Improvement of Yttrium Oxyfluoride Coating with Modified Precursor Solution for Laser-Induced Hydrothermal Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Coated Substrates and Precursor Solutions
2.2. Laser-Induced Single-Step Coating (LISSC) Process
2.3. Surface Characterization
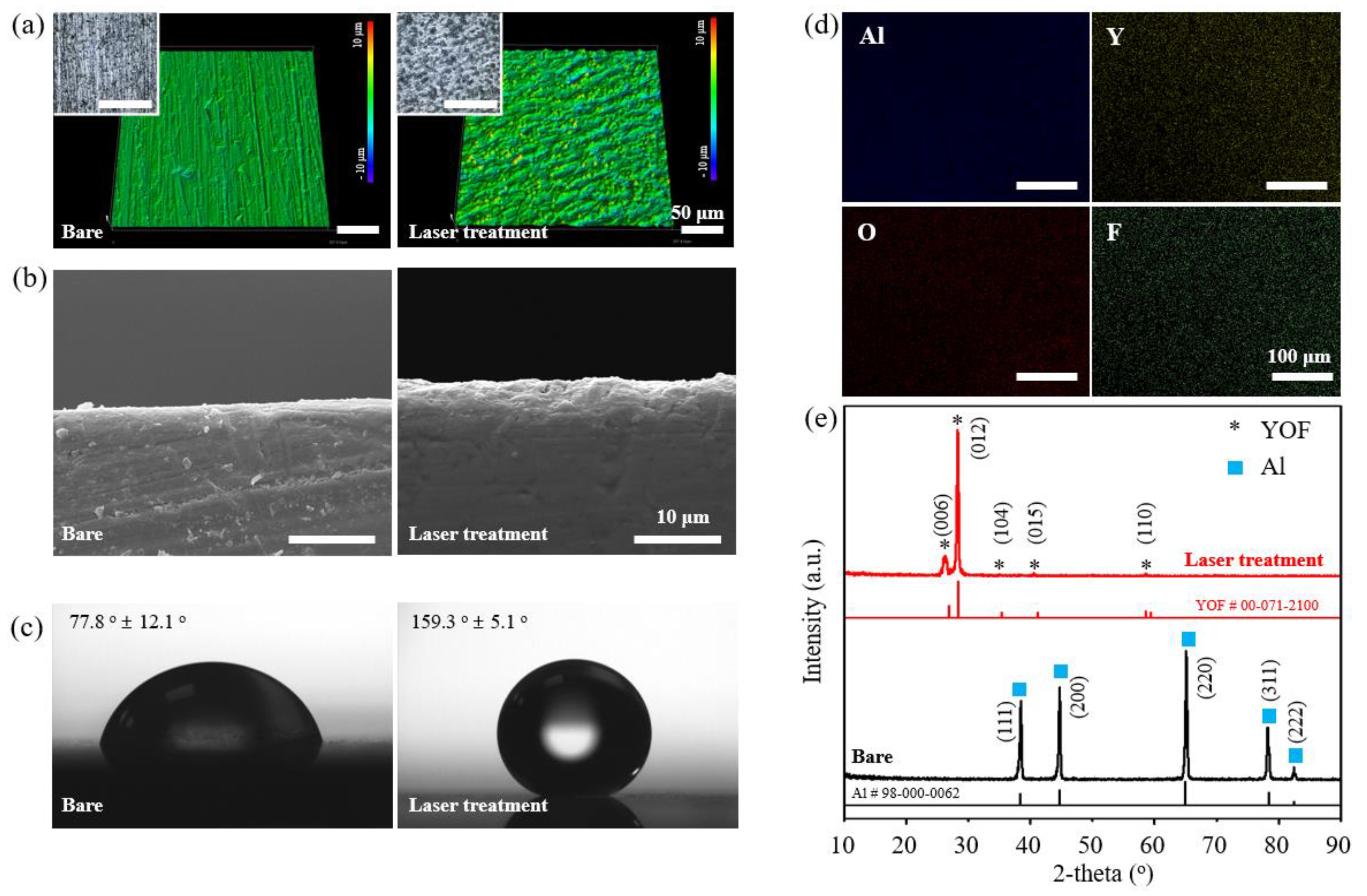
3. Results
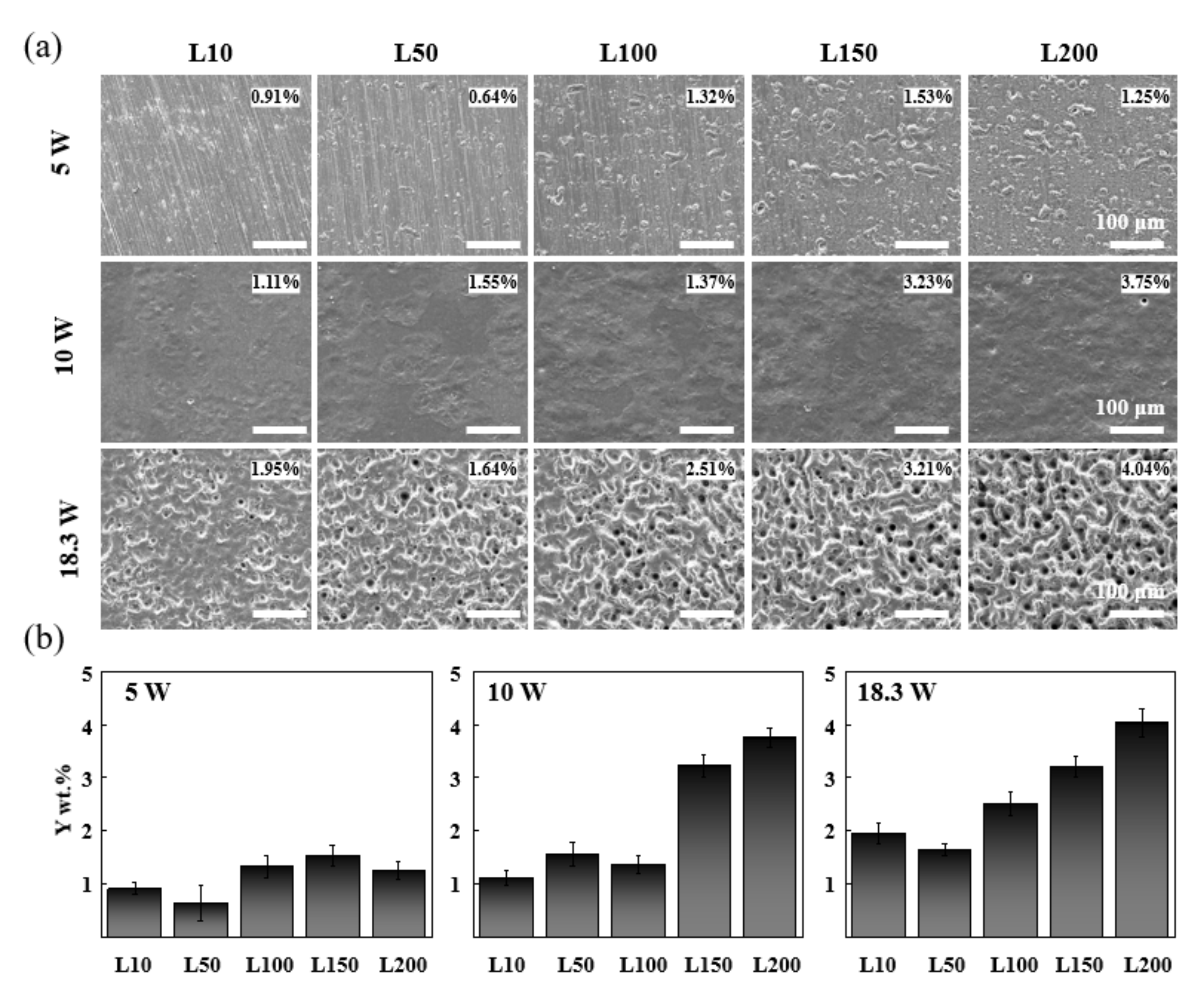
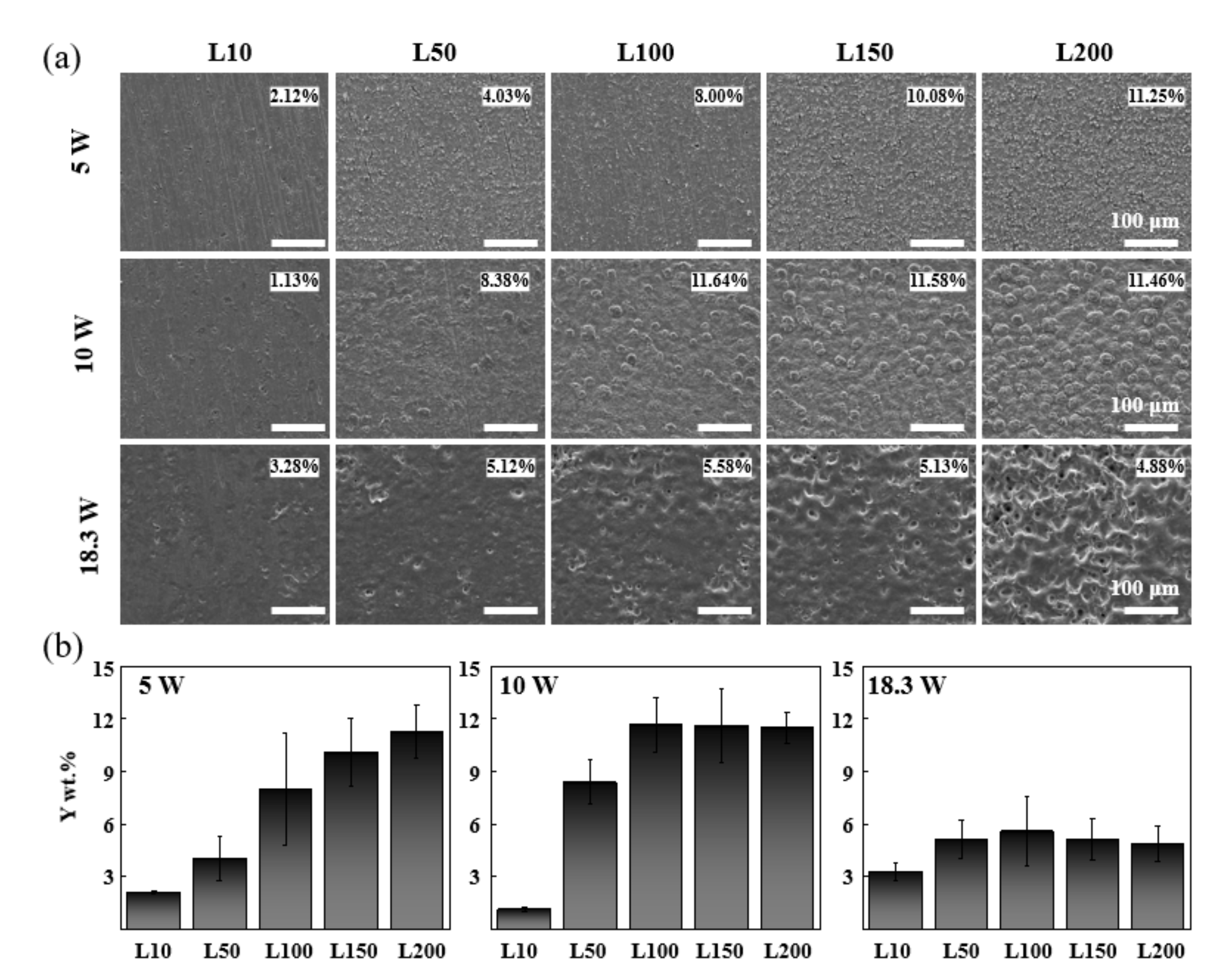
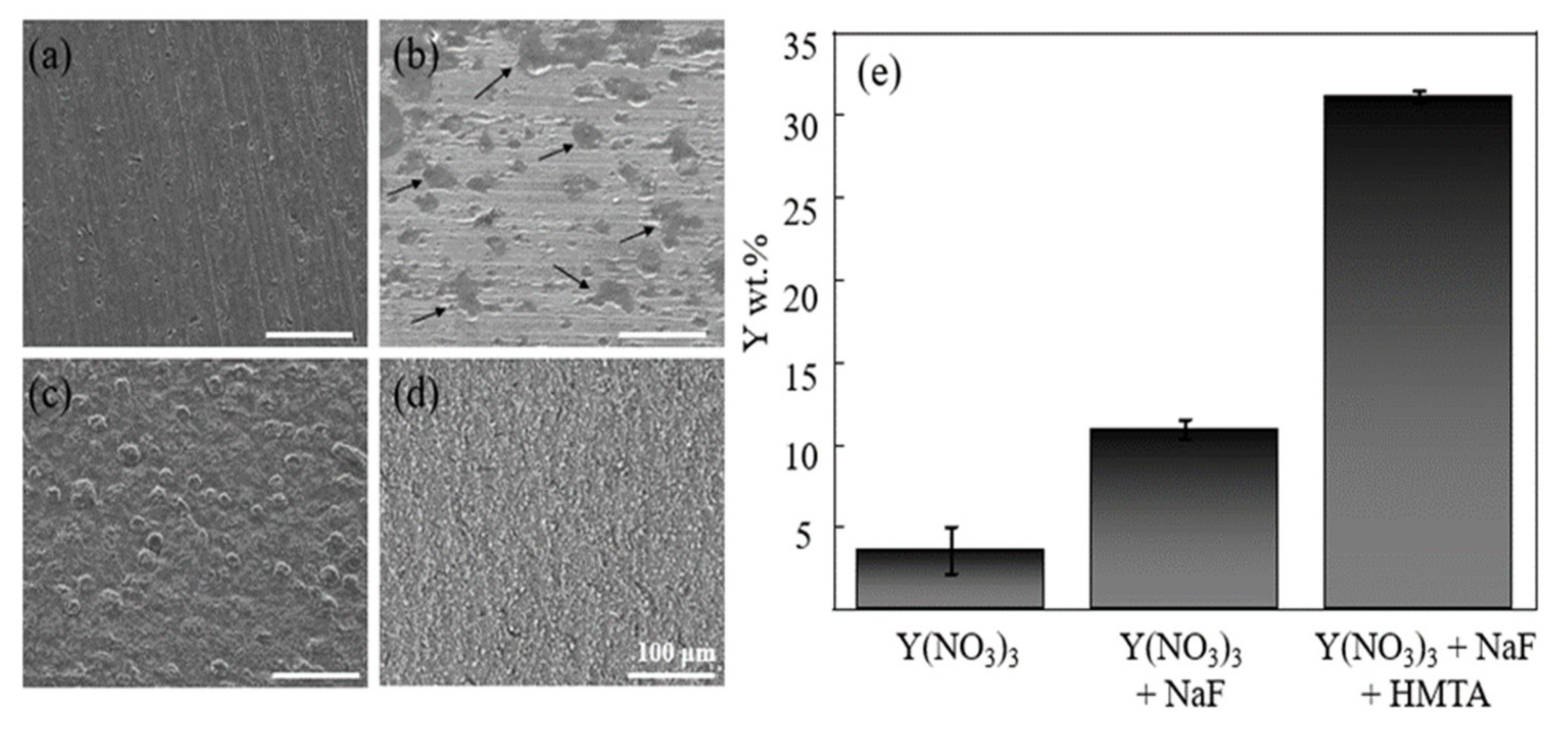
3.1. Optimization for Stable YOF Formation
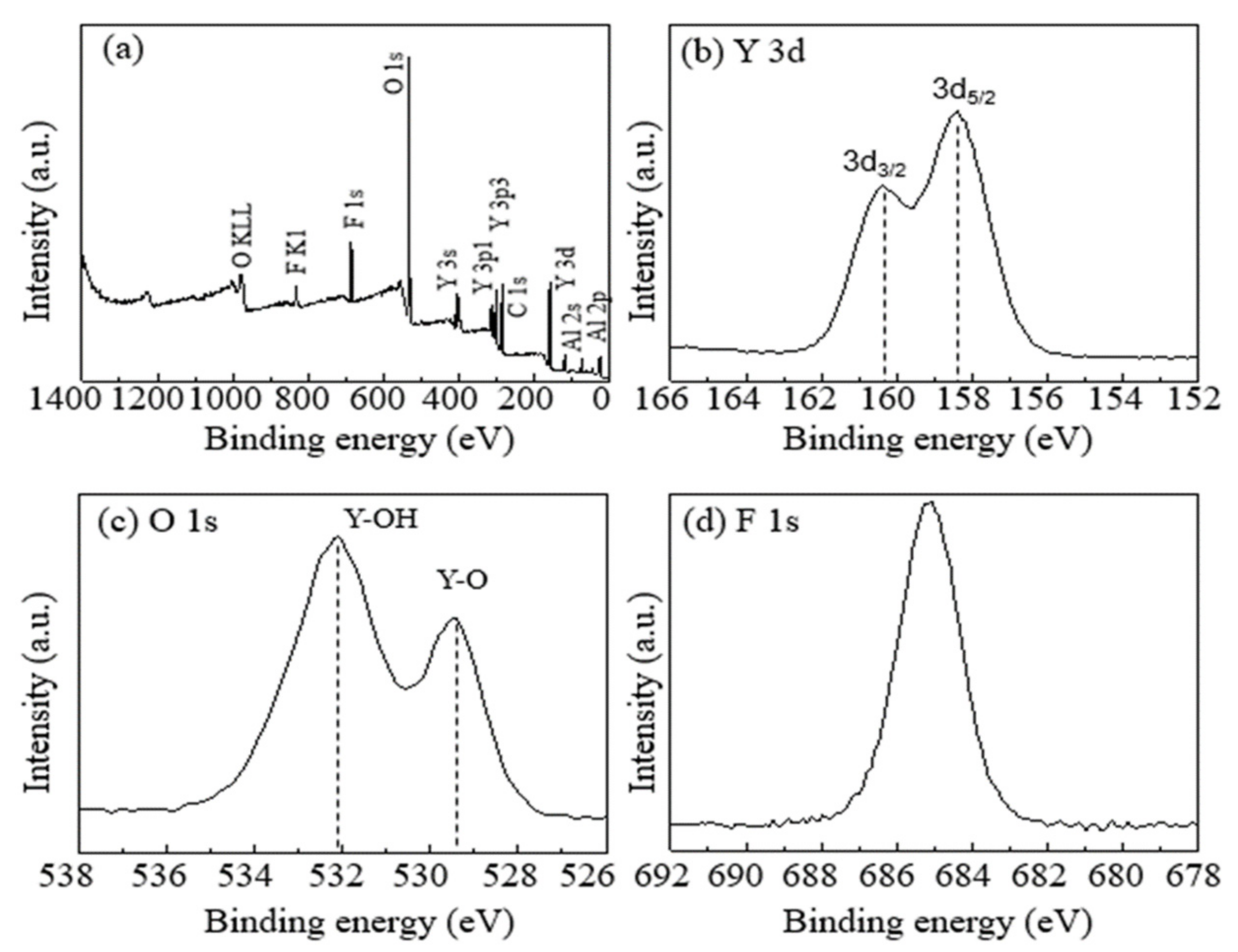
3.2. Component Analysis of Coated Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bu, L.; Gao, S.; Wang, W.; Zhou, L.; Feng, S.; Chen, X.; Yu, D.; Li, S.; Lu, G. Film-Depth-Dependent Light Absorption and Charge Transport for Polymer Electronics: A Case Study on Semiconductor/Insulator Blends by Plasma Etching. Adv. Electron. Mater. 2016, 2, 1600359. [Google Scholar] [CrossRef]
- Economou, D.J. Pulsed plasma etching for semiconductor manufacturing. J. Phys. D Appl. Phys. 2014, 47, 303001. [Google Scholar] [CrossRef] [Green Version]
- Cardinaud, C.; Peignon, M.-C.; Tessier, P.-Y. Plasma etching: Principles, mechanisms, application to micro-and nano-technologies. Appl. Surf. Sci. 2000, 164, 72–83. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Mane, A.U.; Elam, J.W.; Darling, S.B. Enhanced lithographic imaging layer meets semiconductor manufacturing specification a decade early. Adv. Mater. 2012, 24, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, C.; Chen, Y.; Wang, Y. Phase composition, structural, and plasma erosion properties of ceramic coating prepared by suspension plasma spraying. Int. J. Appl. Ceram. Technol. 2018, 15, 1388–1396. [Google Scholar] [CrossRef]
- Miwa, K.; Takada, N.; Sasaki, K. Fluorination mechanisms of Al2O3 and Y2O3 surfaces irradiated by high-density CF4/O2 and SF6/O2 plasmas. J. Vac. Sci. Technol. A Vac. Surf. Film. 2009, 27, 831–835. [Google Scholar] [CrossRef]
- Cao, Y.-C.; Zhao, L.; Luo, J.; Wang, K.; Zhang, B.-P.; Yokota, H.; Ito, Y.; Li, J.-F. Plasma etching behavior of Y2O3 ceramics: Comparative study with Al2O3. Appl. Surf. Sci. 2016, 366, 304–309. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Han, H.N.; Kim, W.; Hwang, N.-M. Yttrium oxyfluoride coatings deposited by suspension plasma spraying using coaxial feeding. Coatings 2020, 10, 481. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, W.; Hwang, N.-M. Plasma Etching Behavior of YOF Coating Deposited by Suspension Plasma Spraying in Inductively Coupled CHF3/Ar Plasma. Coatings 2020, 10, 1023. [Google Scholar] [CrossRef]
- Lin, T.-K.; Wang, W.-K.; Huang, S.-Y.; Tasi, C.-T.; Wuu, D.-S. Comparison of erosion behavior and particle contamination in mass-production CF4/O2 plasma chambers using Y2O3 and YF3 protective coatings. Nanomaterials 2017, 7, 183. [Google Scholar] [CrossRef] [Green Version]
- Tahara, R.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication of dense yttrium oxyfluoride ceramics by hot pressing and their mechanical, thermal, and electrical properties. Jpn. J. Appl. Phys. 2018, 57, 06JF04. [Google Scholar] [CrossRef]
- Ashizawa, H.; Yoshida, K. Plasma-resistance evaluation of yttrium oxyfluoride coating prepared by aerosol deposition method. Int. J. Appl. Ceram. Technol. 2022, 19, 375–382. [Google Scholar] [CrossRef]
- Ma, T.; List, T.; Donnelly, V.M. Comparisons of NF3 plasma-cleaned Y2O3, YOF, and YF3 chamber coatings during silicon etching in Cl2 plasmas. J. Vac. Sci. Technol. A Vac. Surf. Film. 2018, 36, 031305. [Google Scholar] [CrossRef]
- Miyashita, K.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fluorine and oxygen plasma exposure behavior of yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2019, 58, SEEC01. [Google Scholar] [CrossRef]
- Lin, T.-K.; Wuu, D.-S.; Huang, S.-Y.; Wang, W.-K. Preparation and characterization of sprayed-yttrium oxyfluoride corrosion protective coating for plasma process chambers. Coatings 2018, 8, 373. [Google Scholar] [CrossRef] [Green Version]
- Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication, characterization, and fluorine-plasma exposure behavior of dense yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2017, 56, 06HC02. [Google Scholar] [CrossRef] [Green Version]
- Shiba, Y.; Teramoto, A.; Goto, T.; Kishi, Y.; Shirai, Y.; Sugawa, S. Stable yttrium oxyfluoride used in plasma process chamber. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 021405. [Google Scholar] [CrossRef]
- Rajakumar, G.; Mao, L.; Bao, T.; Wen, W.; Wang, S.; Gomathi, T.; Gnanasundaram, N.; Rebezov, M.; Shariati, M.A.; Chung, I.-M. Yttrium oxide nanoparticle synthesis: An overview of methods of preparation and biomedical applications. Appl. Sci. 2021, 11, 2172. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Geng, D.; Shang, M.; Lian, H.; Cheng, Z.; Lin, J. YOF nano/micro-crystals: Morphology controlled hydrothermal synthesis and luminescence properties. CrystEngComm 2014, 16, 2196–2204. [Google Scholar] [CrossRef]
- Andelman, T.; Gordonov, S.; Busto, G.; Moghe, P.V.; Riman, R.E. Synthesis and cytotoxicity of Y2O3 nanoparticles of various morphologies. Nanoscale Res. Lett. 2010, 5, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.P.; Xu, A.W.; You, L.P.; Song, R.Q.; Yu, J.C.; Zhang, H.X.; Li, Q.; Liu, H.Q. Hydrothermal synthesis of rare earth (Tb, Y) hydroxide and oxide nanotubes. Adv. Funct. Mater. 2003, 13, 955–960. [Google Scholar] [CrossRef]
- Ratsimba, A.; Zerrouki, A.; Tessier-Doyen, N.; Nait-Ali, B.; André, D.; Duport, P.; Neveu, A.; Tripathi, N.; Francqui, F.; Delaizir, G. Densification behaviour and three-dimensional printing of Y2O3 ceramic powder by selective laser sintering. Ceram. Int. 2021, 47, 7465–7474. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Yang, H.; Zhang, J.; Wang, L.; Zhang, Q. High optical quality Y2O3 transparent ceramics with fine grain size fabricated by low temperature air pre-sintering and post-HIP treatment. Ceram. Int. 2016, 42, 4238–4245. [Google Scholar] [CrossRef]
- Um, S.H.; Chung, Y.W.; Seo, Y.; Seo, H.; Ok, M.R.; Kim, Y.C.; Han, H.S.; Chung, J.J.; Edwards, J.R.; Jeon, H. Robust Hydroxyapatite Coating by Laser-Induced Hydrothermal Synthesis. Adv. Funct. Mater. 2020, 30, 2005233. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, S.F.; Lo, W.K.; Chen, Y.; Shen, Y.; Kafenda, K.; Su, J.; Xia, K.; Yang, S. A universal method for depositing patterned materials in situ. Nat. Commun. 2020, 11, 5334. [Google Scholar] [CrossRef]
- Lee, B.H.; Oyane, A.; Tsurushima, H.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. A new approach for hydroxyapatite coating on polymeric materials using laser-induced precursor formation and subsequent aging. ACS Appl. Mater. Interfaces 2009, 1, 1520–1524. [Google Scholar] [CrossRef]
- McGee, S.; Lei, Y.; Goff, J.; Wilkinson, C.J.; Nova, N.N.; Kindle, C.M.; Zhang, F.; Fujisawa, K.; Dimitrov, E.; Sinnott, S.B. Single-Step Direct Laser Writing of Multimetal Oxygen Evolution Catalysts from Liquid Precursors. ACS Nano 2021, 15, 9796–9807. [Google Scholar] [CrossRef]
- Armon, N.; Greenberg, E.; Edri, E.; Nagler-Avramovitz, O.; Elias, Y.; Shpaisman, H. Laser-Based Printing: From Liquids to Microstructures. Adv. Funct. Mater. 2021, 31, 2008547. [Google Scholar] [CrossRef]
- Rui, M.; Li, X.; Gan, L.; Zhai, T.; Zeng, H. Ternary Oxide Nanocrystals: Universal Laser-Hydrothermal Synthesis, Optoelectronic and Electrochemical Applications. Adv. Funct. Mater. 2016, 26, 5051–5060. [Google Scholar] [CrossRef]
- Armon, N.; Greenberg, E.; Layani, M.; Rosen, Y.S.; Magdassi, S.; Shpaisman, H. Continuous Nanoparticle Assembly by a Modulated Photo-Induced Microbubble for Fabrication of Micrometric Conductive Patterns. ACS Appl. Mater. Interfaces 2017, 9, 44214–44221. [Google Scholar] [CrossRef]
- Zarzar, L.D.; Swartzentruber, B.S.; Donovan, B.F.; Hopkins, P.E.; Kaehr, B. Using Laser-Induced Thermal Voxels to Pattern Diverse Materials at the Solid-Liquid Interface. ACS Appl. Mater. Interfaces 2016, 8, 21134–21139. [Google Scholar] [CrossRef] [PubMed]
- Edri, E.; Armon, N.; Greenberg, E.; Moshe-Tsurel, S.; Lubotzky, D.; Salzillo, T.; Perelshtein, I.; Tkachev, M.; Girshevitz, O.; Shpaisman, H. Laser Printing of Multilayered Alternately Conducting and Insulating Microstructures. ACS Appl. Mater. Interfaces 2021, 13, 36416–36425. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, L.; Zi, W.; Zhang, J.; Liu, L.; Zou, L.; Gan, S. Morphology control and multicolor-tunable luminescence of YOF: Ln3+ (Ln = Eu, Tb, Dy, Tm) nano-/microcrystals. New J. Chem. 2015, 39, 115–121. [Google Scholar] [CrossRef]
- Chen, L.-J.; Chuang, Y.-J. Hydrothermal synthesis and characterization of hexagonal zinc oxide nanorods with a hexamethylenetetramine (HMTA) template-assisted at a low temperature. Mater. Lett. 2012, 68, 460–462. [Google Scholar] [CrossRef]
- Azlinda, A.A.; Khusaimi, Z.; Abdullah, S.; Rusop, M. Characterization of urea versus HMTA in the preparation of zinc oxide nanostructures by solution-immersion method grown on gold-seeded silicon substrate. Adv. Mater. Res. 2012, 364, 45–49. [Google Scholar] [CrossRef]
- Suder, W.; Williams, S. Investigation of the effects of basic laser material interaction parameters in laser welding. J. Laser Appl. 2012, 24, 032009. [Google Scholar] [CrossRef]
- Zechel, D.L.; Reid, S.P.; Nashiru, O.; Mayer, C.; Stoll, D.; Jakeman, D.L.; Warren, R.A.J.; Withers, S.G. Enzymatic synthesis of carbon−fluorine bonds. J. Am. Chem. Soc. 2001, 123, 4350–4351. [Google Scholar] [CrossRef]
- McPake, C.B.; Sandford, G. Selective continuous flow processes using fluorine gas. Org. Process Res. Dev. 2012, 16, 844–851. [Google Scholar] [CrossRef]
- Greene, L.E.; Yuhas, B.D.; Law, M.; Zitoun, D.; Yang, P. Solution-grown zinc oxide nanowires. Inorg. Chem. 2006, 45, 7535–7543. [Google Scholar] [CrossRef]
- Wen, B.; Huang, Y.; Boland, J.J. Controllable growth of ZnO nanostructures by a simple solvothermal process. J. Phys. Chem. C 2008, 112, 106–111. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Wu, W.; Liu, C.R. The mechanism of controlled integration of ZnO nanowires using pulsed-laser-induced chemical deposition. Nanoscale 2019, 11, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-S.; Jung, Y.C.; Seong, S.; Ahn, J.; Kang, J.; Noh, W.; Lansalot-Matras, C. Atomic layer deposition of Y2O3 films using heteroleptic liquid (iPrCp) 2 Y (iPr-amd) precursor. J. Mater. Chem. C 2014, 2, 9240–9247. [Google Scholar] [CrossRef]
- Basavegowda, N.; Mishra, K.; Thombal, R.S.; Kaliraj, K.; Lee, Y.R. Sonochemical green synthesis of yttrium oxide (Y2O3) nanoparticles as a novel heterogeneous catalyst for the construction of biologically interesting 1,3-thiazolidin-4-ones. Catal. Lett. 2017, 147, 2630–2639. [Google Scholar] [CrossRef]
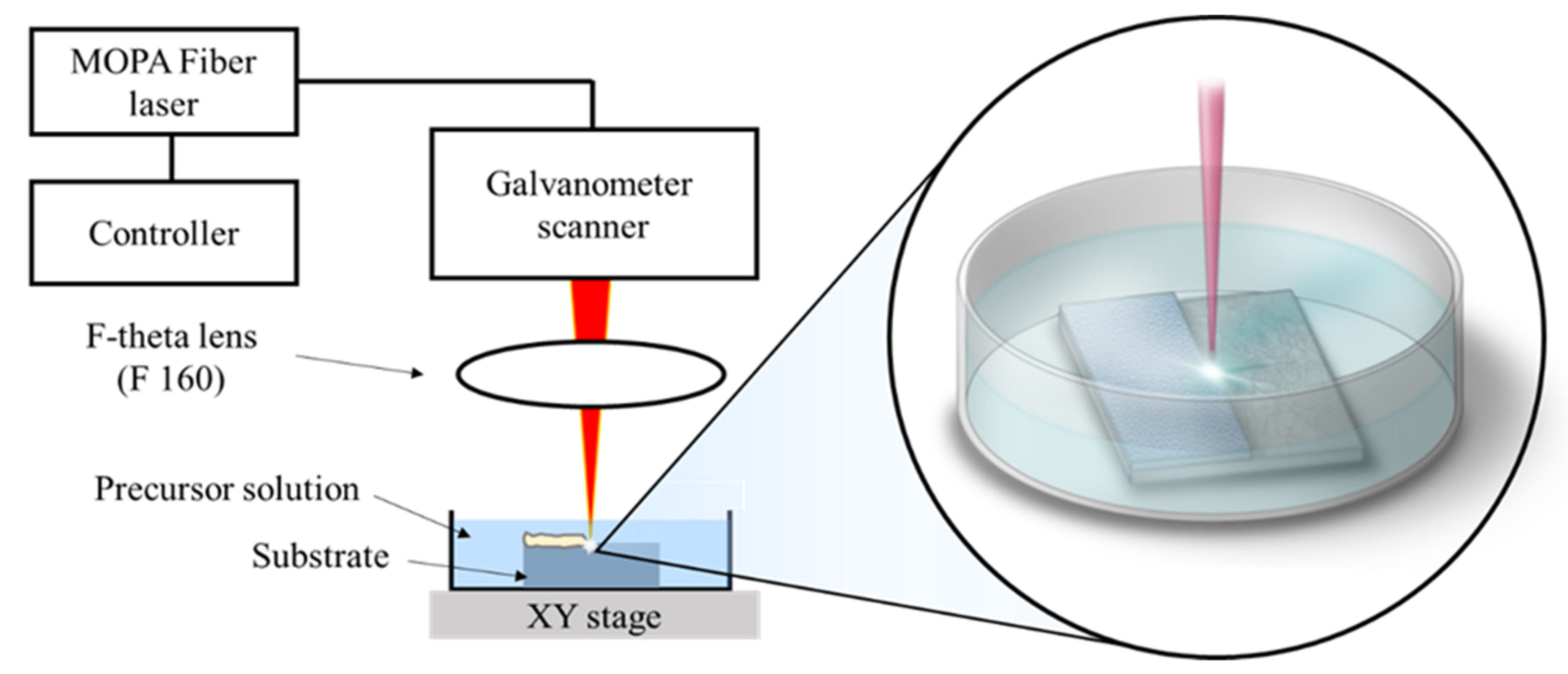

| Laser Parameter | Value, Property |
|---|---|
| Wavelength [nm] | 1064 |
| Laser power [W] | 5, 10, 18.3 |
| Pulse width [ns] | 200 |
| Repetition rate [kHz] | 100 |
| Laser fluence [W/cm2] | 1.77 × 105, 3.53 × 105, 6.47 × 105 |
| Scan speed [mm/s] | 500 |
| Spot diameter [μm] | 60 |
| Focal length [mm] | 160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, K.; Lee, J.; Hwang, H.W.; Jeong, G.; Kim, K.Y.; Kim, Y.-C.; Ok, M.-R.; Han, H.-S.; Sun, J.-Y.; et al. Improvement of Yttrium Oxyfluoride Coating with Modified Precursor Solution for Laser-Induced Hydrothermal Synthesis. Coatings 2022, 12, 740. https://doi.org/10.3390/coatings12060740
Park J, Lee K, Lee J, Hwang HW, Jeong G, Kim KY, Kim Y-C, Ok M-R, Han H-S, Sun J-Y, et al. Improvement of Yttrium Oxyfluoride Coating with Modified Precursor Solution for Laser-Induced Hydrothermal Synthesis. Coatings. 2022; 12(6):740. https://doi.org/10.3390/coatings12060740
Chicago/Turabian StylePark, Jaeho, Kyungwoo Lee, Jaehong Lee, Hae Won Hwang, Goeen Jeong, Kyung Yeun Kim, Yu-Chan Kim, Myoung-Ryul Ok, Hyung-Seop Han, Jeong-Yun Sun, and et al. 2022. "Improvement of Yttrium Oxyfluoride Coating with Modified Precursor Solution for Laser-Induced Hydrothermal Synthesis" Coatings 12, no. 6: 740. https://doi.org/10.3390/coatings12060740
APA StylePark, J., Lee, K., Lee, J., Hwang, H. W., Jeong, G., Kim, K. Y., Kim, Y.-C., Ok, M.-R., Han, H.-S., Sun, J.-Y., & Jeon, H. (2022). Improvement of Yttrium Oxyfluoride Coating with Modified Precursor Solution for Laser-Induced Hydrothermal Synthesis. Coatings, 12(6), 740. https://doi.org/10.3390/coatings12060740






