Facile In Situ Growth of Zif-8 Nanosheets with Enhanced Anti-Corrosion Performance for Carbon Steel in Seawater
Abstract
:1. Introduction
2. Experimental Section
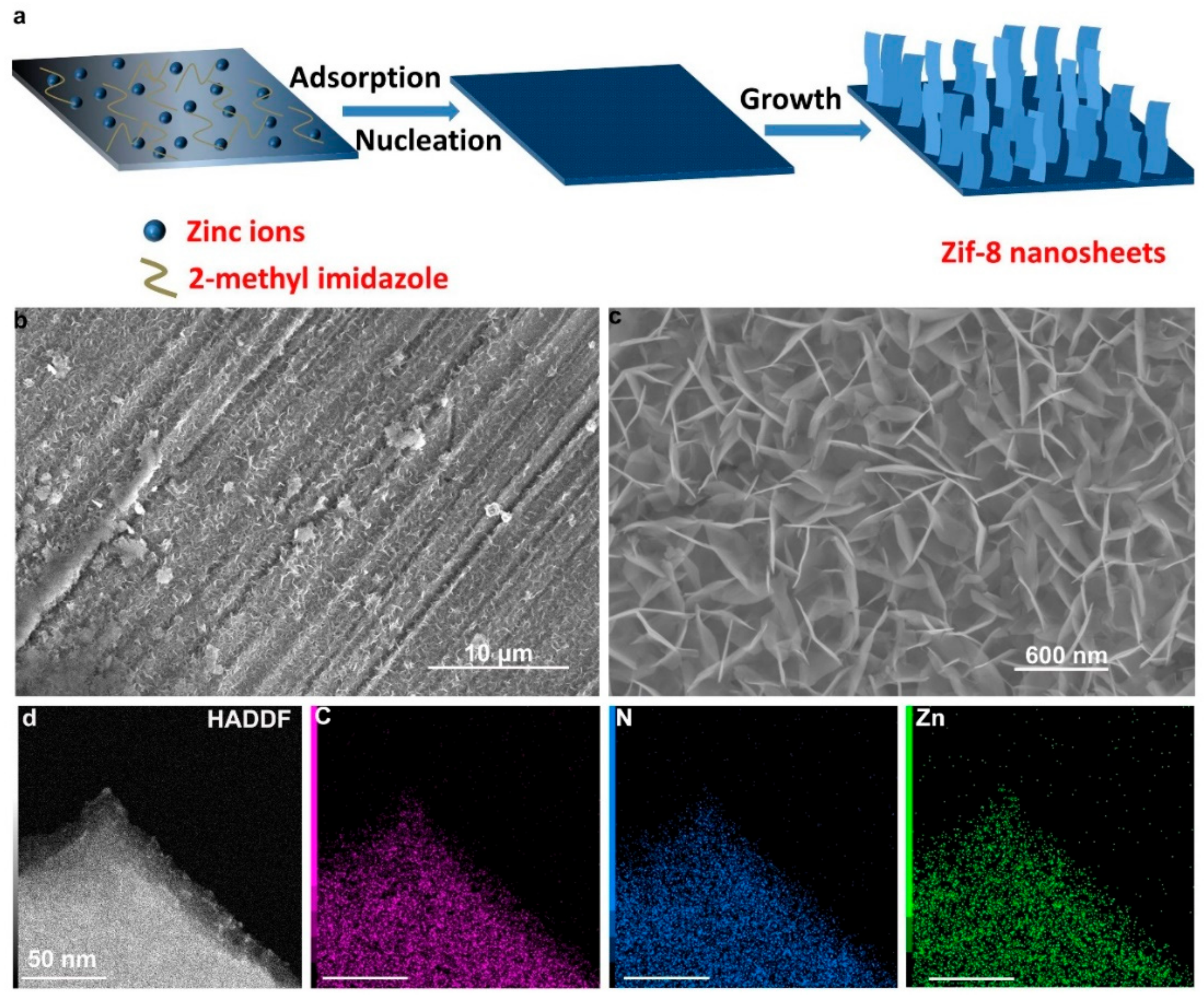
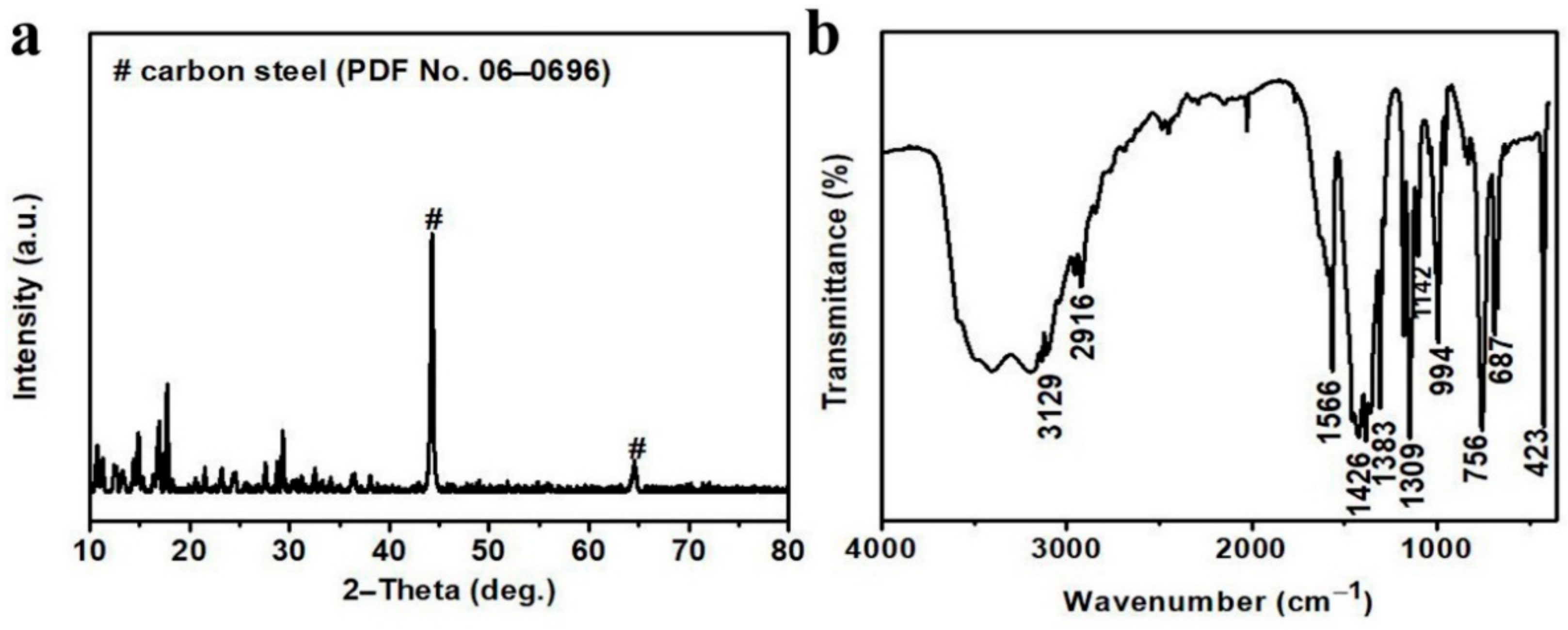
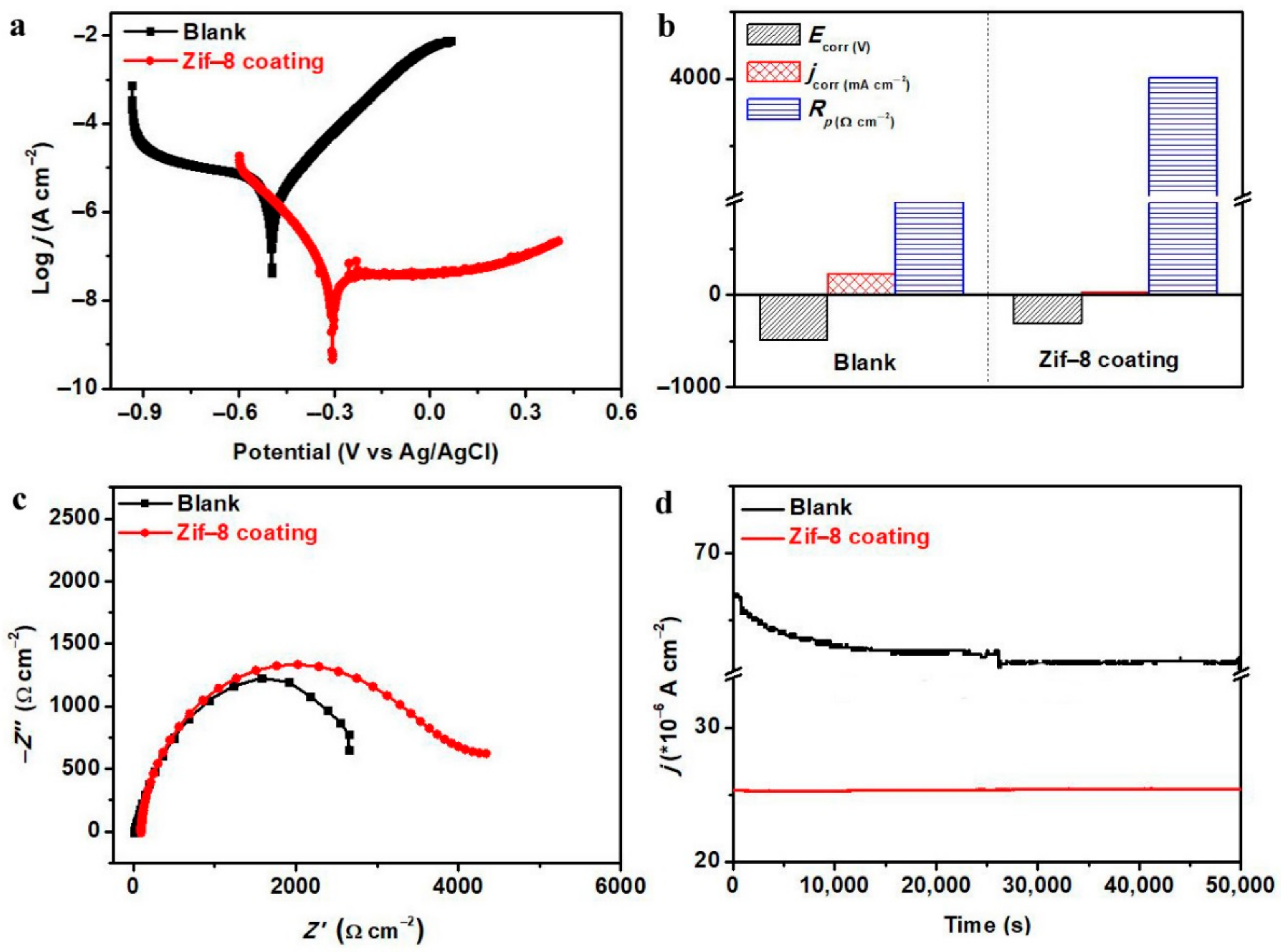
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative invitro Study on Pure Metals (Fe, Mn, Mg, Zn and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, Z.; Tang, C.; Li, W.; You, C.; Chen, M. The mechanical and corrosion resistance of Mg-Zn-Ca-Ag alloys: The influence of Ag content. J. Mater. Res. Technol. 2020, 9, 10863–10875. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Z.; Wang, D.; Wen, Y.; Peng, N.; Shang, W. Zif-8-based micro-arc oxidation composite coatings enhanced the corrosion resistance and superhydrophobicity of a Mg alloy. J. Magnes. Alloy. 2022, in press. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Li, W.; Wang, Q.; Wang, Y.; You, C. Preparation, characteristics and corrosion properties of α-Al2O3 coatings on 10B21 carbon steel by micro-arc oxidation. Surf. Coat. Technol. 2019, 358, 637–645. [Google Scholar] [CrossRef]
- Tian, Y.; Dong, C.; Wang, G.; Cheng, X.; Li, X. Zn–Al–NO2 layered double hydroxide as a controlled-release corrosion inhibitor for steel reinforcements. Mater. Lett. 2019, 236, 517–520. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Zhang, F.; Han, E.-H. Corrosion resistance of layer-by-layer assembled polyvinylpyrrolidone/polyacrylic acid and amorphous silica films on AZ31 magnesium alloys. RSC Adv. 2016, 6, 63107–63116. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Truc, T.A.; Duong, N.T.; Pébère, N.; Olivier, M.-G. Layered double hydroxides as containers of inhibitors in organic coatings for corrosion protection of carbon steel. Prog. Org. Coat. 2012, 74, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Lü, F.; Zhao, S.; Guo, R.; He, J.; Peng, X.; Bao, H.; Fu, J.; Han, L.; Qi, G.; Luo, J.; et al. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media. Nano Energy 2019, 61, 420–427. [Google Scholar] [CrossRef]
- Zheng, J.; Cui, X.; Yang, Q.; Ren, Q.; Yang, Y.; Xing, H. Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage. Chem. Eng. J. 2018, 354, 1075–1082. [Google Scholar] [CrossRef]
- Torad, N.L.; Li, Y.; Ishihara, S.; Ariga, K.; Kamachi, Y.; Lian, H.-Y.; Hamoudi, H.; Sakka, Y.; Chaikittisilp, W.; Wu, K.C.W.; et al. MOF-derived Nanoporous Carbon as Intracellular Drug Delivery Carriers. Chem. Lett. 2014, 43, 717–719. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhuang, W.; Chen, Y.; Wang, Z.; Hernandez, B.V.; Wu, J.; Yang, P.; Liu, D.; Zhu, C.; Ying, H.; et al. Nano-Biocatalysts of Cyt c@ZIF-8/GO Composites with High Recyclability via a de Novo Approach. ACS Appl. Mater. Interfaces 2018, 10, 16066–16076. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, J.; Qin, Y.; Yang, H.; Zhang, S.; Liu, X.; Luo, J. Hollow CoFe-layered double hydroxide polyhedrons for highly efficient CO2 electrolysis. Sci. China Mater. 2022, 65, 536–542. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.-C.; Wu, Y.; Shu, Y.; Gong, X.; Ke, F.-S.; Deng, H. Metal–Organic Frameworks for High Charge–Discharge Rates in Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2018, 57, 3916–3921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Dong, Y.; Yuan, Y.; Zhou, X.; Liu, Y.; Meng, X. Recent advances of metal–organic frameworks in corrosion protection: From synthesis to applications. Chem. Eng. J. 2022, 430, 132823. [Google Scholar] [CrossRef]
- Yu, K.; Tan, X.; Hu, Y.; Chen, F.; Li, S. Microstructure effects on the electrochemical corrosion properties of Mg>—4.1% Ga—2.2% Hg alloy as the anode for seawater-activated batteries. Corros. Sci. 2011, 53, 2035–2040. [Google Scholar] [CrossRef]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal–organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cheng, Q.; Li, W.; Li, Y.; Yang, C.; Tao, K.; Han, L. Construction of 2D ZIF-derived hierarchical and hollow NiCo-LDH “nanosheet-on-nanosheet” arrays on reduced graphene oxide/Ni foam for boosted electrochemical energy storage. J. Alloys Compd. 2021, 850, 156864. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Liang, C.; Pan, A.; Zhang, C.; Tang, Y.; Tan, X.; Liu, J.; Liang, S. MOFs nanosheets derived porous metal oxide-coated three-dimensional substrates for lithium-ion battery applications. Nano Energy 2016, 26, 57–65. [Google Scholar] [CrossRef]
- Kamil, M.P.; Kim, M.J.; Ko, Y.G. Direct electro-co-deposition of Ni-reduced graphene oxide composite coating for anti-corrosion application. Mater. Lett. 2020, 273, 127911. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, Q.; Qi, G. Facile In Situ Growth of Zif-8 Nanosheets with Enhanced Anti-Corrosion Performance for Carbon Steel in Seawater. Coatings 2022, 12, 318. https://doi.org/10.3390/coatings12030318
Zhang Z, Liu Q, Qi G. Facile In Situ Growth of Zif-8 Nanosheets with Enhanced Anti-Corrosion Performance for Carbon Steel in Seawater. Coatings. 2022; 12(3):318. https://doi.org/10.3390/coatings12030318
Chicago/Turabian StyleZhang, Zhishu, Qingjian Liu, and Gaocan Qi. 2022. "Facile In Situ Growth of Zif-8 Nanosheets with Enhanced Anti-Corrosion Performance for Carbon Steel in Seawater" Coatings 12, no. 3: 318. https://doi.org/10.3390/coatings12030318
APA StyleZhang, Z., Liu, Q., & Qi, G. (2022). Facile In Situ Growth of Zif-8 Nanosheets with Enhanced Anti-Corrosion Performance for Carbon Steel in Seawater. Coatings, 12(3), 318. https://doi.org/10.3390/coatings12030318




