Microstructure, Mechanical, and Electrochemical Properties of SiC Particle Reinforced CoCrFeNiCu High-Entropy Alloy Coatings
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials and the Fabrication of the CoCrFeNiCu(SiC)x HEA Coatings
2.2. Characterization Methods of CoCrFeNiCu(SiC)x High-Entropy Alloy Coatings
3. Results and Discussion
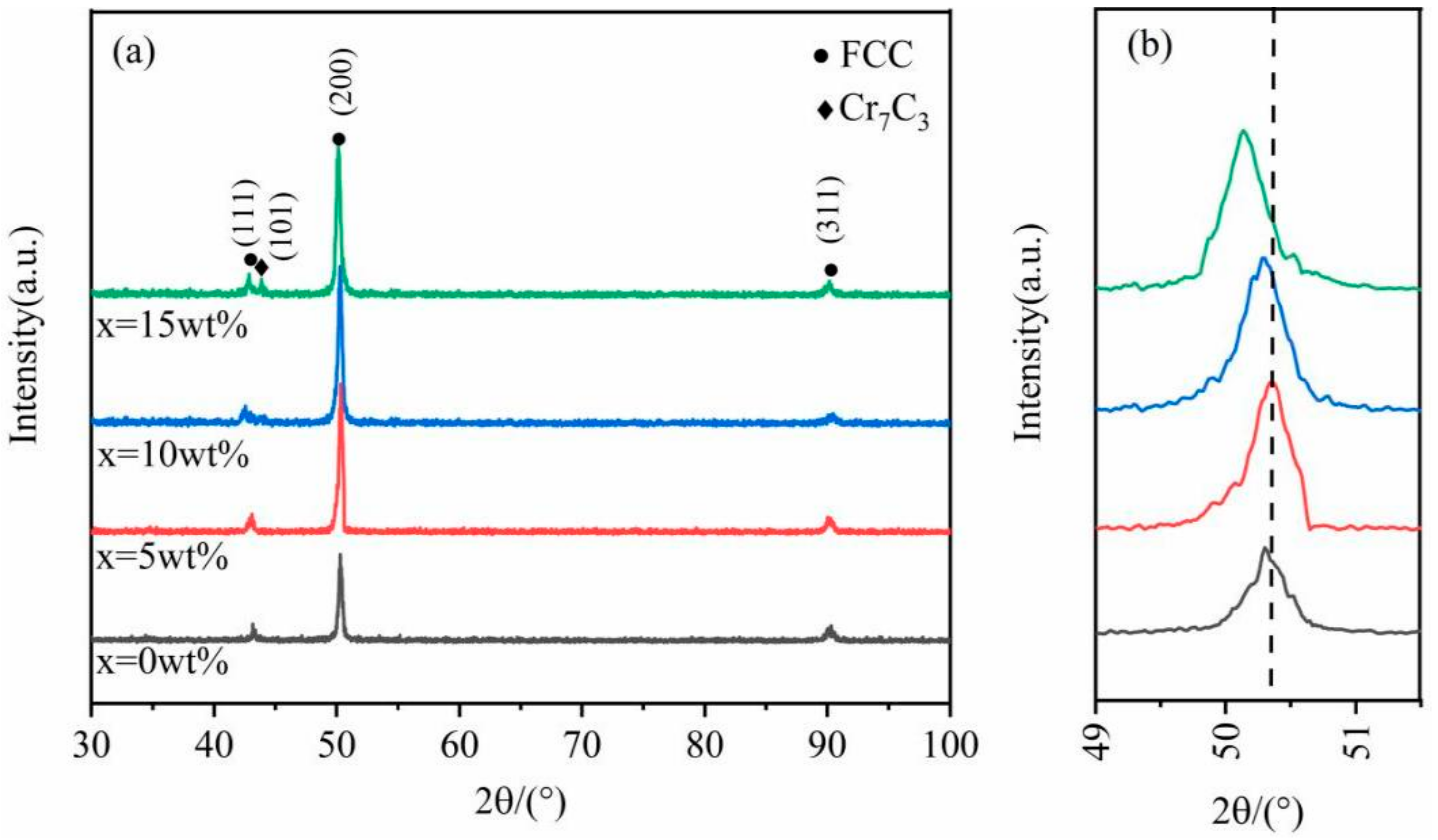
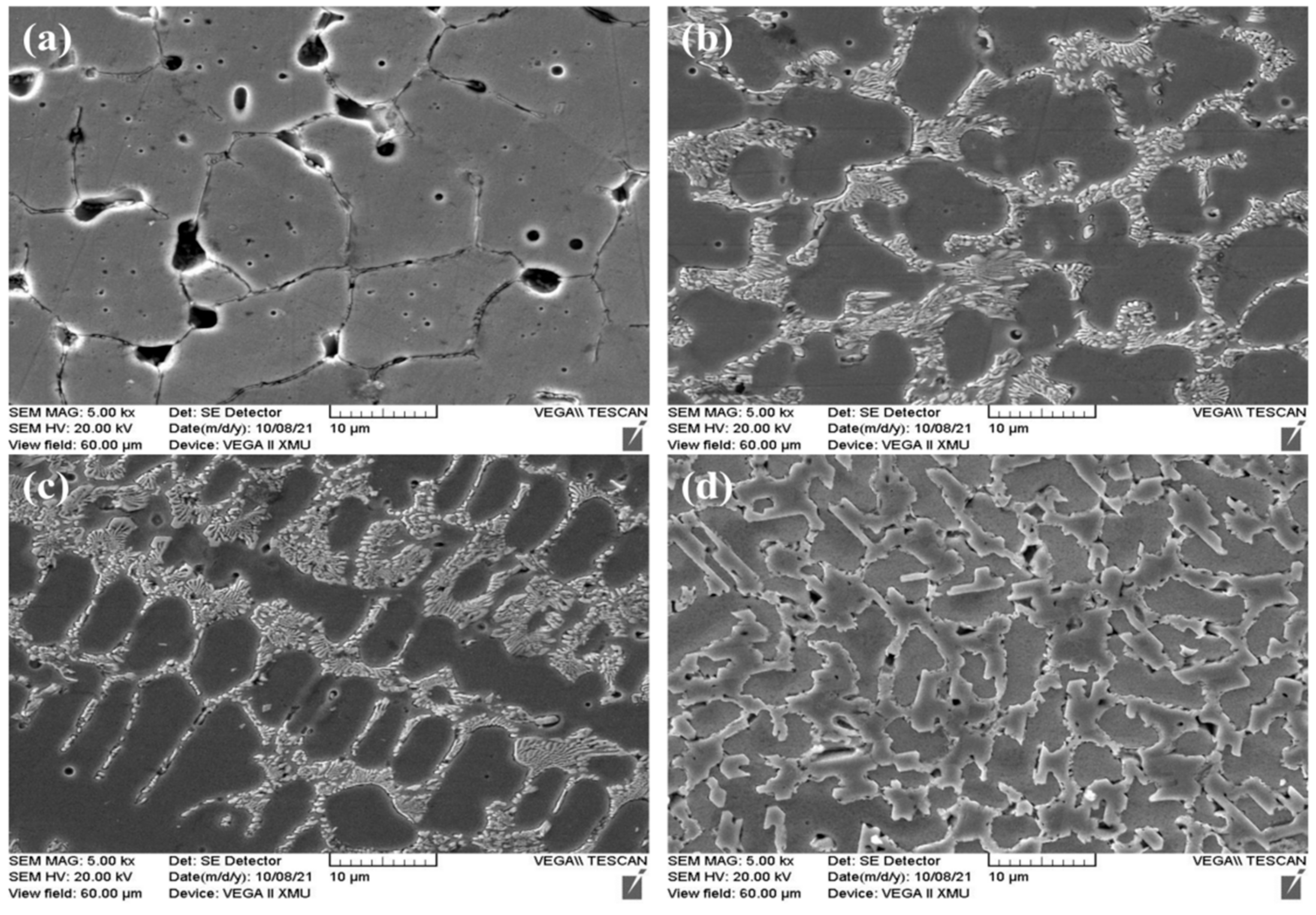
3.1. Phases and Morphologies of the CoCrFeNiCu(SiC)x High-Entropy Alloy Coatings
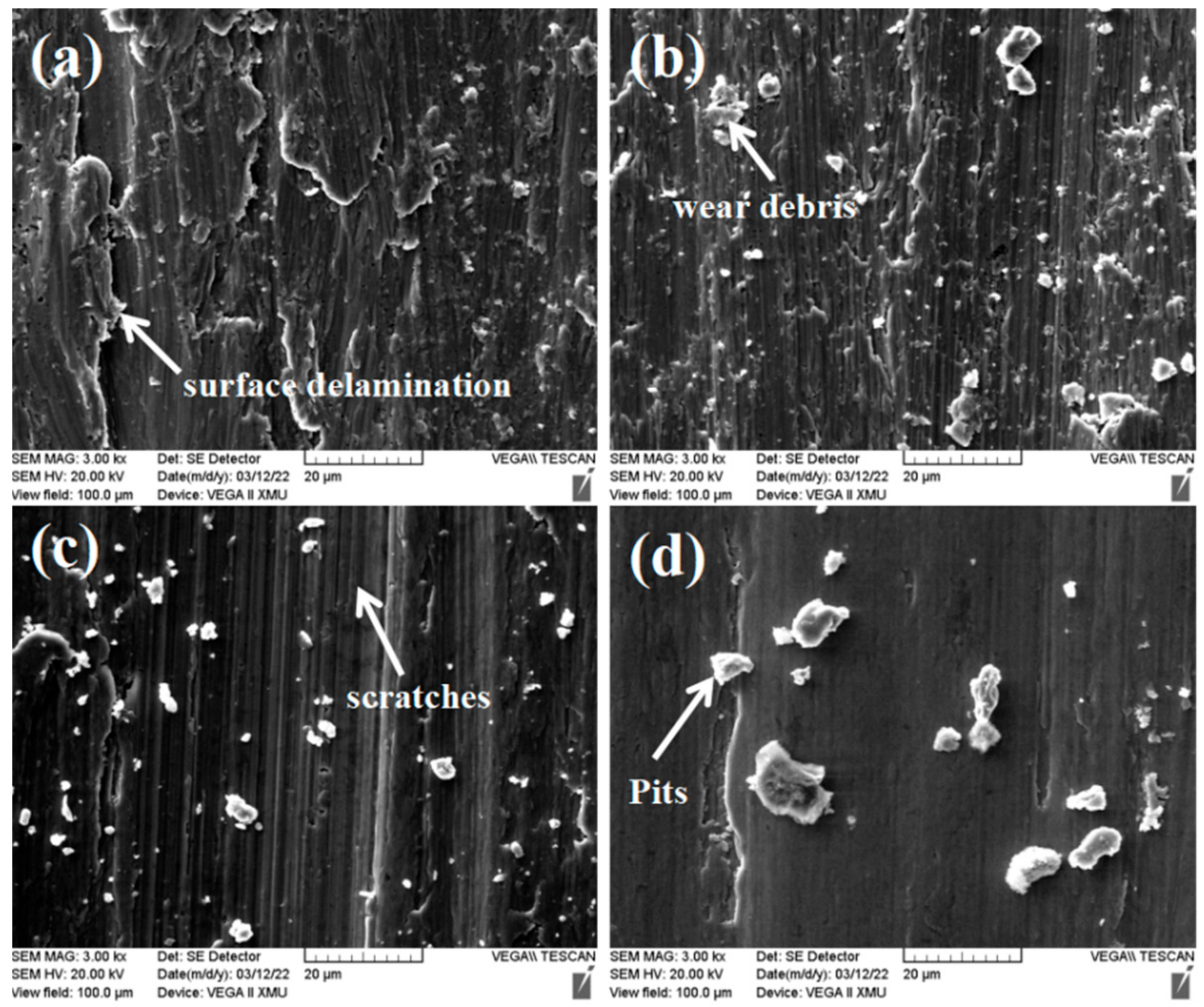
3.2. Mechanical Properties of the CoCrFeNiCu(SiC)x High-Entropy Alloy Coatings
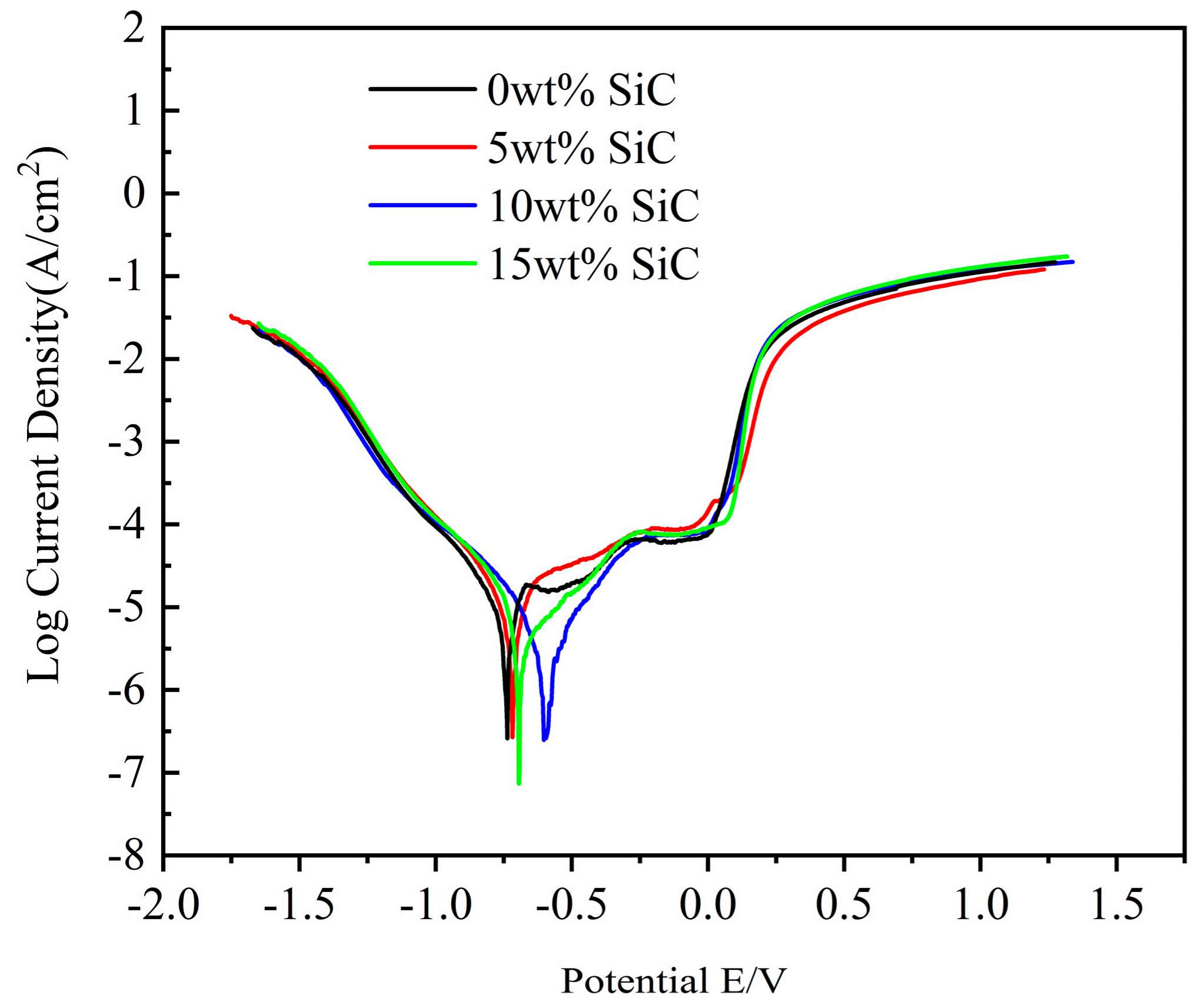
3.3. Electrochemical Properties of the CoCrFeNiCu(SiC)x High-Entropy Alloy Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Nanoslruetured high-entropy alloys novel alloy design eoneepls and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cai, Y.; Ao, S.; Manladan, S.M.; Xue, J.; Luo, Z. Evolution mechanisms of TiC ceramic particles in FeCoCrNiAl high-entropy alloy laser cladding layers. Mater. Res. Express 2019, 6, 1065d2. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, L.; Cui, Y.; Geng, K.; Manladan, S.M.; Luo, Z. High-temperature oxidation behavior of FeCoCrNiAlx high-entropy alloy coatings. Mater. Res. Express 2019, 6, 126552. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, M.-R.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Chang, S.-Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Juan, C.-C.; Wang, W.-R.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. On the superior hot hardness and softening resistance of AlCoCrxFeMo0.5Ni high-entropy alloys. Mater. Sci. Eng. A 2011, 528, 3581–3588. [Google Scholar] [CrossRef]
- Shu, F.Y.; Liu, S.; Zhao, H.Y.; He, W.X.; Sui, S.H.; Zhang, J.; He, P.; Xu, B.S. Structure and high-temperature property of amorphous composite coating synthesized by laser cladding FeCrCoNiSiB high-entropy alloy powder. J. Alloys Compd. 2018, 731, 662–666. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, S.Z.; Fu, H.; Chen, G.Y.; Ran, Q.X.; Wang, S.Q.; Han, R.H. A novel high-entropy alloy coating on Ti-6Al-4V substrate by laser cladding. Mater. Lett. 2022, 308, 131131. [Google Scholar] [CrossRef]
- Abhijith, N.V.; Kumar, D.; Kalyansundaram, D. Development of Single-Stage TiNbMoMnFe High-Entropy Alloy Coating on 304L Stainless Steel Using HVOF Thermal Spray. J. Therm. Spray Technol. 2022. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, T.; Zhu, H.; Xie, Z. Microstructure and mechanical properties of in-situ TiC reinforced FeCoNiCu2.0 high entropy alloy matrix composites. Mater. Sci. Eng. A 2021, 822, 141671. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, L.; Zhong, C.; Song, Q.; Yang, K.; Jiang, Y.; Wang, Z.; Wang, Z.R. Effects of WC on the cavitation erosion resistance of FeCoCrNiB0.2 high entropy alloy coating prepared by laser cladding. Mater. Today Commun. 2021, 26, 102154. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Zhu, S.; Zhang, Z.; Tao, X.; Wang, Z.; Lu, B. Microstructure and wear properties of TiN-Al2O3-Cr2B multiphase ceramics in-situ reinforced CoCrFeMnNi high-entropy alloy coating. Mater. Chem. Phys. 2022, 276, 125352. [Google Scholar] [CrossRef]
- Qu, Y.; Qi, H.; Li, G.; Zhang, Y.; Tian, C.; Nie, S.; Tan, B.; Li, R.; Yu, B. The effect of nanometer phase SiC addition on the microstructure and mechanical properties of Al0.6CrFe2Ni2 high entropy alloys. Mater. Technol. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Szklarz, Z.; Lekki, J.; Bobrowski, P.; Szklarz, M.B.; Rogal, Ł. The effect of SiC nanoparticles addition on the electrochemical response of mechanically alloyed CoCrFeMnNi high entropy alloy. Mater. Chem. Phys. 2018, 215, 385–392. [Google Scholar] [CrossRef]
- Khan, N.A.; Akhavan, B.; Zheng, Z.; Liu, H.; Zhou, C.; Zhou, H.; Chang, L.; Wang, Y.; Liu, Y.; Sun, L.; et al. Nanostructured AlCoCrCu0.5FeNi high entropy oxide (HEO) thin films fabricated using reactive magnetron sputtering. Appl. Surf. Sci. 2021, 553, 149491. [Google Scholar] [CrossRef]
- Böőr, K.; Qiu, R.; Forslund, A.; Bäcke, O.; Larsson, H.; Lindahl, E.; Halvarsson, M.; Boman, M.; von Fieandt, L. Chemical vapor deposition of TiN on a CoCrFeNi multi-principal element alloy substrate. Surf. Coat. Technol. 2020, 393, 125778. [Google Scholar] [CrossRef]
- Xiao, J.-K.; Tan, H.; Wu, Y.-Q.; Chen, J.; Zhang, C. Microstructure and wear behavior of FeCoNiCrMn high entropy alloy coating deposited by plasma spraying. Surf. Coat. Technol. 2020, 385, 125430. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, C.; Chen, L.; Huo, Q. Effect of RE oxides on the microstructure of the coatings fabricated on titanium alloys by laser alloying technique. Scr. Mater. 2006, 54, 847–852. [Google Scholar] [CrossRef]
- Calleja, A.; Urbikain, G.; González, H.; Cerrillo, I.; Polvorosa, R.; Lamikiz, A. Inconel® 718 superalloy machinability evaluation after laser cladding additive manufacturing process. Int. J. Adv. Manuf. Technol. 2018, 97, 2873–2885. [Google Scholar] [CrossRef]
- Lampa, C.; Smirnov, I. High speed laser cladding of an iron based alloy developed for hard chrome replacement. J. Laser Appl. 2019, 31, 022511. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Yan, H.; Lu, Y.; Yu, Z.; Li, C.; Lu, Q.; Qiu, D. Microstructure and wear properties of Ni-W-Si coatings by laser cladding. Appl. Mech. Mater. 2015, 750, 214–219. [Google Scholar] [CrossRef]
- Nair, A.M.; Muvvala, G.; Nath, A.K. A study on in-situ synthesis of TiCN metal matrix composite coating on Ti–6Al–4V by laser surface alloying process. J. Alloys Compd. 2019, 810, 151901. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Liu, X.; He, P.; Ren, X.; Yan, S.; Xu, B. Interface phase evolution during laser cladding of Ni-Cu alloy on nodular cast iron by powder pre-placed method. Opt. Laser. Technol. 2021, 135, 106684. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Wang, Z. Microstructure and mechanical properties of low activation Fe-Ti-Cr-V-W multi-principal element alloys. J. Nucl. Mater. 2020, 533, 152078. [Google Scholar] [CrossRef]
- Pan, Y.; Baptista, J.L. Chemical instability of silicon carbide in the presence of transition metals. J. Am. Ceram. Soc. 1996, 79, 2017–2026. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.-Z.; Pan, Y.; Guo, S. Thermally stable laser cladded CoCrCuFeNi high-entropy alloy coating with low stacking fault energy. J. Alloys Compd. 2014, 600, 210–214. [Google Scholar] [CrossRef]
- Anthonysamy, S.; Ananthasivan, K.; Kaliappan, I.; Chandramouli, V.; Rao, P.R.V.; Mathews, C.K.; Jacob, K.T. Gibbs energies of formation of chromium carbides. Metall. Mater. Trans. A 1996, 27, 1919–1924. [Google Scholar] [CrossRef]
- Aguilar-Hurtado, J.Y.; Vargas-Uscategui, A.; Paredes-Gil, K.; Palma-Hillerns, R.; Tobar, M.J.; Amado, J.M. Boron addition in a non-equiatomic Fe50Mn30Co10Cr10 alloy manufactured by laser cladding: Microstructure and wear abrasive resistance. Appl. Surf. Sci. 2020, 515, 146084. [Google Scholar] [CrossRef]
- Chen, K.; Wang, T.; Wang, X.; Jiang, Y.; Xue, J.; Liu, X.; Jiang, Y.; Chen, Z. Effect of Submicron SiC Particles on the Properties of Alcocrfeni High Entropy Alloy Coatings. Powder Metall. Met. Ceram. 2020, 59, 424–433. [Google Scholar] [CrossRef]
- Lu, J.; Lu, H.; Xu, X.; Yao, J.; Cai, J.; Luo, K. High-performance integrated additive manufacturing with laser shock peening-induced microstructural evolution and improvement in mechanical properties of Ti6Al4V alloy components. Int. J. Mach. Tools Manuf. 2020, 148, 103475. [Google Scholar] [CrossRef]
- Achard, J.F. Contact and rubbing of flat surface. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Qiu, X.-W.; Liu, C.-G. Microstructure and properties ofAl2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Qui, Y.; Thomas, S.; Fabajanic, D.; Barlow, A.J.; Fraser, H.L.; Birbilis, N. Microstructural evolution, electrochemical and corrosion properties of AlxCoCrFeNiTiy high entropy alloys. Mater. Des. 2019, 170, 107698. [Google Scholar] [CrossRef]



| Beam Diameter (mm) | Defocus Distance (mm) | Power (w) | Scanning Speed (mm/min) |
|---|---|---|---|
| 2 × 15 | 20.4 | 2000 | 180 |
| Samples | Ba (mv) | Bc (mv) | Ecorr (mv) | Icorr (mA/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|---|---|
| 0 wt% SiC | 781.2 | 589.16 | −717.69 | 4.3094 × 10−2 | 0.080099 |
| 5 wt% SiC | 209.97 | 168.15 | −707.34 | 3.7538 × 10−2 | 0.058383 |
| 10 wt% SiC | 471.6 | 401.48 | −597.89 | 2.4136 × 10−3 | 0.040087 |
| 15 wt% SiC | 344.1 | 266.69 | −693.97 | 1.5144 × 10−2 | 0.051372 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Du, H.; Liu, J.; Feng, D.; Xia, S. Microstructure, Mechanical, and Electrochemical Properties of SiC Particle Reinforced CoCrFeNiCu High-Entropy Alloy Coatings. Coatings 2022, 12, 519. https://doi.org/10.3390/coatings12040519
Xu L, Du H, Liu J, Feng D, Xia S. Microstructure, Mechanical, and Electrochemical Properties of SiC Particle Reinforced CoCrFeNiCu High-Entropy Alloy Coatings. Coatings. 2022; 12(4):519. https://doi.org/10.3390/coatings12040519
Chicago/Turabian StyleXu, Li, Huiling Du, Jia Liu, Danni Feng, and Siyu Xia. 2022. "Microstructure, Mechanical, and Electrochemical Properties of SiC Particle Reinforced CoCrFeNiCu High-Entropy Alloy Coatings" Coatings 12, no. 4: 519. https://doi.org/10.3390/coatings12040519
APA StyleXu, L., Du, H., Liu, J., Feng, D., & Xia, S. (2022). Microstructure, Mechanical, and Electrochemical Properties of SiC Particle Reinforced CoCrFeNiCu High-Entropy Alloy Coatings. Coatings, 12(4), 519. https://doi.org/10.3390/coatings12040519






