Self-Cleaning Coatings for the Protection of Cementitious Materials: The Effect of Carbon Dot Content on the Enhancement of Catalytic Activity of TiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Techniques
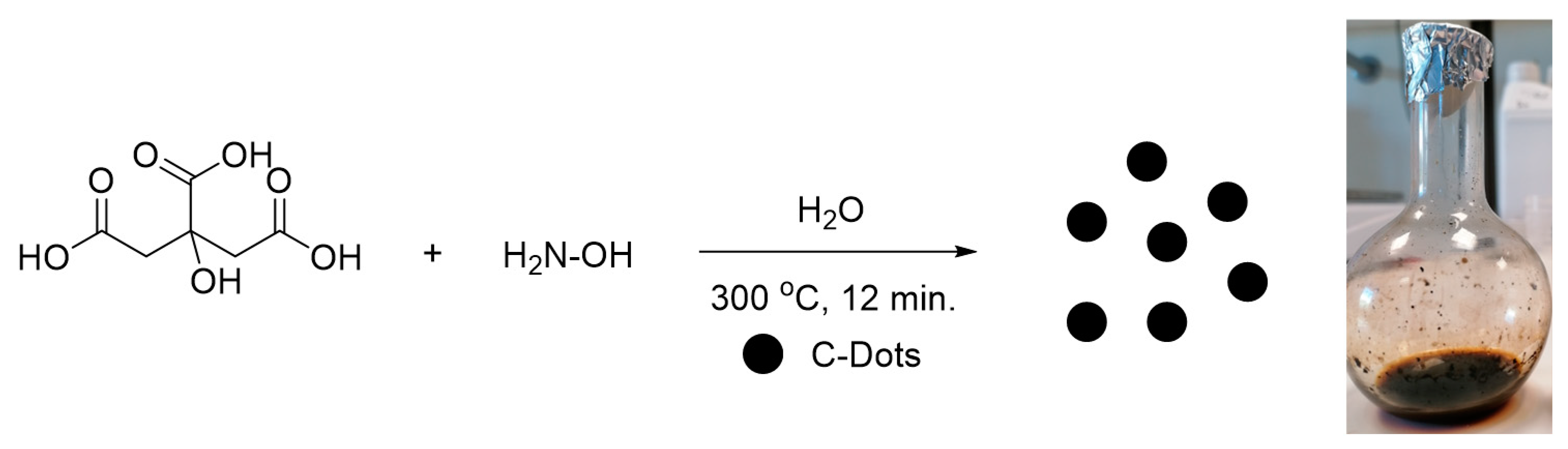
2.3. Synthesis of Carbon Dots
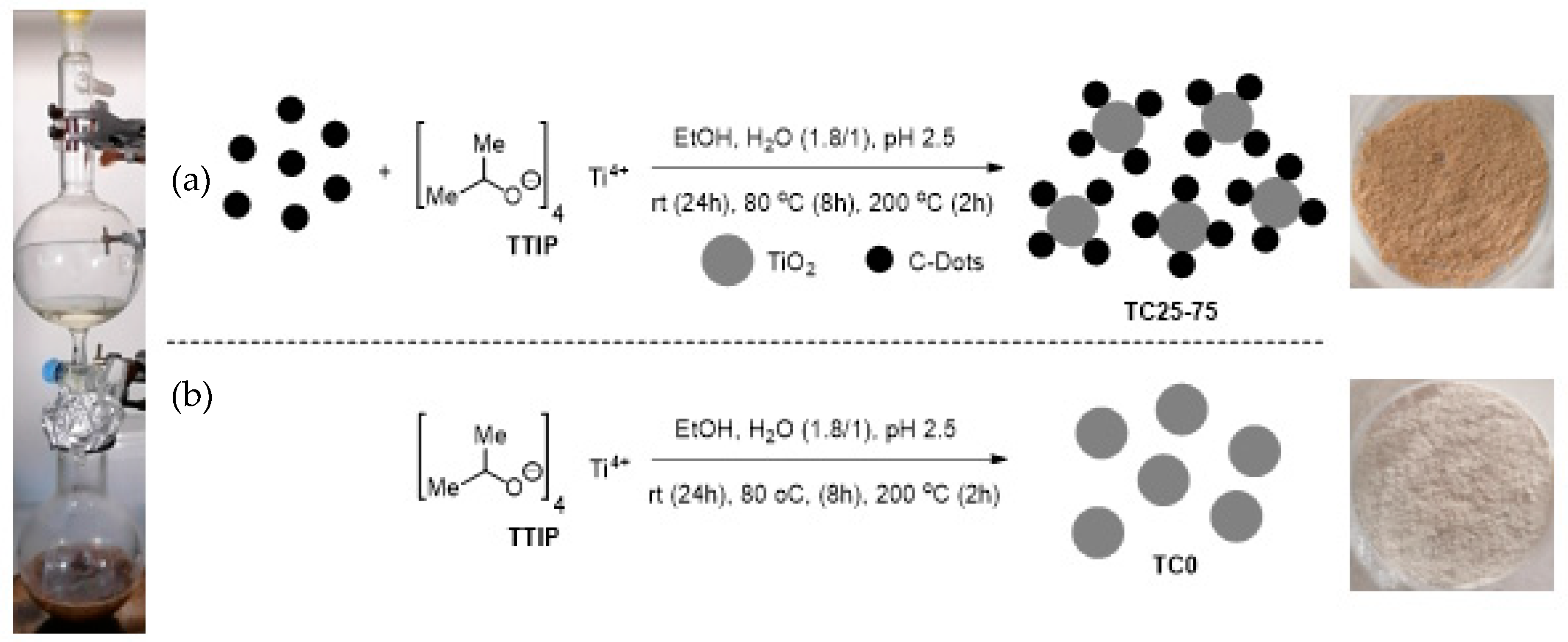
2.4. Synthesis of TiO2 (TC0) and TC25–75 Photocatalysts
2.5. Incorporation of Photocatalysts into the Protective Agent
2.6. Application of the Protective/Photocatalytic Film onto Cementitious Mortars
2.7. Photodegradation of MO Using Photocatalysts and Their Reusability
2.8. Self-Cleaning Performance of Treated Cementitious Mortars
3. Results and Discussion
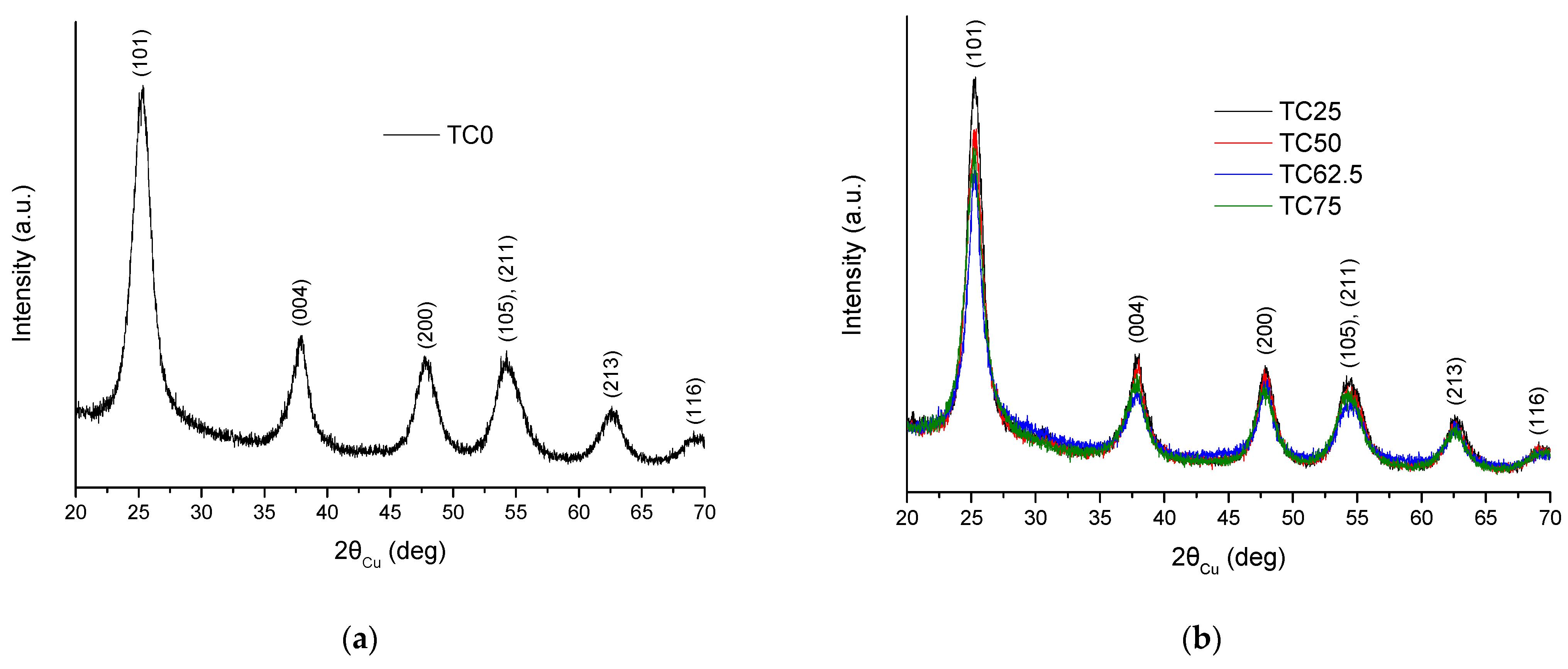
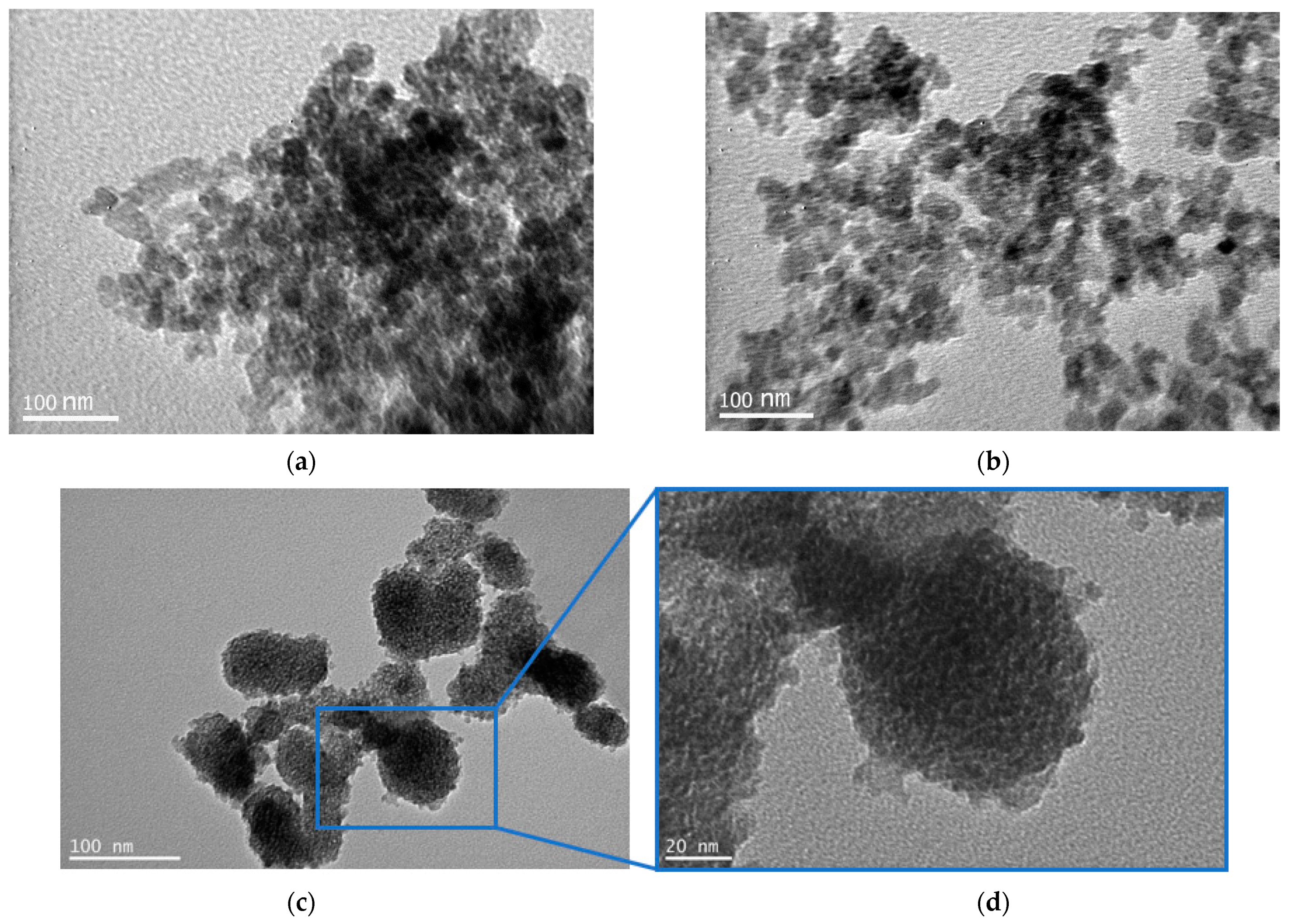
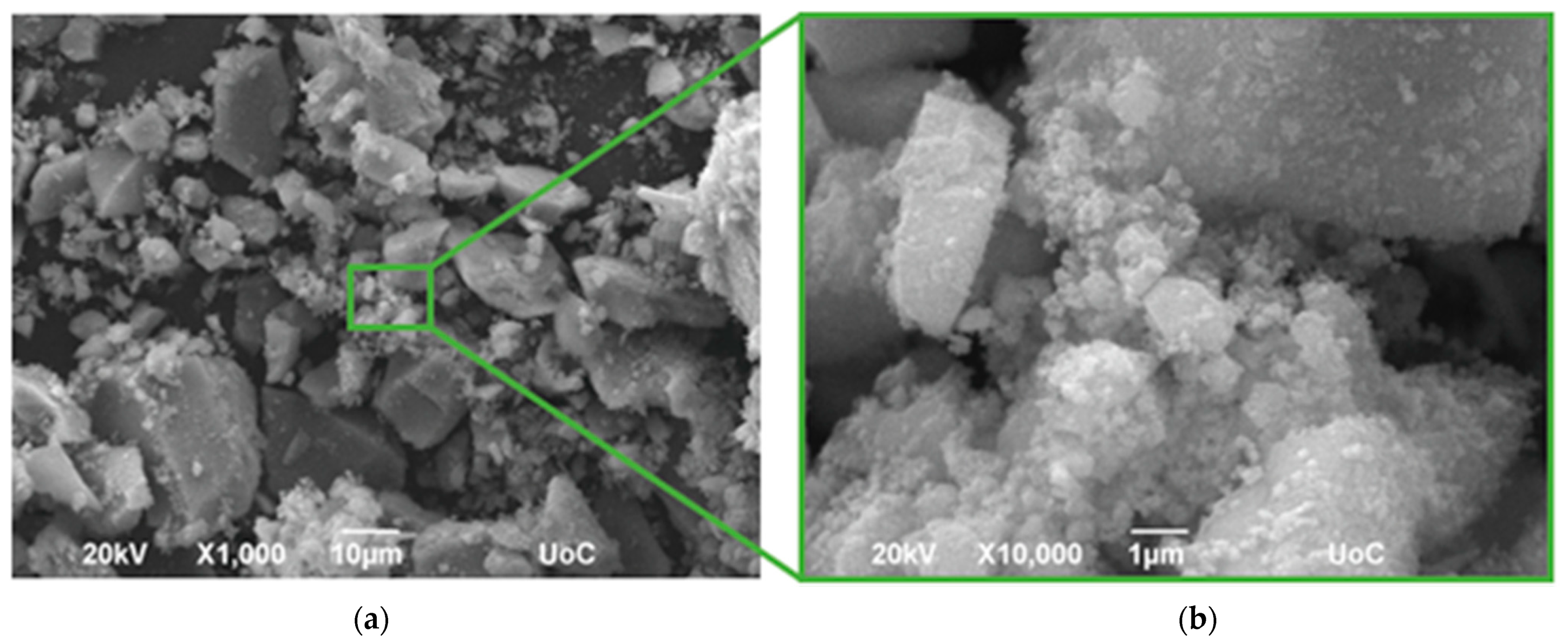
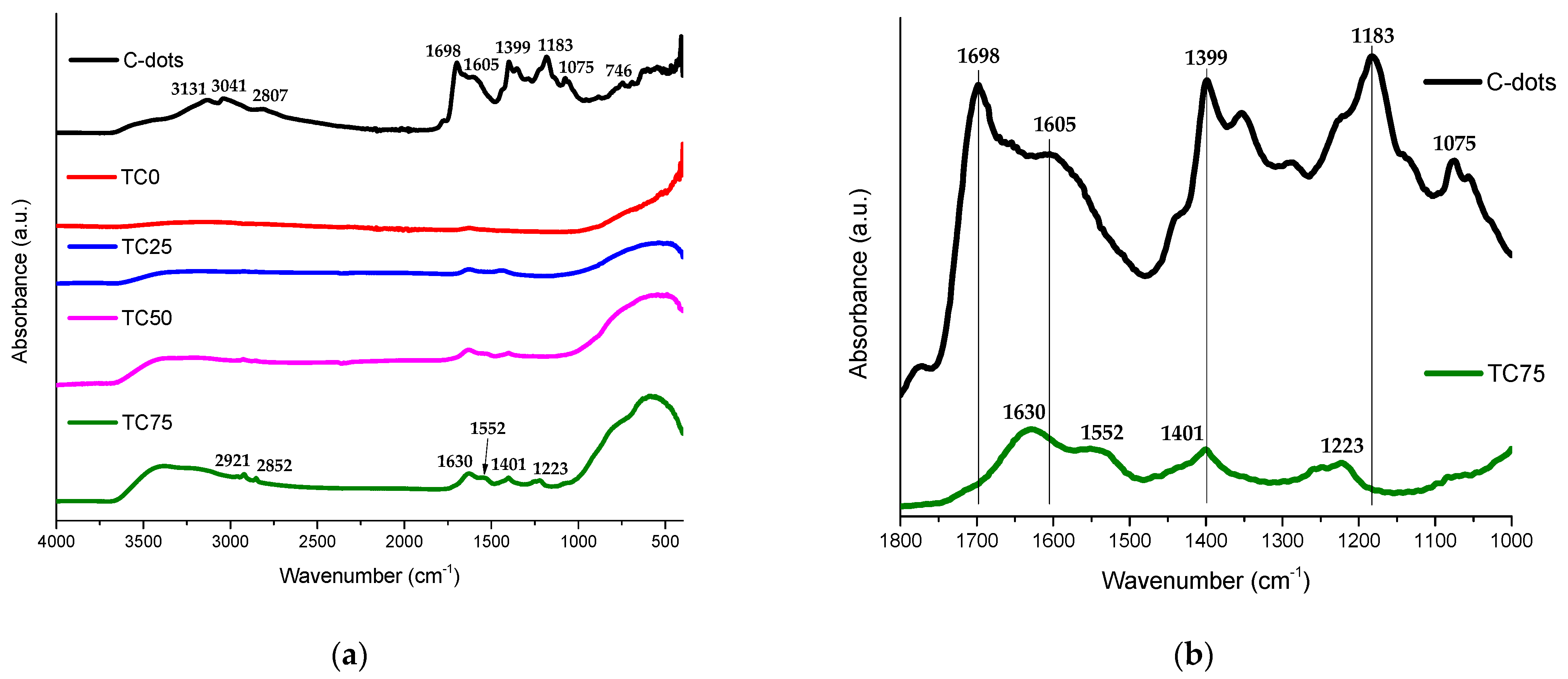
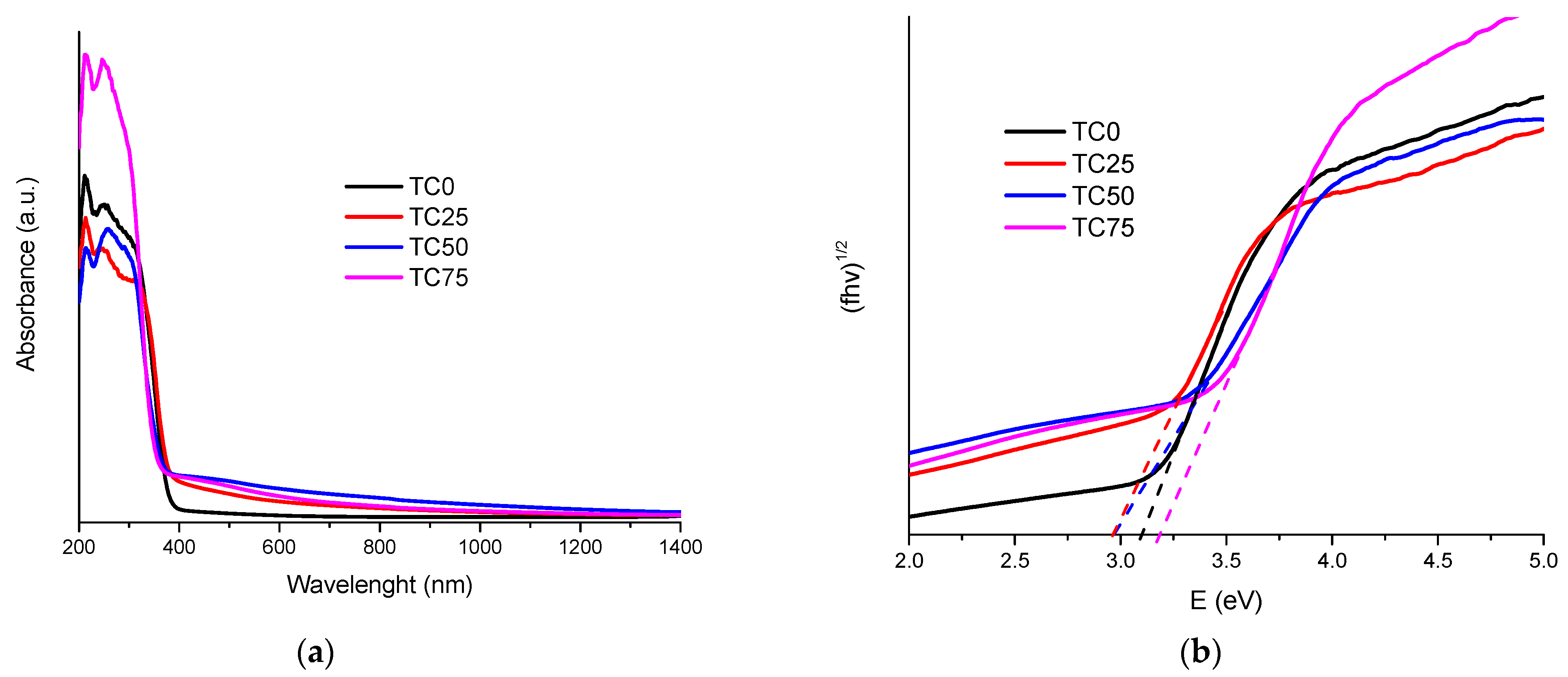
3.1. Structure and Morphology of C-Dots and TC0-75 Photocatalysts
3.2. Photocatalytic Performance of TC25–75 Compared to TCO and TAu
3.3. Photocatalytic Activity of Multifunctional Protective Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [Green Version]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/#FullReport (accessed on 27 February 2022).
- Vandyck, T.; Keramidas, K.; Kitous, A.; Spadaro, J.V.; Van Dingenen, R.; Holland, M.; Saveyn, B. Air quality co-benefits for human health and agriculture counterbalance costs to meet Paris Agreement pledges. Nat. Commun. 2018, 9, 4939. [Google Scholar] [CrossRef] [PubMed]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Sakar, M.; Prakash, R.M.; Do, T.-O. Insights into the TiO2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Msagati, T.A.M.; Mamba, B.B.; Ofomaja, A.E. An efficient and stable narrow bandgap carbon dot-brookite composite over other CD-TiO2 polymorphs in rhodamine B degradation under LED light. Ceram. Int. 2019, 45, 14173–14181. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.-T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Hazarika, D.; Karak, N. Photocatalytic degradation of organic contaminants under solar light using carbon dot/titanium dioxide nanohybrid, obtained through a facile approach. Appl. Surf. Sci. 2016, 376, 276–285. [Google Scholar] [CrossRef]
- Chen, J.; Shu, J.; Anqi, Z.; Juyuan, H.; Yan, Z.; Chen, J. Synthesis of carbon quantum dots/TiO2 nanocomposite for photo-degradation of Rhodamine B and cefradine. Diam. Relat. Mater. 2016, 70, 137–144. [Google Scholar] [CrossRef]
- Miao, R.; Luo, Z.; Zhong, W.; Chen, S.-Y.; Jiang, T.; Dutta, B.; Nasr, Y.; Zhang, Y.; Suib, S.L. Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst. Appl. Catal. B Environ. 2016, 189, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Syafei, D.; Sugiarti, S.; Darmawan, N.; Khotib, M. Synthesis of TiO2/Carbon Nanoparticle (C-dot) Composites as Active Catalysts for Photodegradation of Persistent Organic Pollutant. Indones. J. Chem. 2017, 17, 37–42. [Google Scholar] [CrossRef]
- Wongso, V.; Chung, H.K.; Sambudi, N.S.; Sufian, S.; Abdullah, B.; Wirzal, M.D.H.; Ang, W.L. Silica–carbon quantum dots decorated titanium dioxide as sunlight-driven photocatalyst to diminish acetaminophen from aquatic environment. J. Photochem. Photobiol. A Chem. 2020, 394, 112436. [Google Scholar] [CrossRef]
- Li, F.; Tian, F.; Liu, C.; Wang, Z.; Du, Z.; Li, R.; Zhang, L. One-step synthesis of nanohybrid carbon dots and TiO2 composites with enhanced ultraviolet light active photocatalysis. RSC Adv. 2015, 5, 8389–8396. [Google Scholar] [CrossRef]
- Kumar, M.S.; Yasoda, K.H.; Kumaresan, D.; Kothurkar, N.K.; Batabyal, S.K. TiO2-carbon quantum dots (CQD) nanohybrid: Enhanced photocatalytic activity. Mater. Res. Express 2018, 5, 075502. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, M.; Chen, G.; Zou, W.; Zhao, Y.; Zhang, H.; Zhao, Q. Visible−Ultraviolet Upconversion Carbon Quantum Dots for Enhancement of the Photocatalytic Activity of Titanium Dioxide. ACS Omega 2021, 6, 4247–4254. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Dimitrov, S.; Daboczi, M.; Kim, J.-S.; Guo, Q.; Fang, Y.; Stoeckel, M.-A.; Samorì, P.; Fenwick, O.; Sobrido, A.B.J.; et al. Nitrogen-Doped Carbon Dots/TiO2 Nanoparticle Composites for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2020, 3, 3371–3381. [Google Scholar] [CrossRef]
- Wang, A.; Xiao, X.; Zhou, C.; Lyu, F.; Fu, L.; Wang, C.; Ruan, S. Large-scale synthesis of carbon dots/TiO2 nanocomposites for the photocatalytic color switching system. Nanoscale Adv. 2019, 1, 1819–1825. [Google Scholar] [CrossRef] [Green Version]
- Stefanakis, D.; Krasoudaki, T.; Kaditis, A.-I.; Bakolas, A.; Maravelaki, P.-N. Design of Novel Photocatalytic Films for the Protection of Architectural Surfaces via the Incorporation of Green Photocatalysts. Coatings 2021, 11, 934. [Google Scholar] [CrossRef]
- Barberena-Fernández, A.M.; Carmona-Quiroga, P.M.; Blanco-Varela, M.T. Interaction of TEOS with cementitious materials: Chemical and physical effects. Cem. Concr. Compos. 2015, 55, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Al-Qaradawi, S.; Salman, S.R. Photocatalytic degradation of methyl orange as a model compound. J. Photochem. Photobiol. A Chem. 2002, 148, 161–168. [Google Scholar] [CrossRef]
- Kapetanaki, K.; Vazgiouraki, E.; Stefanakis, D.; Fotiou, A.; Anyfantis, G.C.; García-Lodeiro, I.; Blanco-Varela, M.T.; Arabatzis, I.; Maravelaki, P.N. TEOS Modified With Nano-Calcium Oxalate and PDMS to Protect Concrete Based Cultural Heritage Buildings. Front. Mater. 2020, 7, 16. [Google Scholar] [CrossRef]
- Maravelaki, P.N.; Kapetanaki, K.; Stefanakis, D. TEOS-PDMS-Calcium Oxalate Hydrophobic Nanocomposite for Protection and Stone Consolidation. Heritage 2021, 4, 4068–4075. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials used in conservation and restoration of cultural heritage: An up-to-date overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef] [PubMed]
- Enesca, A.; Andronic, L.; Duta, A. The influence of surfactants on the crystalline structure, electrical and photocatalytic properties of hybrid multi-structured (SnO2, TiO2 and WO3) thin films. Appl. Surf. Sci. 2012, 258, 4339–4346. [Google Scholar] [CrossRef]
- Xu, H.; Hao, Z.; Feng, W.; Wang, T.; Fu, X. The floating photocatalytic spheres loaded with weak light-driven TiO2-based catalysts for photodegrading tetracycline in seawater. Mater. Sci. Semicond. Process 2022, 144, 106610. [Google Scholar] [CrossRef]
- Mugundan, S.; Praveen, P.; Sridhar, S.; Prabu, S.; Mary, K.L.; Ubaidullah, M.; Shaikh, S.F.; Kanagesan, S. Sol-gel synthesized barium doped TiO2 nanoparticles for solar photocatalytic application. Inorg. Chem. Commun. 2022, 139, 109340. [Google Scholar] [CrossRef]
- Rosales, A.; Esquivel, Κ. SiO2@TiO2 Composite Synthesis and Its Hydrophobic Applications: A Review. Catalysts 2020, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Rosales, A.; Maury-Ramírez, A.; Mejía-De Gutiérrez, R.; Guzmán, C.; Esquivel, K. SiO2@TiO2 Coating: Synthesis, Physical Characterization and Photocatalytic Evaluation. Coatings 2018, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Franzoni, E.; Fregni, A.; Gabrielli, R.; Graziani, G.; Sassoni, E. Compatibility of photocatalytic TiO2-based finishing for renders in architectural restoration: A preliminary study. Build. Environ. 2014, 80, 125–135. [Google Scholar] [CrossRef]
- Rosales, A.; Ortiz-Frade, L.; Medina-Ramirez, I.E.; Godínez, L.A.; Esquivel, K. Self-cleaning of SiO2-TiO2 coating: Effect of sonochemical synthetic parameters on the morphological, mechanical, and photocatalytic properties of the films. Ultrason. Sonochem. 2021, 73, 105483. [Google Scholar] [CrossRef] [PubMed]
- Maravelaki-Kalaitzaki, P.; Agioutantis, Z.; Lionakis, E.; Stavroulaki, M.; Perdikatsis, V. Physico-chemical and mechanical characterization of hydraulic mortars containing nano-titania for restoration applications. Cem. Concr. Compos. 2013, 36, 33–41. [Google Scholar] [CrossRef]
- Balliana, E.; Ricci, G.; Pesce, C.; Zendri, E. Assessing the value of green conservation for cultural heritage: Positive and critical aspects of already available methodologies. Int. J. Conserv. Sci. 2016, 7, 185–202. Available online: https://ijcs.ro/public/IJCS-16-SI01_Balliana.pdf (accessed on 14 October 2021).
- Petronella, F.; Pagliarulo, A.; Truppi, A.; Lettieri, M.; Masieri, M.; Calia, A.; Curri, M.L.; Comparelli, R. TiO2 Nanocrystal Based Coatings for the Protection of Architectural Stone: The Effect of Solvents in the Spray-Coating Application for a Self-Cleaning Surfaces. Coatings 2018, 8, 356. [Google Scholar] [CrossRef] [Green Version]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Kapridaki, C.; Verganelaki, A.; Dimitriadou, P.; Maravelaki-Kalaitzaki, P. Conservation of Monuments by a Three-Layered Compatible Treatment of TEOS-Nano-Calcium Oxalate Consolidant and TEOS-PDMS-TiO2 Hydrophobic/Photoactive Hybrid Nanomaterials. Materials 2018, 11, 684. [Google Scholar] [CrossRef] [Green Version]
- Stefanakis, D.; Philippidis, A.; Sygellou, L.; Filippidis, G.; Ghanotakis, D.; Anglos, D. Synthesis of fluorescent carbon dots by a microwave heating process: Structural characterization and cell imaging applications. J. Nanopart. Res. 2014, 16, 12646. [Google Scholar] [CrossRef]
- De Muynck, W.; Ramirez, A.M.; De Belie, N.; Verstraete, W. Evaluation of strategies to prevent algal fouling on white architectural and cellular concrete. Int. Biodeterior. Biodegrad. 2009, 63, 679–689. [Google Scholar] [CrossRef]
- Allena, N.S.; Mahdjoubd, N.; Vishnyakovb, V.; Kellyb, P.J.; Kriekc, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Shah, A.H.; Rather, M.A. Effect of calcination temperature on the crystallite size, particle size and zeta potential of TiO2 nanoparticles synthesized via polyol-mediated method. Mater. Today Proc. 2021, 44, 482–488. [Google Scholar] [CrossRef]
- Esquivel, K.; Nava, R.; Zamudio-Méndez, A.; González, M.V.; Jaime-Acuña, O.E.; Escobar-Alarcón, L.; Peralta-Hernández, J.M.; Pawelec, B.; Fierro, J.L.G. Microwave-assisted synthesis of (S)Fe/TiO2 systems: Effects of synthesis conditions and dopant concentration on photoactivity. Appl. Catal. B Environ. 2013, 140–141, 213–224. [Google Scholar] [CrossRef]
- Górska, P.; Zaleska, A.; Kowalska, E.; Klimczuk, T.; Sobczak, J.W.; Skwarek, E.; Janusz, W.; Hupka, J. TiO2 photoactivity in vis and UV light: The influence of calcination temperature and surface properties. Appl. Catal. B Environ. 2008, 84, 440–447. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Kibasomba, P.M.; Dhlamini, S.; Maaza, M.; Liu, C.-P.; Rashad, M.M.; Rayan, D.A.; Mwakikunga, B.W. Strain and grain size of TiO2 nanoparticles from TEM, Raman spectroscopy and XRD: The revisiting of the Williamson-Hall plot method. Results Phys. 2018, 9, 628–635. [Google Scholar] [CrossRef]
- Munafò, P.; Quagliarini, E.; Goffredo, G.B.; Bondioli, F.; Licciulli, A. Durability of nano-engineered TiO2 self-cleaning treatments on limestone. Constr. Build. Mater. 2014, 65, 218–231. [Google Scholar] [CrossRef]
- Zamudio-Méndez, A.; Sánchez-Palma, E.; Navarro-López, R.; Pérez-Lara, M.A.; Rivera Muñoz, E.M.; Godínez, L.A.; Velázquez-Castillo, R.; Nava, R.; Esquivel, K. Self-Cleaning Activity on Concrete Surfaces Coated with Fe- and S-Doped TiO2 Synthesized by Sol–Gel Microwave Method. J. Nanosci. Nanotechnol. 2017, 17, 5637–5645. [Google Scholar] [CrossRef]
- Shetty, R.; Chavan, V.B.; Kulkarni, P.S.; Kulkarni, B.D.; Kamble, S.P. Photocatalytic Degradation of Pharmaceuticals Pollutants Using N-Doped TiO2 Photocatalyst: Identification of CFX Degradation Intermediates. Indian Chem. Eng. 2017, 59, 177–199. [Google Scholar] [CrossRef]
- Wu, W.-Y.; Chang, Y.-M.; Ting, J.-M. Room-temperature synthesis of single-crystalline anatase TiO2 nanowires. Cryst. Growth Des. 2010, 10, 1646–1651. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Jiang, C.; Wu, S.; Chen, W.; Lv, P.; Wang, Q.; Liu, J.; Narh, C.; Cao, X.; Ghiladi, R.A.; et al. Carbon quantum dots: A bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. J. Photochem. Photobiol. B Biol. 2020, 206, 111864. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties of amorphous semiconductors. In Amorphous and Liquid Semiconductors; Tauc, J., Ed.; Springer: Boston, MA, USA, 1974; pp. 159–220. [Google Scholar] [CrossRef]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Wang, J.; Guo, B.; Zhang, X.; Zhang, Z.; Han, J.; Wu, J. Sonocatalytic degradation of methyl orange in the presence of TiO2 catalysts and catalytic activity comparison of rutile and anatase. Ultrason. Sonochem. 2005, 12, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Priyanshu, V.; Sujoy, K.S. Degradation kinetics of pollutants present in a simulated wastewater matrix using UV/TiO2 photocatalysis and its microbiological toxicity assessment. Res. Chem. Intermed. 2017, 43, 6317–6341. [Google Scholar] [CrossRef]
- NASA’s Power Data Access Viewer. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 18 February 2022).
- Ke, J.; Li, X.; Zhao, Q.; Liu, B.; Liu, S.; Wang, S. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, F. Current and future trends in protective treatments for stone heritage. In Conserving Stone Heritage; Gherardi, F., Maravelaki, P.N., Eds.; Springer: Cham., Switzerland, 2022; pp. 137–176. [Google Scholar] [CrossRef]
- Falchi, L.; Zendri, E.; Müller, U.; Fontana, P. The influence of water-repellent admixtures on the behaviour and the effectiveness of Portland limestone cement mortars. Cem. Concr. Compos. 2015, 59, 107–118. [Google Scholar] [CrossRef]

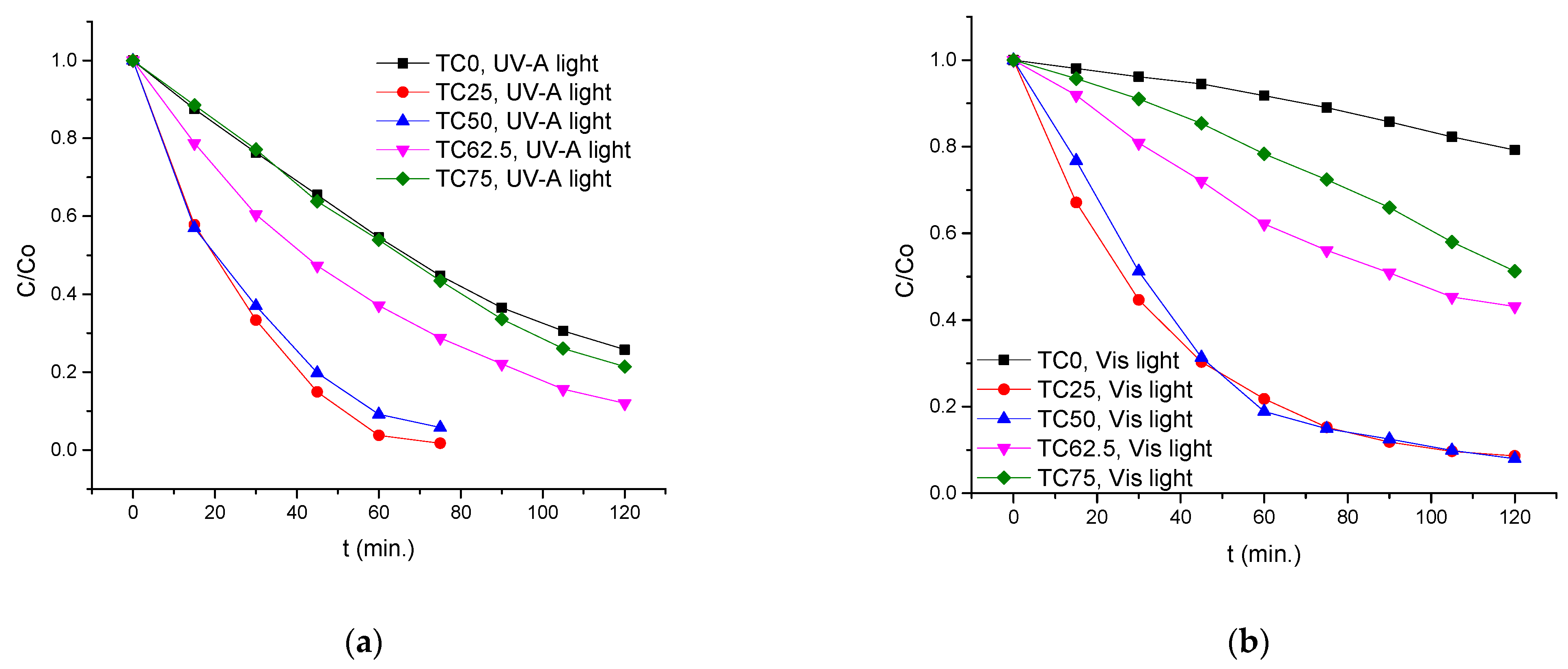
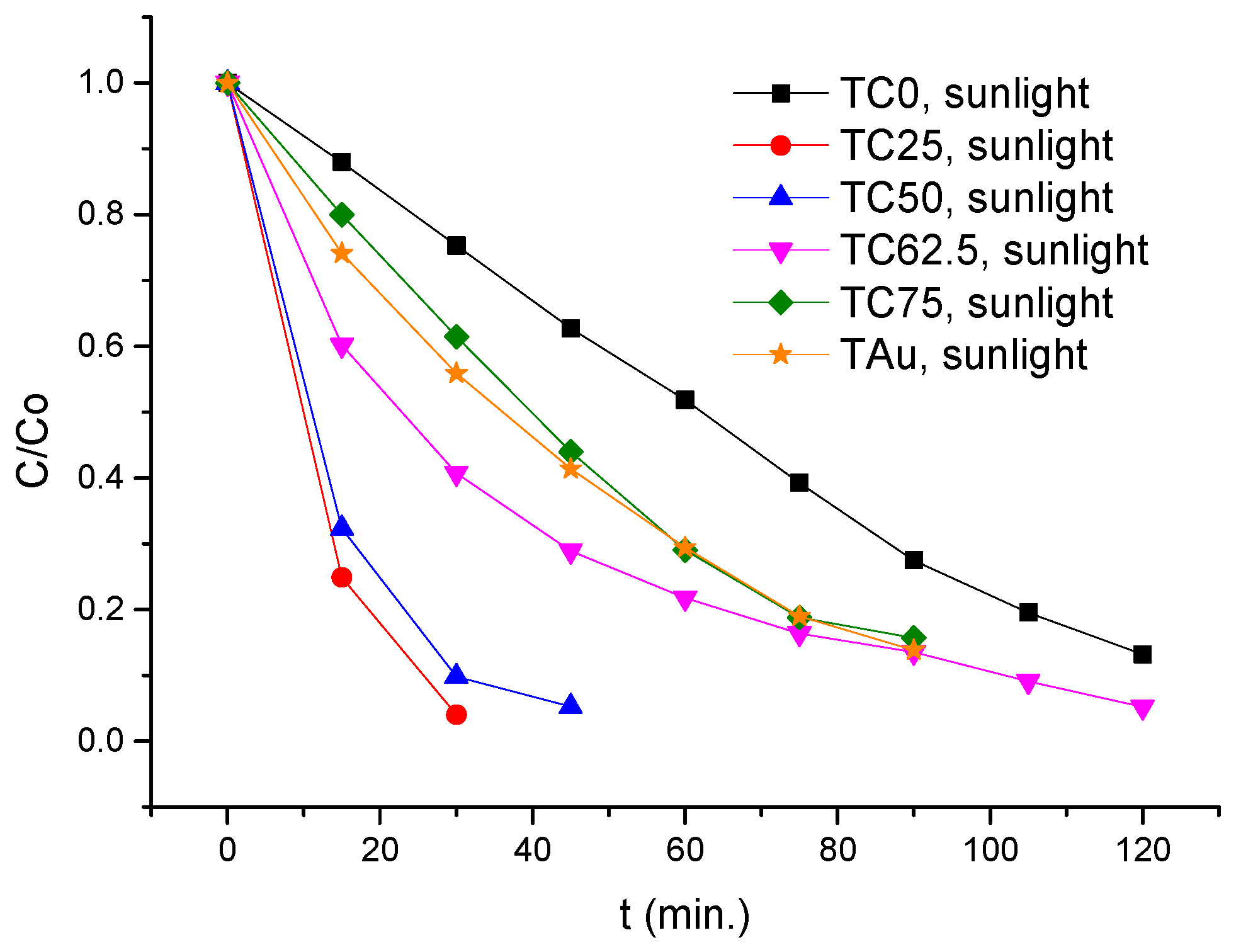
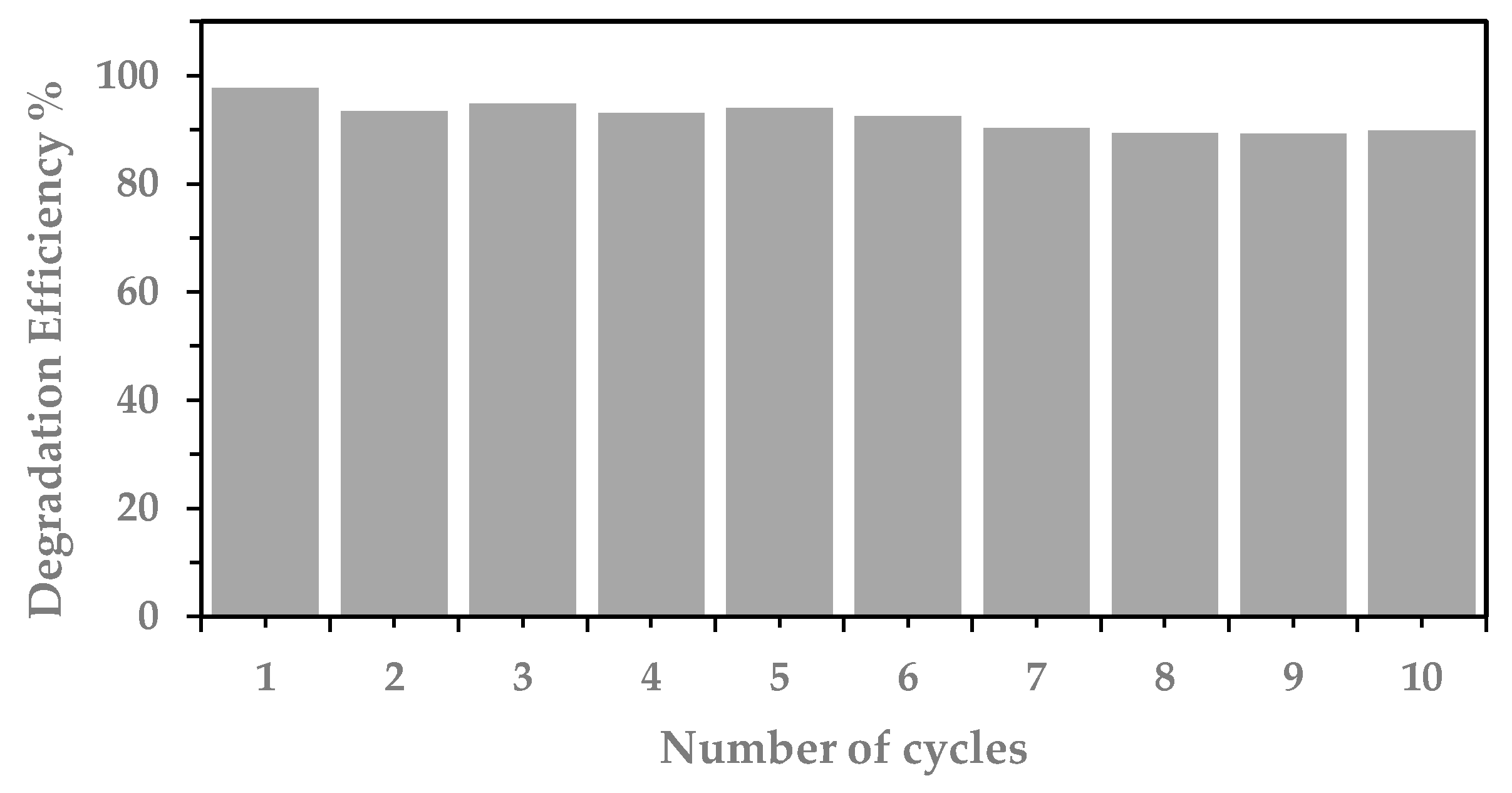
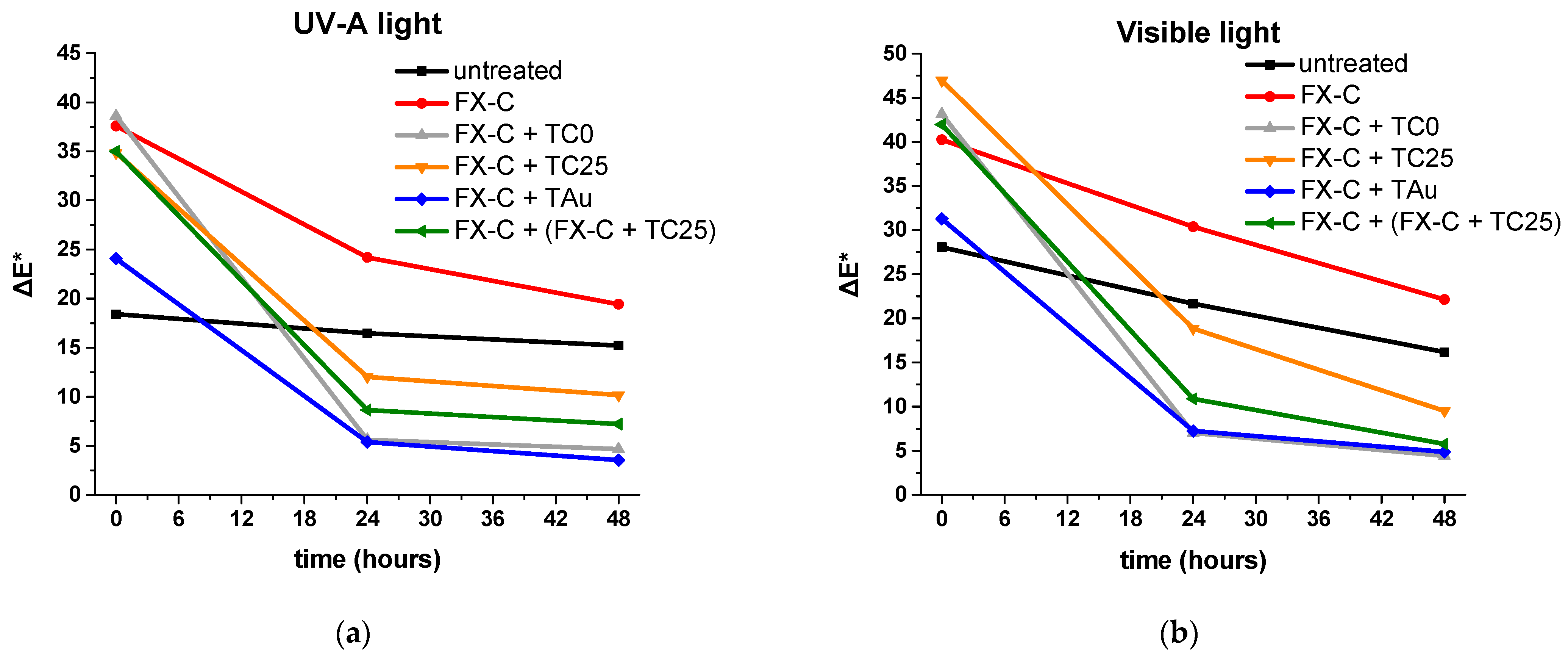
| Type of Irradiation | Photocatalyst | Kapp (min−1) | R2 | Degradation Rate (%)/min |
|---|---|---|---|---|
| UV-A light | TC0 | 1.10 × 10−2 | 0.997 | 45.4/60 |
| TC25 | 5.07 × 10−2 | 0.982 | 96.2/60 | |
| TC50 | 3.79 × 10−2 | 0.997 | 90.9/60 | |
| TC62.5 | 1.72 × 10−2 | 0.999 | 62.9/60 | |
| TC75 | 1.21 × 10−2 | 0.991 | 46.1/60 | |
| Visible light | TC0 | 0.18 × 10−2 | 0.984 | 20.8/120 |
| TC25 | 2.28 × 10−2 | 0.991 | 91.4/120 | |
| TC50 | 2.29 × 10−2 | 0.991 | 92.0/120 | |
| TC62.5 | 0.74 × 10−2 | 0.998 | 56.9/120 | |
| TC75 | 0.49 × 10−2 | 0.980 | 48.7/120 | |
| Solar light | TC0 | 1.59 × 10−2 | 0.971 | 48.1/60 |
| TC25 | 9.13 × 10−2 | 0.983 | 96.0/30 | |
| TC50 | 6.96 × 10−2 | 0.992 | 90.2/30 | |
| TC62.5 | 2.41 × 10−2 | 0.994 | 78.2/60 | |
| TC75 | 2.06 × 10−2 | 0.993 | 71.0/60 | |
| TAu | 2.14 × 10−2 | 0.998 | 70.6/60 |
| Light Irradiation | Time (h) | ΔE* | |||||
|---|---|---|---|---|---|---|---|
| Untreated | FX-C | FX-C + TC0 | FX-C + TC25 | FX-C + TAu | FX-C + (FX-C + TC25) | ||
| UV-A light | 0 | 18.41 | 37.60 | 38.62 | 34.88 | 24.08 | 35.02 |
| 24 | 16.48 | 24.20 | 5.62 | 12.04 | 5.39 | 8.64 | |
| 48 | 15.21 | 19.43 | 4.67 | 10.16 | 3.54 | 7.21 | |
| 0 | 28.07 | 40.24 | 43.14 | 46.97 | 31.28 | 41.95 | |
| Visible light | 24 | 21.67 | 30.39 | 7.00 | 18.85 | 7.24 | 10.88 |
| 48 | 16.17 | 22.14 | 4.40 | 9.50 | 4.87 | 5.77 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryparis, C.; Krasoudaki, T.; Maravelaki, P.-N. Self-Cleaning Coatings for the Protection of Cementitious Materials: The Effect of Carbon Dot Content on the Enhancement of Catalytic Activity of TiO2. Coatings 2022, 12, 587. https://doi.org/10.3390/coatings12050587
Gryparis C, Krasoudaki T, Maravelaki P-N. Self-Cleaning Coatings for the Protection of Cementitious Materials: The Effect of Carbon Dot Content on the Enhancement of Catalytic Activity of TiO2. Coatings. 2022; 12(5):587. https://doi.org/10.3390/coatings12050587
Chicago/Turabian StyleGryparis, Charis, Themis Krasoudaki, and Pagona-Noni Maravelaki. 2022. "Self-Cleaning Coatings for the Protection of Cementitious Materials: The Effect of Carbon Dot Content on the Enhancement of Catalytic Activity of TiO2" Coatings 12, no. 5: 587. https://doi.org/10.3390/coatings12050587
APA StyleGryparis, C., Krasoudaki, T., & Maravelaki, P.-N. (2022). Self-Cleaning Coatings for the Protection of Cementitious Materials: The Effect of Carbon Dot Content on the Enhancement of Catalytic Activity of TiO2. Coatings, 12(5), 587. https://doi.org/10.3390/coatings12050587







