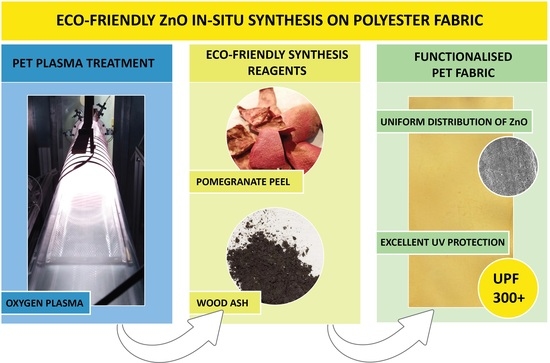
Eco-Friendly In Situ ZnO Synthesis on PET Fabric Using Oxygen Plasma and Plant Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Natural Extracts
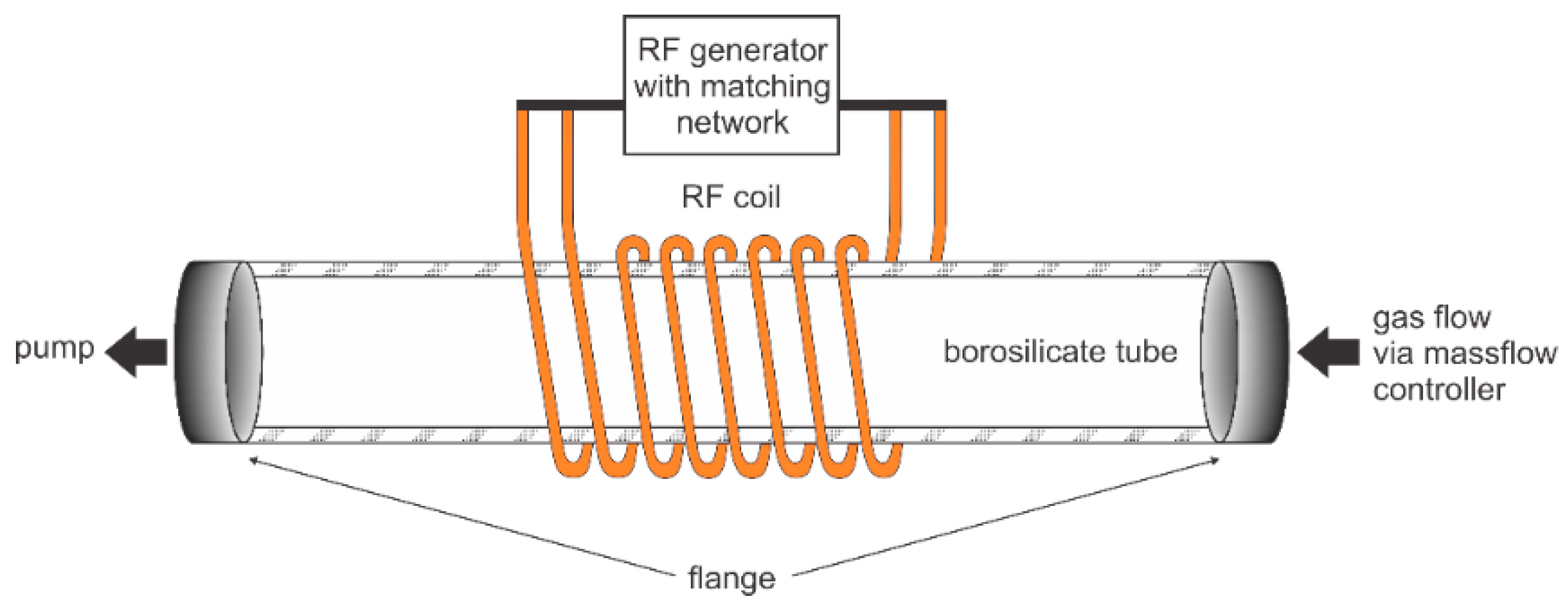
2.3. Plasma Treatment of PET Fabric
2.4. In Situ Synthesis of ZnO on PET Fabric
2.5. UV Protection Factor Measurements
2.6. Colour Measurements
2.7. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDS | Energy dispersive spectroscopy |
| PET | Polyethylene terephthalate |
| SEM | Scanning electron microscopy |
| UPF | Ultraviolet protection factor |
| UV | Ultraviolet |
| VUV | Vacuum ultraviolet |
| ZnO | Zinc oxide |
References
- Štular, D.; Primc, G.; Mozetič, M.; Jerman, I.; Mihelčič, M.; Ruiz-Zepeda, F.; Tomšič, B.; Simončič, B.; Gorjanc, M. Influence of non-thermal plasma treatement on the adsorption of a stimuli-responsive nanogel onto polyethylene terephthalate fabric. Prog. Org. Coat. 2018, 120, 198–207. [Google Scholar] [CrossRef]
- Gorenšek, M.; Gorjanc, M.; Bukošek, V.; Kovač, J.; Jovančić, P.; Mihailović, D. Functionalization of PET fabrics by corona and nano silver. Text. Res. J. 2010, 80, 253–262. [Google Scholar] [CrossRef]
- Azeem, M.; Javed, A.; Morikawa, H.; Noman, M.T.; Khan, M.Q.; Shahid, M.; Wiener, J. Hydrophilization of Polyester Textiles by Nonthermal Plasma. Autex Res. J. 2021, 21, 142–149. [Google Scholar] [CrossRef]
- Weerasinghe, D.U.; Perera, S.; Dissanayake, D.G.K. Application of biomimicry for sustainable functionalization of textiles: Review of current status and prospectus. Text. Res. J. 2019, 89, 4282–4294. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 18. [Google Scholar] [CrossRef]
- Verbič, A.; Šala, M.; Jerman, I.; Gorjanc, M. Novel green in situ synthesis of ZnO nanoparticles on cotton using pomegranate peel extract. Materials 2021, 14, 4472. [Google Scholar] [CrossRef] [PubMed]
- Verbič, A.; Brenčič, K.; Primc, G.; Gorjanc, M. Importance of protocol design for suitable green in situ synthesis of ZnO on cotton using aqueous extract of japanese knotweed leaves as reducing agent. Forests 2022, 13, 143. [Google Scholar] [CrossRef]
- Aladpoosh, R.; Montazer, M. The role of cellulosic chains of cotton in biosynthesis of ZnO nanorods producing multifunctional properties: Mechanism, characterizations and features. Carbohydr. Polym. 2015, 126, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Naz, M.Y.; Saleem, M.; Tanawush, M.; Głowacz, A.; Glowacz, W.; Rahman, S.; Mahnashi, M.H.; Alqahtani, Y.S.; Alyami, B.A.; et al. Statistical study of nonthermal plasma-assisted ZnO coating of cotton fabric through ultrasonic-assisted green synthesis for improved self-cleaning and antimicrobial properties. Materials 2021, 14, 6998. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Y.; Zhu, S.; Abbes, N.; Jing, X.; Zhang, L. A review of the green synthesis of ZnO nanoparticles using plant extracts and their prospects for application in antibacterial textiles. J. Eng. Fibers Fabr. 2021, 16, 15589250211046242. [Google Scholar] [CrossRef]
- Raslan, W.M.; El-Halwagy, A.A.; Elsayad, H. Recent advances in plasma/nanoparticles treatments of textile fibers. J. Text. Coloration Polym. Sci. 2020, 17, 87–105. [Google Scholar] [CrossRef]
- Gorjanc, M.; Gorenšek, M.; Jovančić, P.; Mozetič, M. Multifunctional textiles—Modification by plasma, dyeing and nanoparticles. In Eco-Friendly Textile Dyeing and Finishing; Gunay, M., Ed.; Intech: Rijeka, Croatia, 2013; Volume 1, pp. 3–31. [Google Scholar]
- Kan, C.W. A Novel Green Treatment for Textiles: Plasma Treatment as a Sustainable Technology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- AATCC Test Method: 183. In Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation Through Fabrics; American Association of Textile Chemists and Colorists: Durham, NC, USA, 2020.
- AS/NZS 4399; Sun Protective Clothing—Evaluation and Classification. Standards Australia/Standards New Zealand: Wellington, New Zealand, 2017.
- Longo, R.C.; Ranjan, A.; Ventzek, P.L. Density functional theory study of oxygen adsorption on polymer surfaces for atomic-layer etching: Implications for semiconductor device fabrication. ACS Appl. Nano Mater. 2020, 3, 5189–5202. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishikawa, K.; Mozetič, M.; Tsutsumi, T.; Kondo, H.; Sekine, M.; Hori, M. Polyethylene terephthalate (PET) surface modification by VUV and neutral active species in remote oxygen or hydrogen plasmas. Plasma Process. Polym. 2019, 16, 1800175. [Google Scholar] [CrossRef]
- Urbas, R.; Kostanjšek, K.; Dimitrovski, K. Impact of structure and yarn colour on UV properties and air permeability of multilayer cotton woven fabrics. Text. Res. J. 2011, 81, 1916–1925. [Google Scholar] [CrossRef]
- Gorjanc, M.; Mozetič, M.; Primc, G.; Vesel, A.; Spasić, K.; Puač, N.; Petrović, Z.; Kert, M. Plasma treated polyethylene terephthalate for increased embedment of UV-responsive microcapsules. Appl. Surf. Sci. 2017, 419, 224–234. [Google Scholar] [CrossRef]
- Elabid, A.E.; Zhang, J.; Shi, J.; Guo, Y.; Ding, K.; Zhang, J. Improving the low temperature dyeability of polyethylene terephthalate fabric with dispersive dyes by atmospheric pressure plasma discharge. Appl. Surf. Sci. 2016, 375, 26–34. [Google Scholar] [CrossRef]
- Haji, A.; Ashraf, S.; Nasiriboroumand, M.; Lievens, C. Environmentally friendly surface treatment of wool fiber with plasma and chitosan for improved coloration with cochineal and safflower natural dyes. Fibers Polym. 2020, 21, 743–750. [Google Scholar] [CrossRef]
- Dave, H.; Ledwani, L.; Chandwani, N.; Kikani, P.; Desai, B.; Nema, S.K. Surface modification of polyester fabric by non-thermal plasma treatment and its effect on coloration using natural dye. J. Polym. Mater 2013, 30, 14. [Google Scholar]

| Sample Code | Description | |
|---|---|---|
| Extr | Immersion in pomegranate peel extract |  |
| Ash-ZnAc-Extr | Immersion in wood ash, zinc acetate and pomegranate peel extract |  |
| P-Ash-ZnAc-Extr | Plasma treatment, immersion in wood ash, zinc acetate and pomegranate peel extract | 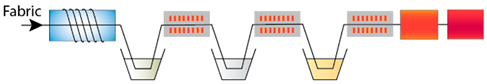 |
| P-Ash-P-ZnAc-Extr | Plasma treatment, immersion in wood ash extract, plasma treatment, immersion in zinc acetate and pomegranate peel extract | 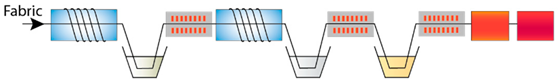 |
| P-Ash-P-ZnAc-P-Extr | Plasma treatment, immersion in wood ash extract, plasma treatment, immersion in zinc acetate, plasma treatment, immersion in pomegranate peel extract | 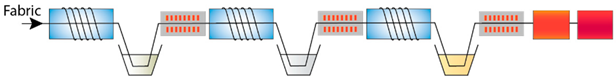 |
| Sample | UPF | K/S | L* | a* | b* | Scanned Image |
|---|---|---|---|---|---|---|
| Raw PET | 16.5 | 0.03 | 93.1 | −0.4 | 0.0 |  |
| Extr | 34.9 | 2.21 | 76.2 | 5.1 | 22.5 |  |
| Ash-ZnAc-Extr | 177.9 | 7.48 | 72.2 | 1.6 | 44.7 |  |
| P-Ash-ZnAc-Extr | 218.6 | 8.32 | 76.2 | −2.5 | 50.5 |  |
| P-Ash-P-ZnAc-Extr | 295.2 | 11.46 | 74.9 | 0.2 | 53.4 |  |
| P-Ash-P-ZnAc-P-Extr | 303.7 | 12.14 | 73.7 | 1.0 | 54.6 |  |
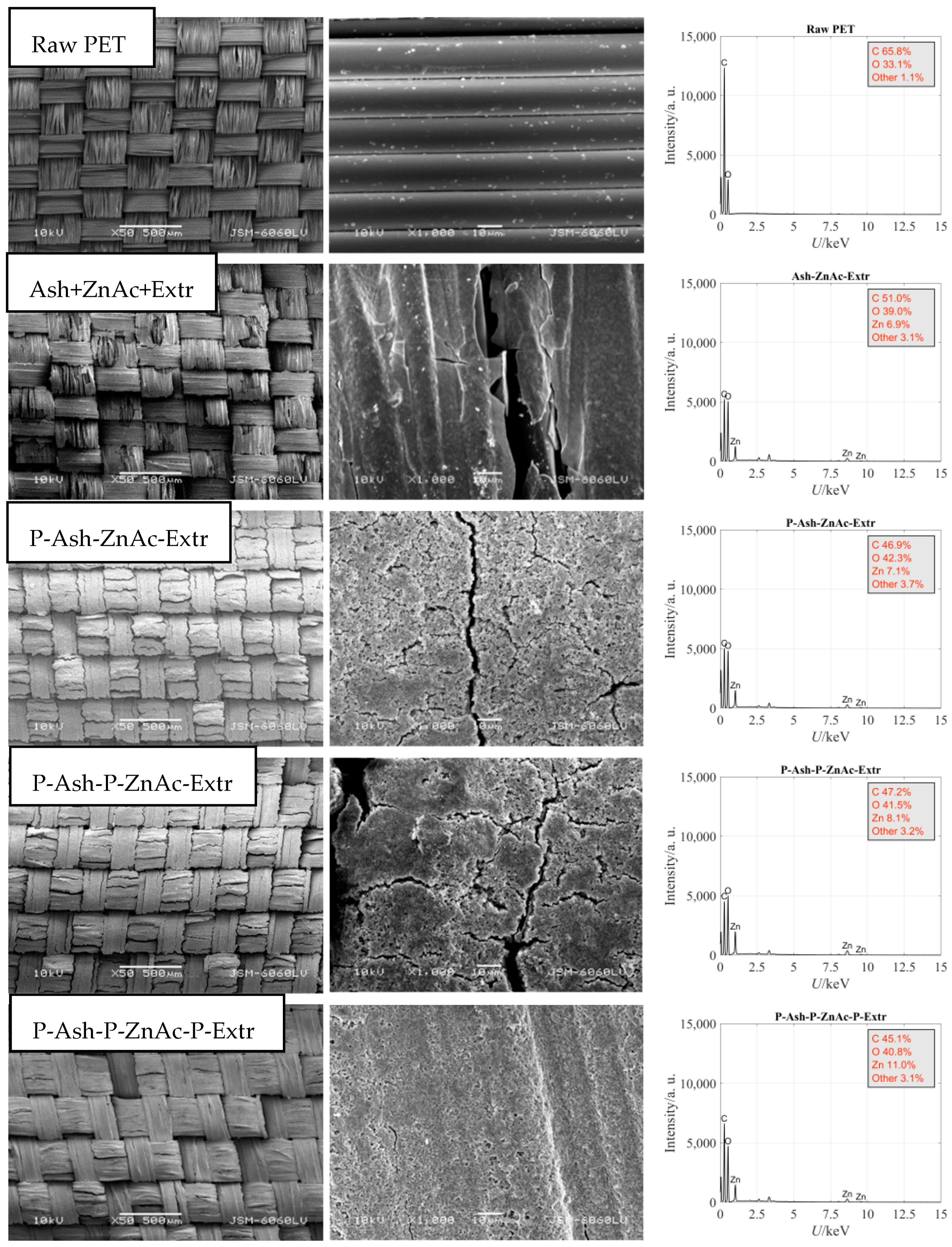
| Sample | C (wt.%) | O (wt.%) | Zn (wt.%) | Other (wt.%) |
|---|---|---|---|---|
| Raw PET | 65.8 | 33.1 | 0.0 | 1.1 |
| Ash-ZnAc-Extr | 51.0 | 39.0 | 6.9 | 3.1 |
| P-Ash-ZnAc-Extr | 46.9 | 42.3 | 7.1 | 3.7 |
| P-Ash-P-ZnAc-Extr | 47.2 | 41.5 | 8.1 | 3.2 |
| P-Ash-P-ZnAc-P-Extr | 45.1 | 40.8 | 11.0 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbič, A.; Brenčič, K.; Primc, G.; Mozetič, M.; Gorjanc, M. Eco-Friendly In Situ ZnO Synthesis on PET Fabric Using Oxygen Plasma and Plant Waste. Coatings 2022, 12, 537. https://doi.org/10.3390/coatings12040537
Verbič A, Brenčič K, Primc G, Mozetič M, Gorjanc M. Eco-Friendly In Situ ZnO Synthesis on PET Fabric Using Oxygen Plasma and Plant Waste. Coatings. 2022; 12(4):537. https://doi.org/10.3390/coatings12040537
Chicago/Turabian StyleVerbič, Anja, Katja Brenčič, Gregor Primc, Miran Mozetič, and Marija Gorjanc. 2022. "Eco-Friendly In Situ ZnO Synthesis on PET Fabric Using Oxygen Plasma and Plant Waste" Coatings 12, no. 4: 537. https://doi.org/10.3390/coatings12040537
APA StyleVerbič, A., Brenčič, K., Primc, G., Mozetič, M., & Gorjanc, M. (2022). Eco-Friendly In Situ ZnO Synthesis on PET Fabric Using Oxygen Plasma and Plant Waste. Coatings, 12(4), 537. https://doi.org/10.3390/coatings12040537