciferous extracts, they can be successful substituted to replace conventional toxic inhibitors and extend the possibility of “smart coatings“ by inducing a response in the coating and/or substrate to improve the inhibition of corrosion.
Li et al. [102] studied a green Brassica oleracea L. extract as a novel corrosion inhibitor for Q235 steel in two typical acid media. This extract also had very good results in the inhibition of pipeline steel and mild steel and zinc in acidic media [103,104].
In addition to the extract of B. oleracea, it proved to be a good corrosion inhibitor, as well as the extract from B. campestris. Caseletto et al. showed that the eco-friendly extract of B. campestris is capable of inhibiting Cor-Ten steel corrosion in HCl and NaCl solutions [105].
Lately, with the use of facemasks and visors, eyeglasses become are fogged, and these cause a major challenge in several optical applications that require excellent light transmission characteristics. Ren et al. [106] studied a bioinspired coating with antifogging capabilities. The bioinspired adhesive was based on tannic acid, which is also found in rapeseed but also in other crucifers [107].
Starting with tannic acid, too, Behboodi-Sadabad et al. studied the biomimetic surface coatings base of combinations of polyphenols and catecholamines (e.g., dopamine) from plants [108]. Dopamine is found in different parts of plants of Brassica species: broccoli (Brassica olereacea var. italica) (1 μg/g FW) and Brussels sprouts (Brassica olereacea var. gemmifera) (1 μg/g FW); in addition, polyphenols are found in abundance in the genus Brassica as we showed in the previous chapter; thus, crucifers are a serious candidate for their use for obtaining the biomimetic surface coatings such as anti-fouling coatings referring to the solid surfaces repelling liquids [109], and is related to the chemical composition and the coatings morphology; they have a high potential in various domains such as the food industry.
Anti-fouling coatings [110] in food technology are very useful to reduce or prevent cross-contamination and in marine anti-fouling applications. Zulkifli et al. [111] studied the potential of B. oleracea as an anti-fouling agent for mild steel in seawater and, in conclusion, the cabbage extract has potential as an additive in paint.
4. Conclusions and Future Perspectives
In recent years, developing a sustainable use of wastes has gained great interest and has become an attractive research topic all over the world. Moreover, the discovery of new applications of these residues led to new opportunities for plant-derived natural compounds as drug agents with diverse chemical structures and novel mechanisms of action, new approaches for environmental applications, or for other industries. Green synthesis is using bio-reduction that is 100% eco-friendly and is energy-saving and economically viable. This review is useful for developing a new nano-bio-coating with increased antioxidant, antimicrobial, and other important qualities, according to the end users’ needs. If the final users and the manufacturer are aware of the main biological activities of Brassica vegetables, they will act accordingly considering the final goal.
The biggest challenges for the synthesis of new compounds based on plant extracts are related to the obtaining of controlled morphologies for the nanoparticles, lower minimum inhibitory concentrations for medical applications, decreased toxicity, and bioavailability. The phytosynthesis of nanoparticles usually leads to a reduced toxicity of the NPs and to lower MIC values, due to the contribution of the bioactive compounds acting as capping agents. According to the presented data, metallic nanoparticles presented notable medical applications, such as antimicrobial activity against different bacterial lines or efficiency in environmental approaches. As such, the evaluation of different plant materials is necessary, as different plants can lead to different results regarding the NPs properties. At the same time, the evaluation of plants wastes is promising, both from the nanotechnological point of view, as well as from the perspective of environmental protection and wastes reduction.
Future perspectives must be targeted toward the scale-up processes, from the laboratory level to industry, under the conditions that some extraction methods of bioactive compounds responsible for NP synthesis are already scaled up. In addition, research must be conducted in order to obtain stable formulations with no or less toxic by-products with efficient cost, energy, and reagents consumption. The scale-up of phytosynthesis procedures using cruciferous vegetables wastes would represent a valuable approach for the development of tailored nanomaterials, as the slight modification of the phytosynthesis parameters can lead to the achievement of the desired properties. These approaches will have great promise considering large amounts of wastes produced all over the world due to increased population and globalization.
Overall, major emphasis must be oriented toward the design of the next generation of nanoparticles with special properties and applications, as well as for their application in day-by-day products. Given the importance of this application field, the research and development of nanocoatings based on natural extracts has been continuously increasing. However, advances related to nanomaterials biofunctionalization are crucial to achieve a further high specificity in different areas.
Author Contributions
Conceptualization, C.U. and R.C.F.; methodology, C.U. and I.F.; validation, C.U., I.F. and R.C.F.; investigation, C.U. and R.C.F.; data curation, C.U. and I.F.; writing—original draft preparation, C.U. and I.F.; writing—review and editing, C.U., I.F. and R.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
R.C.F. and I.F. gratefully acknowledge the support of a grant of the Ministry of Research, Innovation and Digitization, CNCS - UEFISCDI, project number PN-III-P4-PCE-2021-0292, within PNCDI III.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| spp. | subspecies |
| var. | varieties (in Latin: varietas) |
| LDL | low-density lipoprotein |
| COVID 19 | Coronavirus disease 2019 |
| AgNPs | silver nanoparticles |
| ZnO | zinc oxide |
| MTCC | Microbial Type Culture Collection |
| IZD | inhibition zone diameter |
| IC50 | the half maximal inhibitory concentration |
| A549 | cell lines |
| CuI | copper iodide |
| XRD | X-ray diffraction analysis |
| nm | nanometer |
| TEM | Transmission electron microscopy |
| HR- TEM | High-resolution transmission electron microscopy |
| AFM | Atomic force microscopy |
| DLS | Dynamic light scattering |
| Z potential | zeta potential |
| °C | degrees Celsius |
| h | hours |
| mL | milliliter |
| mV | millivolt |
| MDR | multidrug-resistant |
| w/v | mass concentration of solution is expressed as % w/v for weight per volume |
| rpm | rotation per minute |
| min. | minutes |
| eV | electron Volt |
| Au | gold |
| Ag | silver |
| NaBH4 | Sodium borohydride |
| W | Watt |
| E. coli | Escherichia coli |
| S. aureus | Staphylococcus aureus |
| AuNP | gold nanoparticles |
| μg | microgram |
References
- Niculescu, A.G.; Chircov, C.; Bîrca, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Sutan, N.A.; Manolescu, D.S.; Fierascu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fierascu, R.C. Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. rhizomes alcoholic extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 746. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Anuta, V.; Velescu, B.S.; Jinga, M.; Jinga, V. A Short Overview of Recent Developments on Antimicrobial Coatings Based on Phytosynthesized Metal Nanoparticles. Coatings 2019, 9, 787. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, I.; Fierascu, R.C.; Ungureanu, C.; Draghiceanu, O.A.; Soare, L.C. Application of Polypodiopsida Class in Nanotechnology–Potential towards Development of More Effective Bioactive Solutions. Antioxidants 2021, 10, 748. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Organization of the United Nations. Top exports of Cabbages and Other Brassicas. In FAOSTAT Database; FAOSTAT: Rome, Italy, 2009. [Google Scholar]
- Hayek, A. Entwurf eines Cruciferensystems auf phylogenetischer Grundlage. Beih. Zum Bot. Cent. 1911, 27, 127. [Google Scholar]
- Schulz, O.E. Cruciferae. In Die Naturlichen Pflanzenfamilien; Engler, A., Harms, H., Eds.; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1936; Volume 17B, pp. 227–658. [Google Scholar]
- Goel, U.; Kawatra, B.L. Nutrition evaluation of a cauliflower leaf protein. J. Sci. Food Agr. 1997, 28, 786. [Google Scholar] [CrossRef]
- FAOSTAT—Food and Agriculture Organization of the United Nations. Top exports of Cabbages and Other Brassicas. In FAOSTAT Database; FAOSTAT: Rome, Italy, 2011. [Google Scholar]
- Francisco, M.; Tortosa, M.; Martinez-Ballesta, M.; Velasco, P.; Garcia-Viguera, C.; Moreno, D.A. Nutritional, and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2017, 170, 273. [Google Scholar] [CrossRef]
- Fahey, J.W. Brassicas. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 606–615. ISBN 9780122270550. [Google Scholar]
- Van Popple, G.; Verhoeven, D.T.; Verhagen, H.; Goldbohm, R.A. Brassica vegetables and cancer prevention. Adv. Exp. Med. Biol. 1999, 472, 159. [Google Scholar]
- Bjorkman, M.; Klingen, I.; Birch, A.; Bones, A.; Bruce, T.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phyto-chemicals of Brassicaceae in plant protection and human health. Influences of climate; environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Bortoloti, G.A.; Baron, D. Phytoremediation of toxic heavy metals by brassica plants: A biochemical and physiological approach. Environ. Adv. 2022, 8, 100204. [Google Scholar] [CrossRef]
- Jo, J.S.; Bhandari, S.R.; Kang, G.H.; Shin, Y.K.; Lee, J.G. Selection of broccoli (brassica oleracea var. italica) on composition and content of glucosinolates and hydrolysates. Sci. Hortic. 2022, 298, 110984. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Wu, C.; Rao, Z.; Du, L.; Zhou, Y. Cruciferous vegetable and isothiocyanate intake and multiple health outcomes. Food Chem. 2022, 375, 131816. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Traditional uses of wild and tended plants in maintaining ecosystem services in agricultural landscapes of the eastern cape province in South Africa. J. Ethnobiol. Ethnomed. 2022, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Q.; Fu, J.; Wang, Y.; Zhang, Y.; Sun, Y.; Tong, H.; Dhankher, O.P. Co-exposure of sulfur nanoparticles and cu alleviate cu stress and toxicity to oilseed rape brassica napus L. J. Environ. Sci. 2023, 124, 319. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and Glucosinolates as Strong Determinants of the Nutritional Quality in Rocket Leafy Salads. Nutrients 2014, 6, 1519. [Google Scholar] [CrossRef] [Green Version]
- Argento, S.; Melilli, M.G.; Branca, F. Enhancing greenhouse tomato-crop productivity by using Brassica macrocarpa guss. Leaves forcontrolling root-knot nematodes. Agronomy 2019, 9, 820. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, H.; Shad, M.A.; Muzaffar, S. Phytochemical composition and antioxidant potential of Brassica. In Brassica Germplasm—Characterization, Breeding, and Utilization; El-Esawi, M.A., Ed.; Intech Open: London, UK, 2018; pp. 7–26. [Google Scholar]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Salopek-Sondi, B. Cruciferous (Brassicaceae) vegetables. In Nonvitaminand Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 195–202. [Google Scholar]
- Chang, C.T.; Hseueh, Y.L.; Shung, H.Y. Purification and properties of chitinase from cabbage stems with roots. Biochem. Mol. Biol. Int. 1996, 40, 417. [Google Scholar]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367. [Google Scholar] [CrossRef] [Green Version]
- Nestle, M. Broccoli sprouts in cancer prevention. Nutr. Rev. 1998, 56, 127. [Google Scholar] [CrossRef]
- Datta, A.; Gopal, M. Safety evaluation of the fungicide iprodioneon cauliflower. Bull. Environ. Contamination. Toxicol. 1999, 62, 496. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Roller, S. Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl. Environ. Microbiol. 2000, 66, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roller, S.; Covil, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67. [Google Scholar] [CrossRef]
- Wang, G.H. Inhibition, and inactivation of five species of food borne pathogens by chitosan. J. Food Prot. 1992, 55, 916. [Google Scholar] [CrossRef]
- Lucca, A.J.D.; Walsh, T.J. Antifungal peptides: Novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 1999, 43, 1. [Google Scholar] [CrossRef] [Green Version]
- Wright, C. Mediterranean Vegetables; Harvard Common Press: Boston, MA, USA, 2012; p. 27. ISBN 9781558321960. [Google Scholar]
- Can, H.; Sari, B.; Kavuşan, H.S.; Serdaroǧlu, M. Arugula and pre-converted arugula extract as natural Nitrate/Nitrite sources for heat-treated sausages. In Proceedings of the 61st International Meat Industry Conference, Zlatibor, Serbia, 26–29 September 2021. [Google Scholar]
- Maggioni, L.; Bothmer, R.; Poulsen, G.; Branca, F. Origin and Domestication of Cole Crops (Brassica oleracea L.): Linguistic and Literary Considerations. Econ. Bot. 2010, 64, 109. [Google Scholar] [CrossRef]
- López-Hernández, A.A.; Ortega-Villarreal, A.S.; Vázquez Rodríguez, J.A.; López-Cabanillas Lomelí, M.; González-Martínez, B.E. Application of different cooking methods to improve nutritional quality of broccoli (brassica oleracea var. italica) regarding its compounds content with antioxidant activity. Int. J. Gastron. Food Sci. 2022, 28, 100510. [Google Scholar] [CrossRef]
- Ortner, E.; Granvogl, M. Thermally induced generation of desirable aroma-active compounds from the glucosinolate sinigrin. J. Agric. Food Chem. 2018, 66, 2485. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Dunkel, A.; Szwengiel, A.; Czaczyk, K.; Drożdżyńska, A.; Zawirska-Wojtasiak, R.; Jeleń, H.H. The relation between phytochemical composition and sensory traits of selected brassica vegetables. LWT 2022, 156, 113028. [Google Scholar] [CrossRef]
- USDA Database Table for Raw Cabbage per 100 g, US Department of Agriculture, National Nutrient Database for Standard Reference, Version SR-27. 1 April 2019. Available online: http://www.ars.usda.gov/nea/bhnrc/mafcl (accessed on 5 January 2020).
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A Mechanistic Perspective on Process-Induced Changes in Glucosinolate Content in Brassica Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; An, R.; Zhou, H.; Zhang, Y.; Ling, J.; Hu, H.; Li, P. The glucosinolate profiles of brassicaceae vegetables responded differently to quick-freezing and drying methods. Food Chem. 2022, 383, 132624. [Google Scholar] [CrossRef] [PubMed]
- Armesto, J.; Gómez-Limia, L.; Carballo, J.; Martínez, S. Effects of different cooking methods on the antioxidant capacity and flavonoid, organic acid and mineral contents of Galega kale (Brassica oleracea var. acephala cv. Galega). Int. J. Food Sci. Nutr. 2018, 70, 136. [Google Scholar] [CrossRef] [PubMed]
- Soengas, P.; Velasco, P.; Fernández, J.C.; Cartea, M.E. New Vegetable Brassica Foods: A Promising Source of Bioactive Compounds. Foods 2021, 10, 2911. [Google Scholar] [CrossRef]
- Hien, T.T.; Truc, T.T.; Muoi, N.V. Effect of salt concentration and pH value on the lactic fermentation process of kohlrabi (brassica oleracea L.). J. Food Sci. Technol. 2022, 10, 399. [Google Scholar]
- Popović-Djordjević, J.B.; Kostić, A.Ž.; Rajković, M.B.; Miljković, I.; Krstić, Đ.; Caruso, G.; Moghaddam, S.S.; Brčeski, I. Organically vs. conventionally grown vegetables: Multi-elemental analysis and nutritional evaluation. Biol. Trace Elem. Res. 2022, 200, 426. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Kim, J.; Moon, B. Effect of main vegetable ingredient on the glucosinolate, carotenoids, capsaicinoids, chlorophylls, and ascorbic acid content of kimchis. J. Food Compos. Anal. 2022, 110, 104523. [Google Scholar] [CrossRef]
- McAlvay, A.C.; Ragsdale, A.P.; Mabry, M.E.; Qi, X.; Bird, K.A.; Velasco, P.; An, H.; Pires, J.C.; Emshwiller, E. Brassica rapa Domestication: Untangling Wild and Feral Forms and Convergence of Crop Morphotypes. Mol. Biol. Evol. 2021, 38, 3358. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Perez, R. Raphanus sativus (radish): Their chemistry and biology. Sci. World J. 2004, 4, 811. [Google Scholar] [CrossRef]
- Ungureanu, C.; Fierascu, I.; Fierascu, R.C.; Costea, T.; Avramescu, S.M.; Călinescu, M.F.; Somoghi, R.; Pirvu, C. In Vitro and In Vivo Evaluation of Silver Nanoparticles Phytosynthesized Using Raphanus sativus L. Waste Extracts. Materials 2021, 14, 1845. [Google Scholar] [CrossRef]
- Hilliard, S.B. Hog Meat and Hoecake: Food Supply in the Old South, 1840-1860. In Southern Foodways Alliance Studies in Culture, People, and Place; Southern Illinois University Press: Carbondale, IL, USA, 1972; ISBN 978-08203-4702-8. [Google Scholar]
- Khadivi, A.; Mirheidari, F.; Moradi, Y. Selection of superior accessions of turnip (Brassica rapa var. rapa L.) based on tuber quality-related characters. Food Sci. Nutr. 2022, online first. [Google Scholar] [CrossRef]
- Bladh, K.W.; Olssonb, K.M. Introduction and use of horseradish (Armoracia rusticana) as food and medicine from antiquity to the present: Emphasis on the nordic countries. J. Herbs Spices Med. Plants. 2011, 17, 197. [Google Scholar] [CrossRef]
- Kurilich, A.C.; Tsau, G.J.; Brown, A.; Howard, L.; Klein, B.P.; Jeffery, E.H.; Kushad, M.; Walling, M.A.; Juvik, J.A. Carotene; tocopherol; and ascorbate contents in subspecies of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1576. [Google Scholar] [CrossRef] [PubMed]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1. [Google Scholar] [CrossRef]
- Muller, H. Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection. Food Res. Technol. 1997, 204, 88. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Rimm, E.B. Epidemiologic evidence for vitamin E in prevention of cardiovascular disease. Am. J. Clin. Nutr. 1995, 62, 1365. [Google Scholar] [CrossRef]
- Piironen, V.; Syvaoja, E.L.; Varo, P.; Salminen, K.; Koivistoinen, P. Tocopherols and tocotrienols in Finnish foods: Vegetables; fruits; and berries. J. Agric. Food Chem. 1986, 34, 742. [Google Scholar] [CrossRef]
- Moreno, D.A.; Carvajal, M.; Lopez-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251. [Google Scholar] [CrossRef]
- Sousa, C.; Lopes, G.; Pereira, D.M.; Taveira, M.; Valentao, P.; Seabra, R.M.; Pereira, J.A. Screening of antioxidant compounds during sprouting of Brassica oleracea L. var. costata DC. Comb. Chem. High Throughput Screen. 2007, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob Agents. 2005, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Biondi, F.; Balducci, F.; Capocasa, F.; Visciglio, M.; Mei, E.; Vagnoni, M.; Mezzetti, B.; Mazzoni, L. Environmental Conditions and Agronomical Factors Influencing the Levels of Phytochemicals in Brassica Vegetables Responsible for Nutritional and Sensorial Properties. Appl. Sci. 2021, 11, 1927. [Google Scholar] [CrossRef]
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, A.; García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins Contents, Profiles, and Color Characteristics of Red Cabbage Extracts from Different Cultivars and Maturity Stages. J. Agric. Food Chem. 2014, 62, 7524. [Google Scholar] [CrossRef]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model system. Food Chem. 2001, 72, 431. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O.I. Total phenol; flavonoid; proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553. [Google Scholar] [CrossRef]
- Ackland, M.L.; Van De Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavanols quercetin and kaempferol in cultured human cancer cell lines. In Vivo 2005, 19, 69. [Google Scholar]
- Kim, D.O.; Padilla-Zakour, O.I.; Griffiths, P.D. Flavonoids and antioxidant capacity of various cabbage genotypes at juvenile stage. J. Food Sci. 2004, 69, C685. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Pérez-Guerrero, C.; Lòpez-Lazaro, M. A review on the dietary flavonoid kaempferol. Med. Chem. 2011, 11, 298. [Google Scholar] [CrossRef]
- Sanlier, N.; Guler Saban, M. The Benefits of Brassica Vegetables on Human Health. J. Hum. Health Res. 2018, 1, 104. [Google Scholar]
- Blaževic, I.; Montaut, S.; Burcul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Abdel-Farida, I.B.; Kima, H.K.; Choia, Y.H.; Verpoortea, R. Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 2009, 67, 23. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337. [Google Scholar] [CrossRef] [PubMed]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordàs, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2007, 68, 536. [Google Scholar] [CrossRef]
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and Functionalized Films/Coatings—Performances and Perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Islam, S.S.; Midya, S.; Sinha, S.; Saadi, S.M.A.I. Natural medicinal plant products as an immune-boosters: A possible role to lessen the impact of Covid-19. Chem. Environ. Eng. 2021, 4, 100105. [Google Scholar] [CrossRef]
- De Carvalho Lima, E.N.; Octaviano, A.L.M.; Piqueira, J.R.C.; Diaz, R.S.; Justo, J.F. Coronavirus and Carbon Nanotubes: Seeking Immunological Relationships to Discover Immunotherapeutic Possibilities. Int. J. Nanomed. 2022, 17, 751. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, J.; Bhatia, A.K. Azadirachta Indica and Brassica Oleracea Mediated Green Synthesis vs. Chemical Synthesis of Silver Nanoparticles and their Antibacterial Properties. Nanosci. Nanotechnol. Asia 2019, 9, 393. [Google Scholar] [CrossRef]
- Ansar, S.; Tabassum, H.; Aladwan, N.S.M.; Naiman, A.M.; Almaarik, B.; AlMahrouqi, S.; Abudawood, M.; Banu, N.; Alsubki, R. Eco friendly silver nanoparticles synthesis by Brassica oleracea and its antibacterial, anticancer and antioxidant properties. Sci Rep. 2020, 10, 18564. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R. Green synthesis of silver nanoparticles from seed extract of Brassica nigra and its antibacterial activity. Nusant. Biosci. 2015, 7, 1. [Google Scholar] [CrossRef]
- Bali, R.; Harris, A.T. Biogenic synthesis of Au nanoparticles using vascular plants. Ind. Eng. Chem. Res. 2010, 49, 12762. [Google Scholar] [CrossRef]
- Haq, S.; Dildar, S.; Ben Ali, M.; Mezni, A.; Hedfi, A.; Shahzad, M.I.; Shahzad, N.; Shah, A. Antimicrobial and antioxidant properties of biosynthesized of NiO nanoparticles using Raphanus sativus (R. sativus) extract. Mater. Res. Express 2021, 8, 055006. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Fierăscu, R.C.; Fierăscu, I.; Lungulescu, E.M.; Nicula, N.; Șomoghi, R.; Dițu, L.; Ungureanu, C.; Șuțan, A.N.; Drăghiceanu, O.A.; Păunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1. [Google Scholar] [CrossRef]
- Gong, H.; Liu, M.; Li, H. In situ green preparation of silver nanoparticles/chemical pulp fiber composites with excellent catalytic performance. J. Mater. Sci. 2019, 54, 6895. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Annamalai, S.K.; Arunachalam, A.M.; Kennedy, S. Green synthesis of crystalline silver nanoparticles using Indigofera aspalathoides-medicinal plant extract for wound healing applications. Asian J. Chem. 2013, 25, S311. [Google Scholar]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf. B Biointerfaces 2010, 80, 94. [Google Scholar] [CrossRef]
- Umamaheswari, A.; Lakshmana Prabu, S.; AdharshJohn, S.; Puratchikody, A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 2021, 29, e00595. [Google Scholar] [CrossRef]
- Kiran Kumar, A.B.V.; Sre Saila, E.; Narang, P.; Aishwarya, M.; Raina, R.; Gautam, M.; Shankar, E.G. Biofunctionalization and biological synthesis of the ZnO nanoparticles: The effect of Raphanus sativus (white radish) root extract on antimicrobial activity against MDR strain for wound healing applications. Inorg. Chem. Commun. 2019, 21, 2079. [Google Scholar] [CrossRef]
- Singh, T.; Jyoti, K.; Patnaik, A.; Singh, A.; Chauhan, R.; Chandel, S.S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. 2017, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.C.; Archana, K.M.; Rajagopal, R. Green synthesis, characterization, catalytic and antibacterial studies of copper iodide nanoparticles synthesized using Brassica oleracea var. capitata f. rubra extract. Chem. Data Collect 2020, 29, 100538. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Ri, H.; Khan, A.U.; Khan, U.A.; Yuan, Q. Green synthesis of catalytic zinc oxide nano-flowers and their bacterial infection therapy. Appl. Organomet. Chem. 2019, 34, e5298. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, T.; Liu, W.; Liu, Y.; Zhao, Y.; Liu, Y.; Li, W.; Ding, K.; Ma, F.; Li, C. Functions of dopamine in plants: A review. Plant Signal. Behav. 2020, 15, 1827782. [Google Scholar] [CrossRef]
- Perveen, R.; Shujaat, S.; Qureshi, Z.; Nawaz, S.; Khand, M.I.; Iqbal, M. Green versus sol-gel synthesis of ZnO nanoparticles and antimicrobial activity evaluation against panel of pathogens. J. Mater. Res. Technol. 2020, 9, 7817. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Ichwan, S.J.; Parine, N.R.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. 2015, 29, 151. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Phytosynthesis of Silver Nanoparticles using Andean Cabbage: Structural Characterization and its Application. Mater. Today Proc. 2020, 21, 2079. [Google Scholar] [CrossRef]
- Unal, I.S.; Demirbas, A.; Onal, Y.; Ildiz, N.; Ocsoy, I. One step preparation of stable gold nanoparticle using red cabbage extracts under UV light and its catalytic activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111800. [Google Scholar] [CrossRef]
- Lokhande, A.C.; Babar, P.T.; Karade, V.C.; Jang, J.S.; Lokhande, V.C.; Lee, D.J.; Kim, I.-C.; Patole, S.P.; Qattan, I.A.; Lokhande, C.D.; et al. A viable green route to produce Ag nanoparticles for antibacterial and electrochemical supercapacitor applications. Mater. Today Bio. 2019, 14, 100181. [Google Scholar] [CrossRef]
- Jain, A.; Sinilal, B.; Starnes, D.L.; Sanagala, R.; Krishnamurthy, S.; Sahi, S.V. Role of Fe-responsive genes in bioreduction and transport of ionic gold to roots of Arabidopsis thaliana during synthesis of gold nanoparticles. Plant. Physiol. Biochem. 2014, 84, 189. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M.; Ganjloo, A.; Bimakr, M.; Moradi, N.; Nikoomanesh, N. Effect of active coating containing radish leaf extract with or without vacuum packaging on the postharvest changes of sweet lemon during cold storage . J. Food Process. Preserv. 2021, 45, e15252. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. A green Brassica oleracea L extract as a novel corrosion inhibitor for Q235 steel in two typical acid media. Colloids Surf. A Physicochem. Eng. 2021, 616, 126077. [Google Scholar] [CrossRef]
- Ngobiri, N.C.; Oguzie, E.E.; Li, Y.; Liu, L.; Oforka, N.C.; Akaranta, O. Eco-friendly corrosion inhibition of pipeline steel using brassica oleracea. Int. J. Corros. 2015, 2015, 404139. [Google Scholar] [CrossRef] [Green Version]
- Chinweuba, A.J. Corrosion inhibition potentials of Brassica oleracea capitata extract on mild steel and zinc in acidic media. Chem. Mater. Res. 2014, 6, 62. [Google Scholar]
- Casaletto, M.P.; Figà, V.; Privitera, A.; Bruno, M.; Napolitano, A.; Piacente, S. Inhibition of Cor-Ten steel corrosion by “green” extracts of Brassica campestris. Corros. Sci. 2018, 136, 91. [Google Scholar] [CrossRef]
- Ren, J.; Kong, R.; Gao, Y.; Zhang, L.; Zhu, J. Bioinspired adhesive coatings from polyethylenimine and tannic acid complexes exhibiting antifogging, self-cleaning. J. Colloid Interface Sci. 2021, 602, 406. [Google Scholar] [CrossRef]
- Bojanowska, M. Changes in chemical composition of rapeseed meal during storage, influencing nutritional value of its protein and lipid fractions. J. Anim. Feed. Sci. 2017, 26, 157. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Li, S.; Lei, W.; Liu, Y.; Sommer, T.; Friederich, P.; Sobek, C.; Messersmith, P.B.; Levkin, P.A. High-throughput screening of multifunctional nanocoatings based on combinations of polyphenols and catecholamines. Mater. Today Bio. 2021, 10, 100108. [Google Scholar] [CrossRef]
- Nikoo, A.H.; Malayeri, M.R. Incorporation of surface energy properties into general crystallization fouling model for heat transfer surfaces. Chem. Eng. Sci. 2020, 215, 115461. [Google Scholar] [CrossRef]
- DeFlorio, W.; Liu, S.; White, A.R.; Taylor, T.M.; Cisneros-Zevallos, L.; Min, Y.; Scholar, E.M.A. Recent developments in antimicrobial and antifouling coatings to reduce or prevent contamination and cross-contamination of food contact surfaces by bacteria. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3093. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, M.F.R.; Radzi, N.M.; Jusoh, S.M.; Saidin, J.; Nik, W.M.N.W. Potential of cabbage extract (Brassica oleracea) as anti-fouling agent in alkyd undercoat for mild steel in seawater. Malays. J. Anal. Sci. 2019, 23, 451. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).