The Effect of Complex Emulsifier on the Structure of Tung Oil and Phenolic Amides Containing Microcapsules and Its Anti-Fouling and Anti-Corrosion Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of MC(T) and MC(P and T)
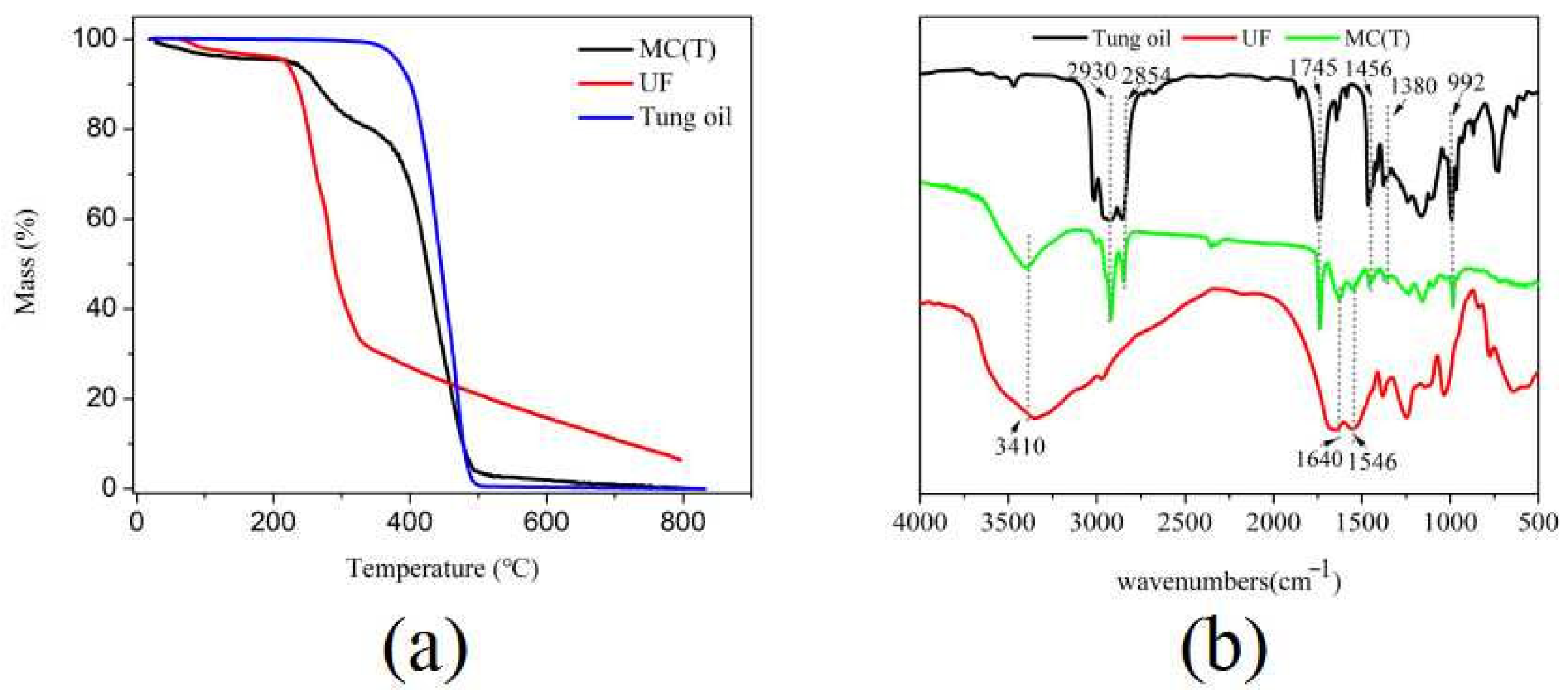
2.3. Characterization of MC(T) and MC(P and T)
2.4. Preparation of the Coating Samples
2.5. Characterization of Coating Samples
3. Results and Discussion
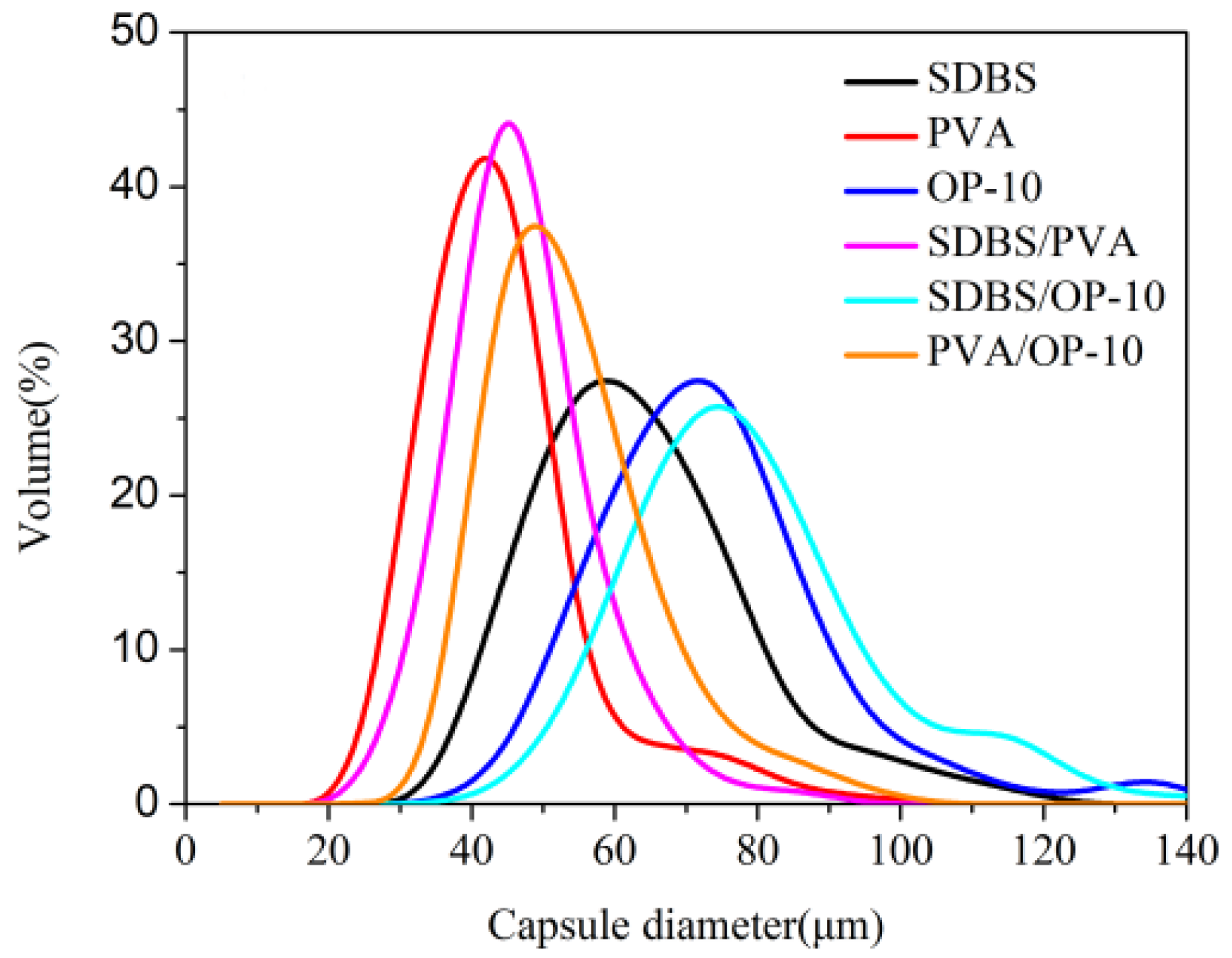
3.1. Emulsification System
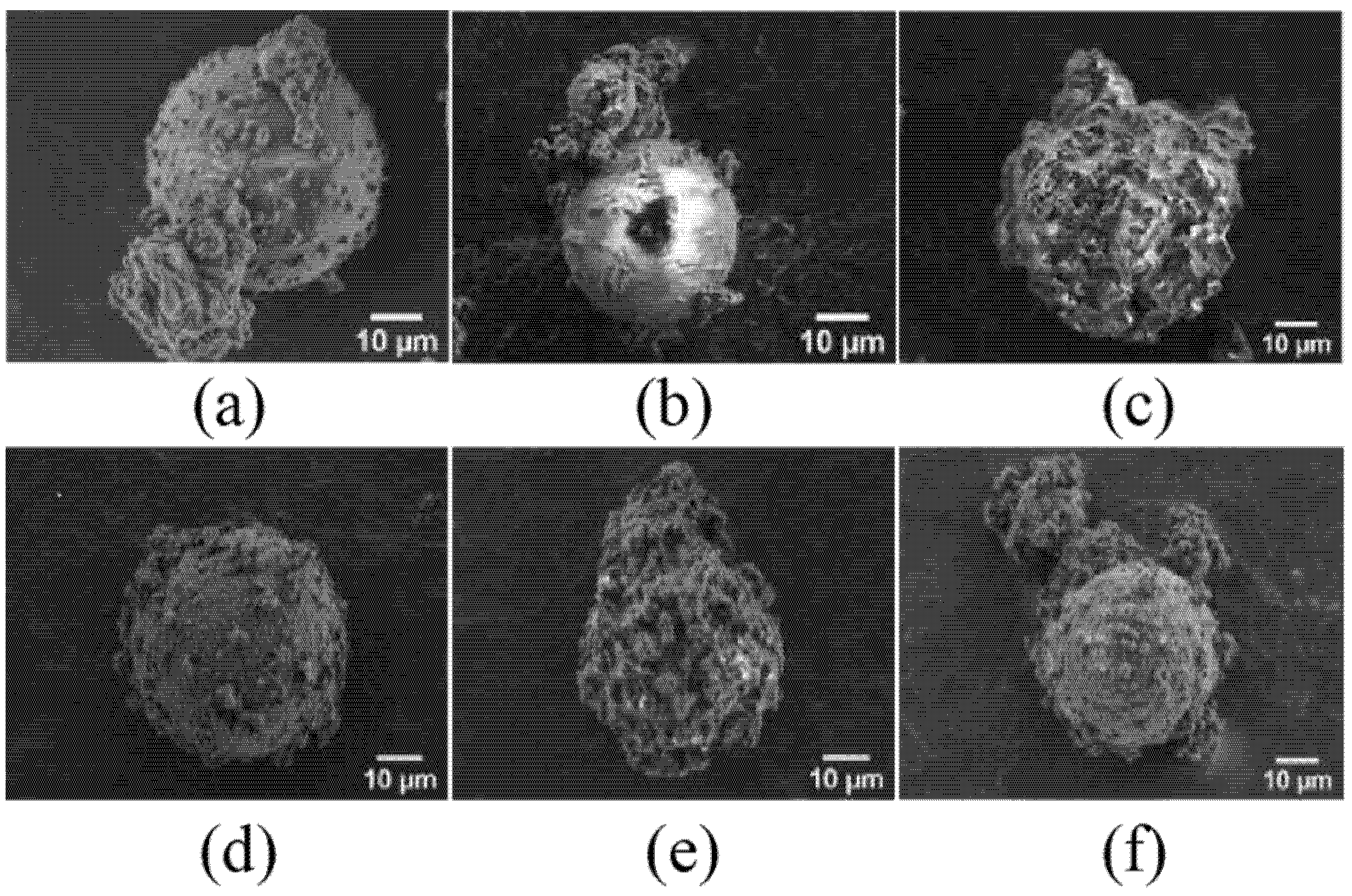
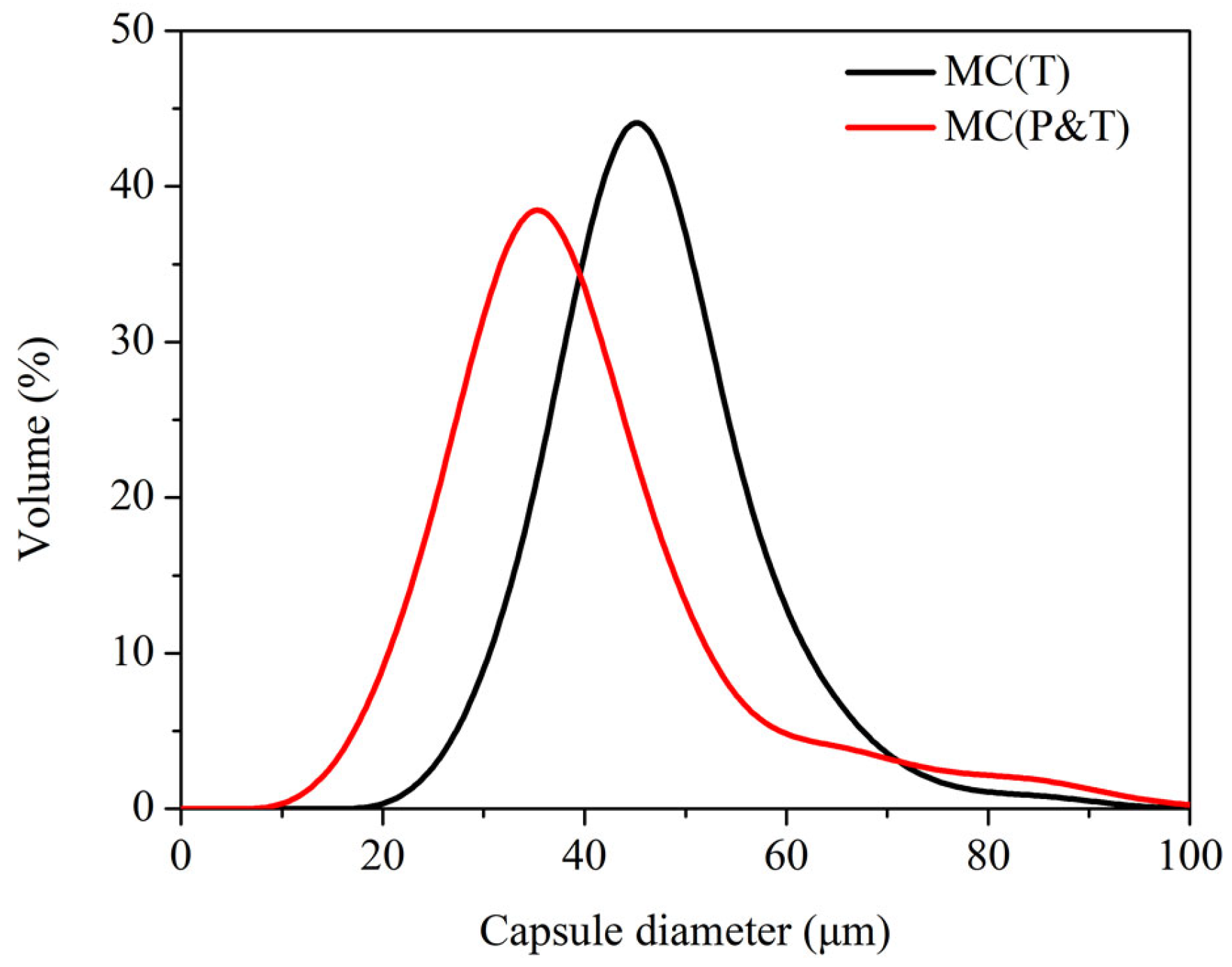
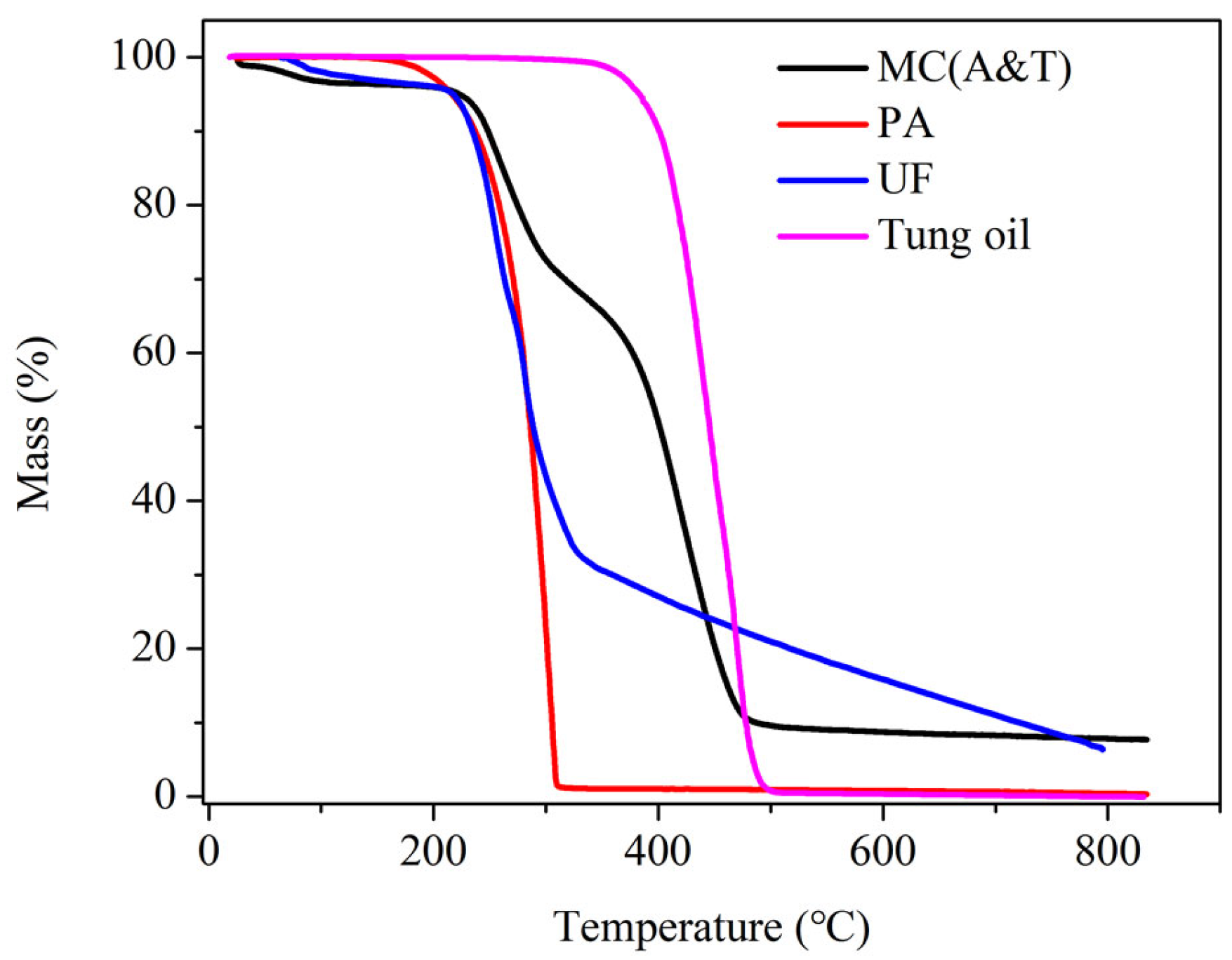
3.2. Characterizations of Synthesized MC(P and T)
3.3. Characterization of Self-Healing Coating
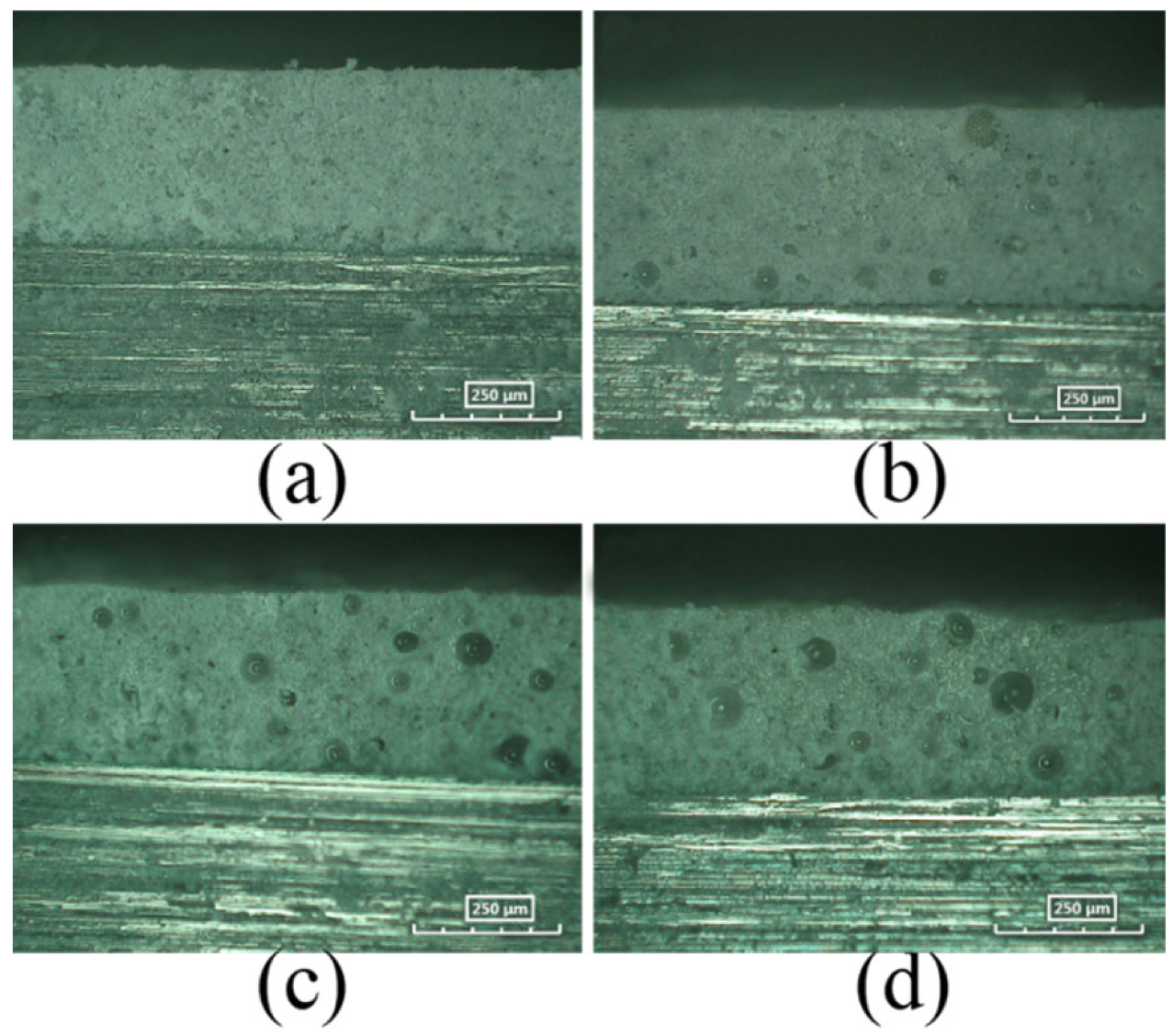
3.3.1. Microstructure of Coating with MC(P and T)
3.3.2. Bonding Strength of Coating with MC(P and T)
3.3.3. EIS of Coating with MC(P and T)
3.3.4. Neutral Salt Test of Coating with MC(P and T)
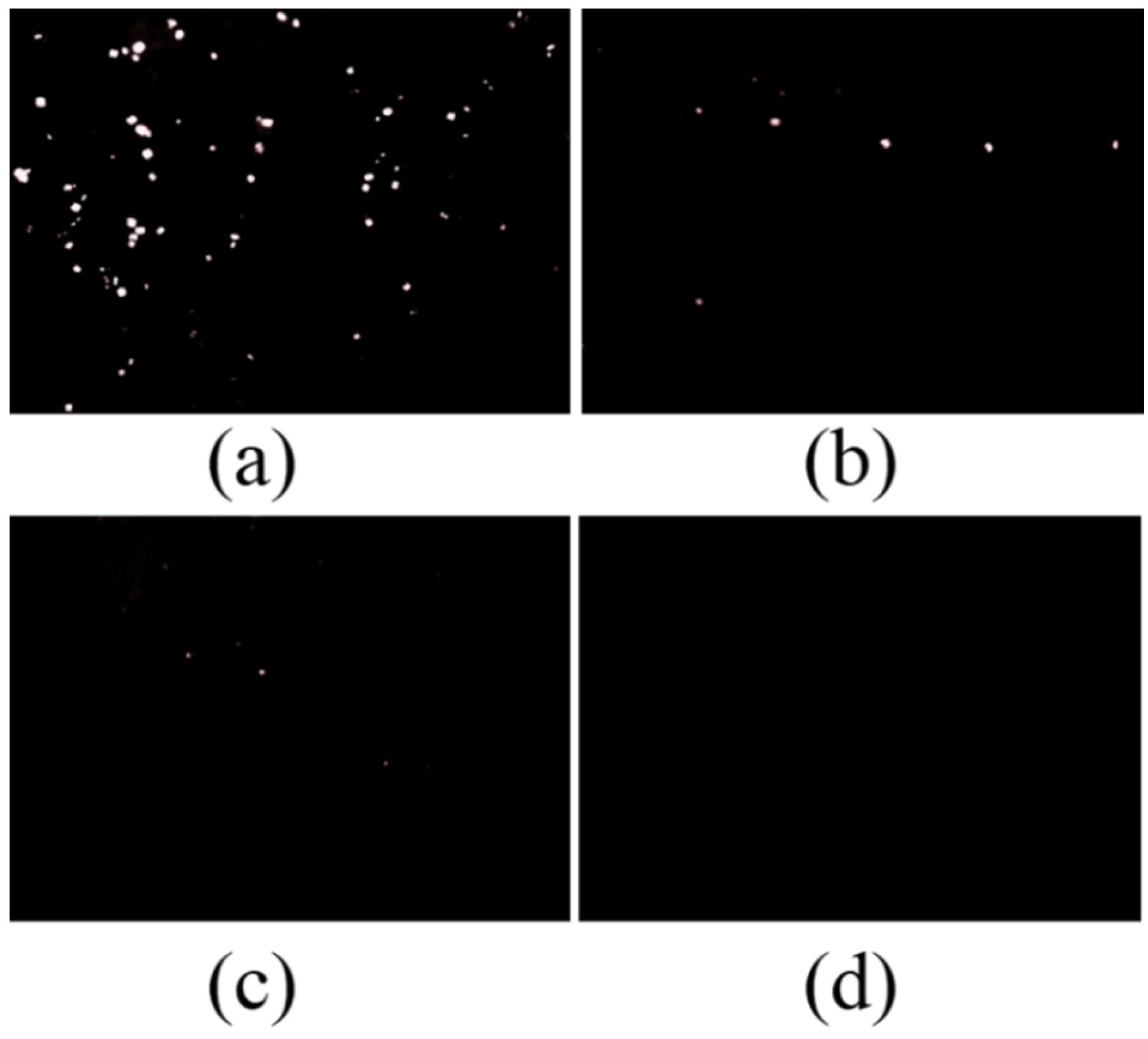
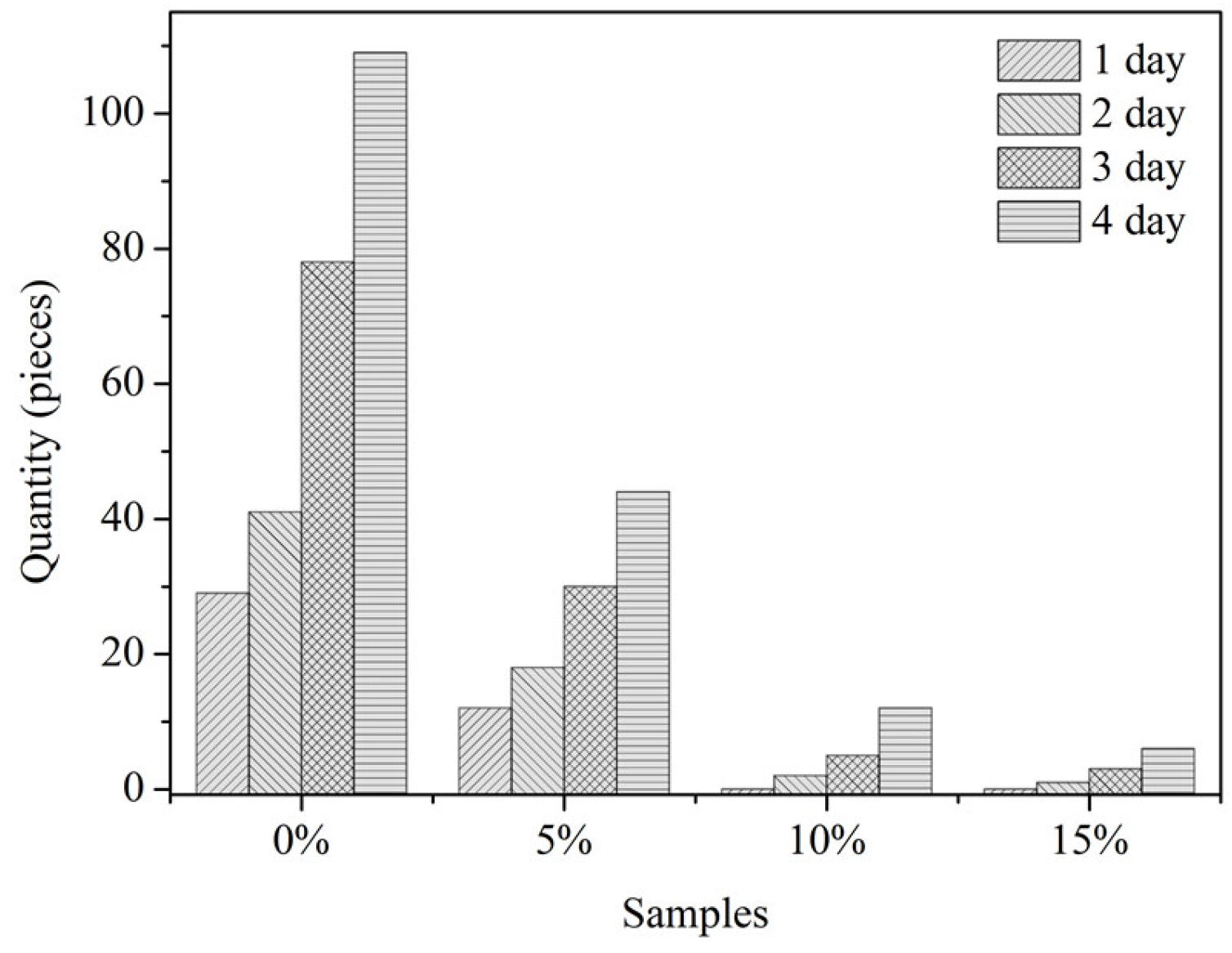
3.3.5. Anti-Fouling of Coatings with MC(P and T)
4. Conclusions
- The complex emulsifier of SDBS/PVA had the best emulsifying effect among the six emulsifiers, which could achieve the narrowest particle size distribution of the microcapsules (from 24.07 to 71.33 µm) with 75% core content.
- The addition of 10–15 wt% of microcapsules in the epoxy coating could provide enough healing agent to repair the damage, which made coatings have better anti-corrosion and anti-fouling effects, and the amide included in the healing area was sufficient for preventing 90% of diatoms and 80% of mussel foot silk from adhering to the scratching.
- The anti-corrosion and anti-fouling integration of the self-healing coating was realized, which provides a practical direction for future engineering applications, but the addition of microcapsules will reduce the bonding strength of the coating. The future research direction is to solve the problem of how to ensure the bonding strength of the coating while adding microcapsules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, G.H.; Hu, J.F.; Zhou, J.H.; Wang, G.W.; Guan, S.L. The study on the mechanical properties of PU/MF double shell self-healing microcapsules. Chem. Eng. 2020, 28, 1459–1473. [Google Scholar] [CrossRef]
- Tong, X.M.; Zhang, T.; Yang, M.Z.; Zhang, Q. Preparation and characterization of novel melamine modified poly(urea-formaldehyde) self-repairing microcapsules. Coll Surf. A Phys. Eng. Asp. 2010, 371, 91–97. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.F.; Pan, M.Q.; Zhang, D.W.; Huang, Y.; Li, G.L.; Li, X.G. Self-healing mechanisms in smart protective coatings. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Yu, S.W.; Mou, J.G.; Wu, D.H.; Zheng, S.H. Research progress on the collaborative drag reduction effect of polymers and surfactants. Materials 2020, 13, 444. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Cui, X.; Jin, G.; Cai, Z.; Liu, E.; Li, X.; Chen, Y.; Lu, B. Self-healing conversion coating with gelatin–chitosan microcapsules containing inhibitor on AZ91D alloy. Surf. Eng. 2018, 34, 79–84. [Google Scholar] [CrossRef]
- Diba, M.; Spaans, S.; Ning, K.; Ippel, B.D.; Yang, F.; Loomans, B.; Dankers, P.Y.W. Self-healing biomaterials: From molecular concepts to clinical applications. Adv. Mater Interfaces 2018, 5, 1800118. [Google Scholar] [CrossRef]
- Mozaffari, M.; Beheshty, M.; Mirabedini, M. Effect of pro-cessing conditions on the microencapsulation of 1-methylimidazole curing agent using solid epoxy resins. Iran. Polym. J. 2017, 26, 629–637. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Ashrafi, A.; Kasiriha, M. Tung oil: An autonomous repairing agent for self-healing epoxy coatings. Prog. Org. Coat. 2011, 70, 383–387. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Wang, H.; Zhu, Y.; Wang, B. Preparation and application of polysulfone microcapsules containing tung oil in self-healing and self-lubricating epoxy coating. Colloids Surf. A Physicochem. Eng. Asp. 2017, 51, 181–187. [Google Scholar] [CrossRef] [Green Version]
- McDonald, S.A.; Coban, S.B.; Sottos, N.R.; Withers, P.J. Tracking capsule activation and crack healing in a microcapsule-based self-healing polymer. Sci. Rep. 2019, 9, 17773. [Google Scholar] [CrossRef]
- Ma, Y.X.; Zhang, Y.R.; Liu, J.T.; Sun, Y.; Ge, Y.J.; Yan, X.N.; Wu, J. Preparation and charaterization of ethylenediamine-polyurea microcapsule epoxy self-healing coating. Materials 2020, 13, 326. [Google Scholar] [CrossRef] [Green Version]
- Behzadnasab, M.; Esfandeh, M.; Mirabedini, S.; Zohuriaan-Mehr, M.J.; Farnood, R.R. Preparation and characterization of linseed oil-filled urea-formaldehyde microcapsules and their effect on mechanical properties of an epoxy-based coating. Colloids Surf. A Phys. Eng. Asp. 2014, 457, 16–26. [Google Scholar] [CrossRef]
- Mahboobeh, A.; Lenia, C.; Maryna, T.; Morozov, Y.; Shakoor, R.A.; Kahraman, R.; Marques, A.C.; Montemor, M.F. Autonomous self-healing in epoxy coatings provided by high efficiency isophorone diisocyanate (IPDI) microcapsules for protection of carbon steel. Prog. Org. Coat. 2019, 19, 105445. [Google Scholar]
- Lee, S.H.; Shin, S.R.; Lee, D.S. Self-healing of cross-linked PU via dual-dynamic covalent bonds of a Schiff base from cystine and vanillin. Mater. Des. 2019, 172, 107774. [Google Scholar] [CrossRef]
- Ye, Z.P.; Zhang, P.S.; Zhang, J.H.; Deng, L.D.; Zhang, J.W.; Lin, G.G.; Guo, R.W.; Dong, A.J. Novel dual-functional coating with underwater self-healing and anti-protein-fouling properties by combining two kinds of microcapsules and a zwitterionic copolymer. Prog. Org. Coat. 2019, 127, 211–221. [Google Scholar] [CrossRef]
- Mao, Q.J.; Feng, X.J.; Liang, P.; Wang, R.Z.; Wang, M.; Cui, S.P.; Lan, M.Z. Characteristics of self-healing microcapsules for cementitious composites. J. Wuhan Univ. Technol. 2018, 5, 1108–1112. [Google Scholar] [CrossRef]
- Li, J.Y.; Shi, H.W.; Liu, F.C.; Han, E.H. Self-healing epoxy coating based on tung oil-containing microcapsules for corrosion protection. Prog. Org. Coat. 2021, 156, 106236. [Google Scholar] [CrossRef]
- Behzadnasab, M.; Mirabedini, S.M.; Esfandeh, M.; Farnood, R.R. Evaluation of corrosion performance of a self-healing epoxy-based coating linseed oil-filled microcapsules via electrochemical impedance spectroscopy. Prog. Org. Coat. 2017, 105, 212–224. [Google Scholar] [CrossRef]
- Guo, H.S.; Yang, J.; Zhao, W.Q.; Xu, T.; Lin, C.G.; Zhang, J.W.; Zhang, L. Direct formation of amphiphilic crosslinked networks based on PVP as a marine anti-biofouling coating. Chem. Eng. J. 2019, 374, 1353–1363. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Yu, L.Z.; Mou, J.G.; Wu, D.H.; Xu, M.S.; Zhou, P.J. Research strategies to develop environmentally friendly marine antifouling coatings. Mar. Drugs 2020, 18, 371. [Google Scholar] [CrossRef]
- Zhang, J.; Ling, W.; Yang, Z.Q.; Liang, Y.L.; Zhang, Y. Isolation and structure-activity relationship of subergorgic acid and synthesis of its derivatives as antifouling agent. Mar. Drugs 2019, 17, 101. [Google Scholar] [CrossRef] [Green Version]



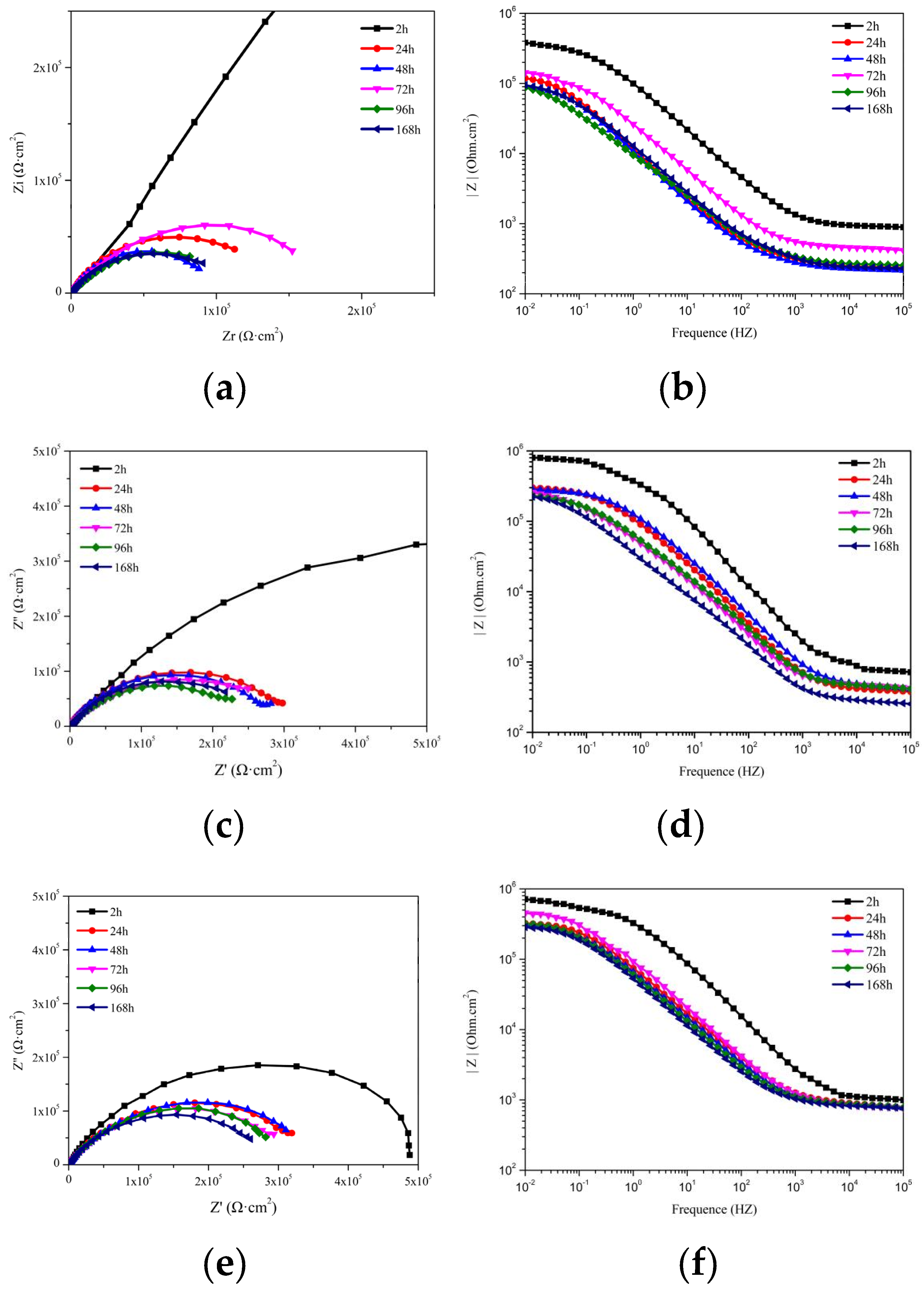
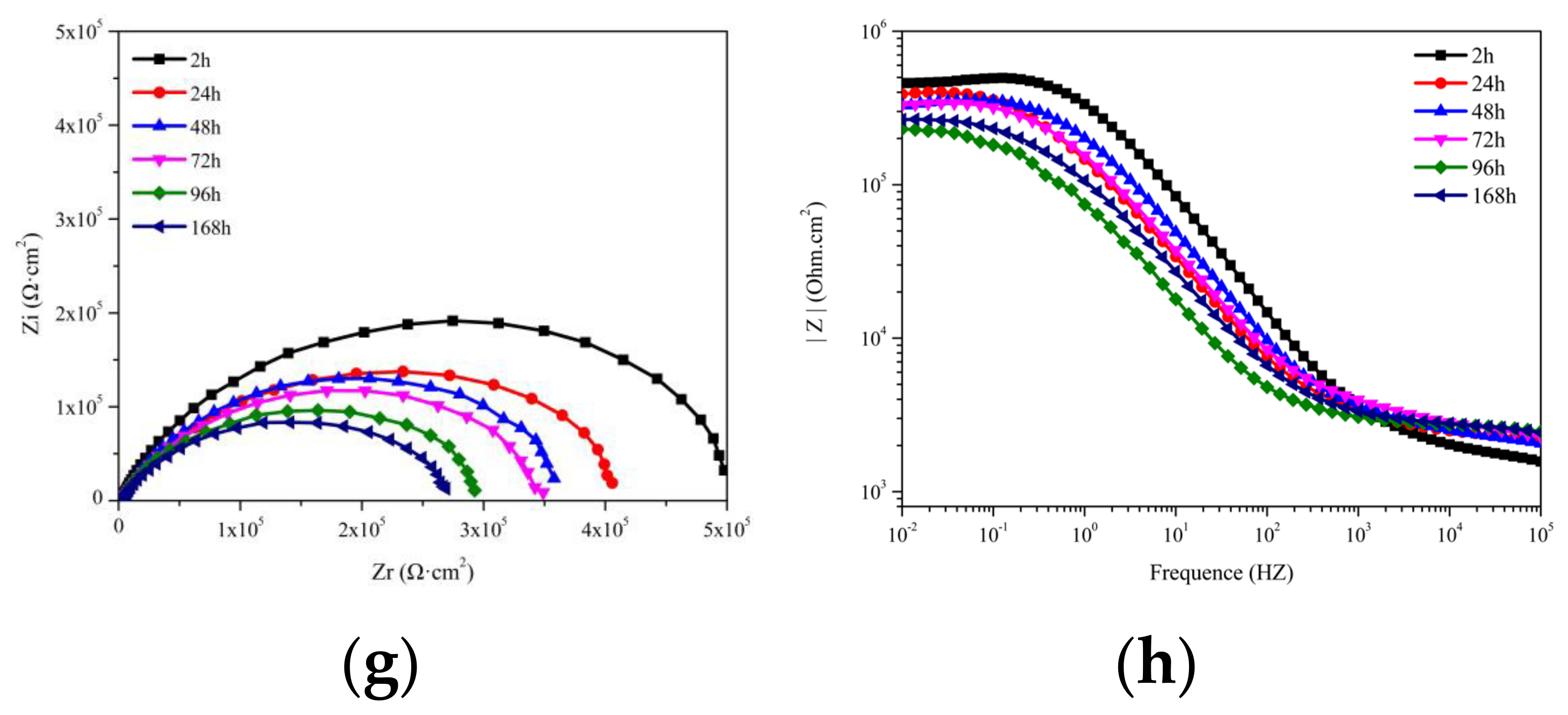
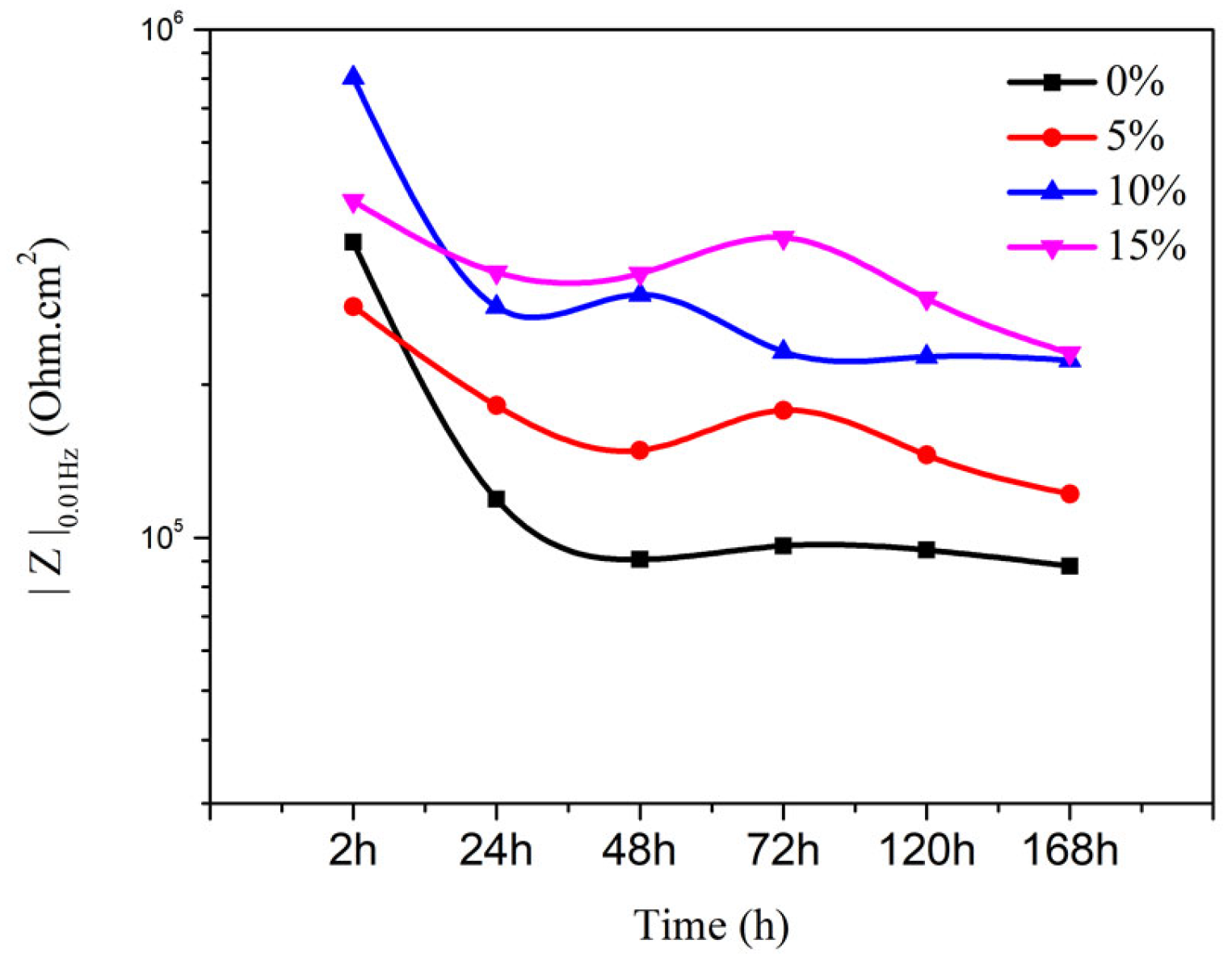


| Time (h) Samples | 24 | 48 | 72 | 96 | 168 |
|---|---|---|---|---|---|
| 0% | 1.47 × 105 | 1.11 × 105 | 1.75 × 105 | 1.30 × 105 | 1.17 × 105 |
| 5% | 3.39 × 105 | 3.12 × 105 | 3.63 × 105 | 2.86 × 105 | 2.54 × 105 |
| 10% | 3.69 × 105 | 3.62 × 105 | 3.32 × 105 | 3.23 × 105 | 2.93 × 105 |
| 15% | 4.26 × 105 | 3.67 × 105 | 3.63 × 105 | 2.97 × 105 | 2.75 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Jiang, D.; Yang, Y.; Ma, L.; Zhou, J.; Huang, G. The Effect of Complex Emulsifier on the Structure of Tung Oil and Phenolic Amides Containing Microcapsules and Its Anti-Fouling and Anti-Corrosion Performances. Coatings 2022, 12, 447. https://doi.org/10.3390/coatings12040447
Ma Y, Jiang D, Yang Y, Ma L, Zhou J, Huang G. The Effect of Complex Emulsifier on the Structure of Tung Oil and Phenolic Amides Containing Microcapsules and Its Anti-Fouling and Anti-Corrosion Performances. Coatings. 2022; 12(4):447. https://doi.org/10.3390/coatings12040447
Chicago/Turabian StyleMa, Yingxiang, Dan Jiang, Yuping Yang, Li Ma, Jian Zhou, and Guosheng Huang. 2022. "The Effect of Complex Emulsifier on the Structure of Tung Oil and Phenolic Amides Containing Microcapsules and Its Anti-Fouling and Anti-Corrosion Performances" Coatings 12, no. 4: 447. https://doi.org/10.3390/coatings12040447
APA StyleMa, Y., Jiang, D., Yang, Y., Ma, L., Zhou, J., & Huang, G. (2022). The Effect of Complex Emulsifier on the Structure of Tung Oil and Phenolic Amides Containing Microcapsules and Its Anti-Fouling and Anti-Corrosion Performances. Coatings, 12(4), 447. https://doi.org/10.3390/coatings12040447






