Effect of Ammonia Addition on the Growth of an AlO(OH) Film during Steam Coating Process
Abstract
1. Introduction
2. Materials and Methods
3. Results
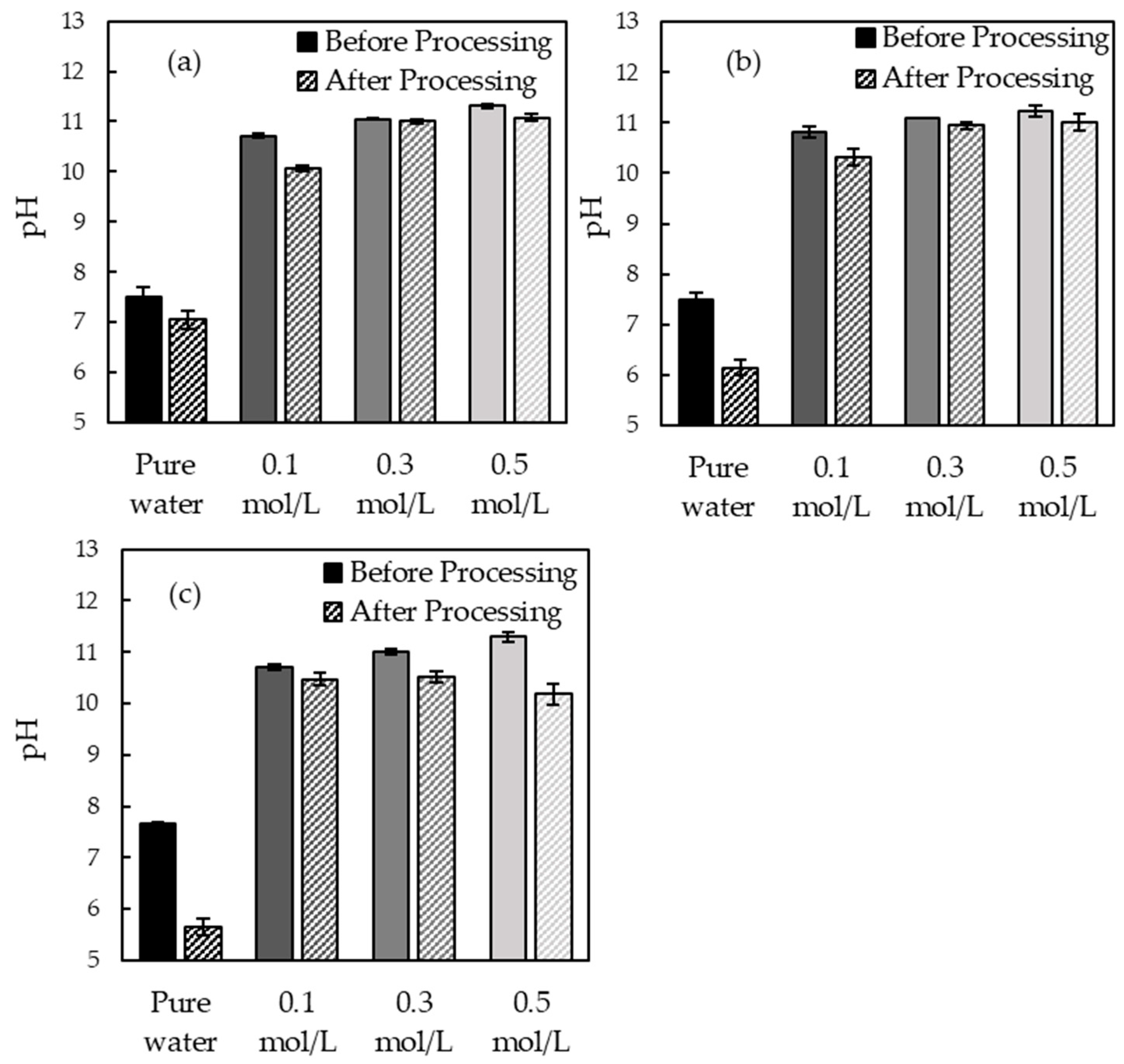
3.1. pH Measurement
3.2. Surface Observation
3.3. Growth Rate of AlO(OH) Film Thickness
4. Discussion
4.1. Relationship between pH and the Surface Morphology
4.2. Relationship between the Surface Morphology of the Film and the Rate Constant, K
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Zhang, J.; Lian, J.; Lei, Y. Welding of aluminum alloy to zinc coated steel by cold metal transfer. Mater. Des. 2013, 49, 602–612. [Google Scholar] [CrossRef]
- Sato, T.; Ogura, T.; Naeba, M. Aluminum Basics. Aluminum Compendium; Nikkan Kogyo Shimbun: Tokyo, Japan, 2018; pp. 10–13. [Google Scholar]
- Lloyd, D. Recent developments in controlling the architecture for property optimization in Al-based materials. Scr. Mater. 2013, 68, 13–16. [Google Scholar] [CrossRef]
- Serizawa, A.; Sato, T.; Miller, M. Effect of cold rolling on the formation and distribution of nanoclusters during pre-aging in an Al–Mg–Si alloy. Mater. Sci. Eng. A 2013, 561, 492–497. [Google Scholar] [CrossRef]
- Zhang, X.H.; Su, G.C.; Ju, C.W.; Wang, W.C.; Yan, W.L. Effect of modification treatment on the microstructure and mechanical properties of Al–0.35%Mg–7.0%Si cast alloy. Mater. Des. 2010, 31, 4408–4413. [Google Scholar] [CrossRef]
- Karabay, S. Modification of AA-6201 alloy for manufacturing of high conductivity and extra high conductivity wires with property of high tensile stress after artificial aging heat treatment for all-aluminum alloy conductors. Mater. Des. 2006, 27, 821–832. [Google Scholar] [CrossRef]
- Dutta, I.; Allen, S.M. A calorimetric study of precipitation in commercial aluminum alloy 6061. J. Mater. Sci. Lett. 1991, 10, 323–326. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al–Mg–Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-precipitate clusters and precipitation processes in Al–Mg–Si alloy. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Esmaeili, S.; Wang, X.; Lloyd, D.J.; Poole, W.J. On the precipitation-hardening behavior of the Al-Mg-Si-Cu alloy AA6111. Met. Mater. Trans. A 2003, 34, 751–763. [Google Scholar]
- Serizawa, A.; Hirosawa, S.; Sato, T. Three-Dimensional Atom Probe Characterization of Nanoclusters Responsible for Multistep Aging Behavior of an Al-Mg-Si Alloy. Met. Mater. Trans. A 2008, 39, 243–251. [Google Scholar] [CrossRef]
- Serizawa, A.; Sato, T.; Poole, W.J. The characterization of dislocation–nanocluster interactions in Al–Mg–Si(–Cu/Ag) alloys. Philos. Mag. Lett. 2010, 90, 279–287. [Google Scholar] [CrossRef]
- Uchiyama, T.; Isoyama, E.; Otsuka, T. Surface treatment of aluminum. J. JILM 1980, 30, 592–605. [Google Scholar] [CrossRef]
- Takahashi, H. Structure and property of oxide films formed on aluminum. J. Surf. Sci. Soc. Jpn. 1988, 9, 720–726. [Google Scholar] [CrossRef][Green Version]
- Evertsson, J.; Bertram, F.; Zhang, F.; Rullik, L.; Merte, L.; Shipilin, M.; Soldemo, M.; Ahmadi, S.; Vinogradov, N.; Carlà, F.; et al. The thickness of native oxides on aluminum alloys and single crystals. Appl. Surf. Sci. 2015, 349, 826–832. [Google Scholar] [CrossRef]
- Woo, L.; Ran, J.; Kornelius, N. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar]
- Chung, C.K.; Tsai, C.H.; Hsu, C.R.; Kuo, E.H.; Chen, Y.; Chung, I.C. Impurity and temperature enhanced growth behaviour of anodic aluminum oxide from AA5052 Al-Mg alloy using hybrid pulse anodization at room temperature. Corros. Sci. 2017, 125, 40–47. [Google Scholar] [CrossRef]
- Liu-Ho, C.; Chun-Chin, C.; Chih-Fu, Y. Improvement of corrosion properties in an aluminum-sprayed AX31 magnesium alloy by a post-hot pressing and anodizing treatment. Surf. Coat. Tech. 2005, 191, 181–187. [Google Scholar]
- Poznyak, A.; Pligovka, A.; Turavets, U.; Norek, M. On-Aluminum and Barrier Anodic Oxide: Meeting the Challenges of Chemical Dissolution Rate in Various Acids and Solutions. Coatings 2020, 10, 875. [Google Scholar] [CrossRef]
- Zahra, S.; Ali, D. Uniform nucleation of zincate layer through the optimized etching process to prevent failure in electroless plating on 2024 aluminum alloy. Eng. Fail. Anal. 2021, 124, 1–13. [Google Scholar]
- Kania, H.; Saternus, M.; Kudláček, J.; Svoboda, J. Microstructure Characterization and Corrosion Resistance of Zinc Coating Obtained in a Zn-AlNiBi Galvanizing Bath. Coatings 2020, 10, 758. [Google Scholar] [CrossRef]
- Twite, R.; Bierwagen, G. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Santa Coloma, P.; Fernández-Carretero, F.J.; Brusciotti, F.; Brizuela, M. Design of Corrosion Protective and Antistatic Hybrid Sol-Gel Coatings on 6XXX AlMgSi Alloys for Aerospace Application. Coatings 2020, 10, 441. [Google Scholar] [CrossRef]
- Aristia, G.; Hoa, L.Q.; Nofz, M.; Sojref, R.; Bäßler, R. Study of Al2O3 Sol-Gel Coatings on X20Cr13 in Artificial North German Basin Geothermal Water at 150 °C. Coatings 2021, 11, 526. [Google Scholar] [CrossRef]
- Yabu, T.; Higa, H.; Doi, M.; Tanabe, H.; Chiba, K. Investigation of Facilities for Industrial Waste Treatment in Aircraft Maintenance. Kawasaki Tech. Rep. 1991, 10, 112–117. [Google Scholar]
- Sugawara, H. Ecofriendly Surface Treatment for Automobile Parts. J. Surf. Finish. Soc. Jpn. 2006, 57, 831–836. [Google Scholar] [CrossRef]
- Sagawa, K. Japan’s Implementation of SDGs, focusing on Material Cycles and Waste Management. JSMCWM 2017, 28, 403–411. [Google Scholar] [CrossRef][Green Version]
- Serizawa, A.; Oda, T.; Watanabe, K.; Mori, K.; Yokomizo, T.; Ishizaki, T. Formation of Anticorrosive Film for Suppressing Pitting Corrosion on Al-Mg-Si Alloy by Steam Coating. Coatings 2018, 8, 23. [Google Scholar] [CrossRef]
- Li, H.; Takata, N.; Kobashi, M.; Serizawa, A. In Situ Scanning Electron Microscopy Observation of Crack Initiation and Propagation in Hydroxide Films Formed by Steam Coating on Aluminum-Alloy Sheets. Materials 2020, 13, 1238. [Google Scholar] [CrossRef]
- Misono, M.; Saito, Y. Catalytic Chemistry; How Is the Rate of a Catalytic Reaction Determined; Maruzen: Tokyo, Japan, 2009; pp. 1–2. [Google Scholar]
- Yamada, H.; Okamoto, T. Composition and structure of boehmite films. J. Jpn. Inst. Light Met. 1971, 21, 535–542. [Google Scholar] [CrossRef]
- Manasa, R.L.; Alka, M. Current perspectives of anoxic ammonia removal and blending of partial nitrifying and denitrifying bacteria for ammonia reduction in wastewater treatment. J. Water Process. Eng. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Chen, P. The impact of alkali and alkaline earth metals on green ammonia synthesis. Chem 2021, 7, 3203–3220. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Innocenti, M.; Lavacchi, A. Measuring the Thickness of Metal Coatings: A Review of the Methods. Coatings 2020, 10, 1211. [Google Scholar] [CrossRef]
- Raybaud, P.; Digne, M.; Iftimie, R.; Wellens, W.; Euzen, P.; Toulhoat, H. Morphology and Surface Properties of Boehmite (γ-AlOOH): A Density Functional Theory Study. J. Catal. 2001, 201, 236–246. [Google Scholar] [CrossRef]
- Takata, N.; Li, H.; Kobashi, M.; Shimada, Y.; Serizawa, A.; Ishizaki, T. Transmission electron microscopic observation of layered double hydroxide films formed on aluminum alloys prepared by steam coating process. Tetsu-To-Hagane/ISIJ 2019, 105, 177–182. [Google Scholar] [CrossRef]
- Morioka, S. Growth of aluminum oxide film. Corros. Eng. 1966, 15, 481–485. [Google Scholar]
- Uchiyama, H. Morphological control of metal oxides via crystal growth in a solution. Rikogakkai Kansai Univ. Eng. Technol. 2010, 17, 63–67. [Google Scholar]
- Tsuruta, T. Calculation and Measurement of pH at Elevated Temperatures. Corros. Eng. Dig. 1985, 34, 135–139. [Google Scholar] [CrossRef][Green Version]
- Kudoh, M. Current Researches on the Formation of Solidification Microstructure in Alloys. Tetsu-to-Hagane/ISIJ 2002, 88, 229–235. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Cui, W.; Page, K.L.; Pearce, C.I.; Bowden, M.E.; Graham, T.R.; Shen, Z.; Li, P.; Wang, Z.; Kerisit, S.; et al. Size and Morphology Controlled Synthesis of Boehmite Nanoplates and Crystal Growth Mechanisms. Cryst. Growth Des. 2018, 18, 6, 3596–3606. [Google Scholar] [CrossRef]
- Corbató, C.E.; Tettenhorst, R.T.; Christoph, G.G. Structure Refinement of Deuterated Boehmite. Clays Clay Miner. 1985, 33, 71–75. [Google Scholar] [CrossRef]
- Luo, Y.; Deng, Y.; Guan, L.; Ye, L.; Guo, X.; Luo, A. Effect of grain size and crystal orientation on the corrosion behavior of as-extruded Mg-6Gd-2Y-0.2Zr alloy. Corros Sci. 2020, 164, 108338. [Google Scholar] [CrossRef]









| Mg | Si | Cu | Mn | Fe | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|
| 0.59 | 0.96 | 0.01 | 0.05 | 0.18 | 0.04 | 0.01 | 0.02 | Bal. |
| Processing Time | Steam Source | Before Processing | After Processing |
|---|---|---|---|
| 8 h | Pure Water | 0.20 | 0.18 |
| 0.1 mol/L | 0.040 | 0.060 | |
| 0.3 mol/L | 0.010 | 0.045 | |
| 0.5 mol/L | 0.050 | 0.070 | |
| 16 h | Pure Water | 0.14 | 0.15 |
| 0.1 mol/L | 0.11 | 0.16 | |
| 0.3 mol/L | 0.0 | 0.070 | |
| 0.5 mol/L | 0.11 | 0.18 | |
| 24 h | Pure Water | 0.050 | 0.16 |
| 0.1 mol/L | 0.050 | 0.12 | |
| 0.3 mol/L | 0.060 | 0.13 | |
| 0.5 mol/L | 0.10 | 0.20 |
| Treatment Time | Pure Water | Ammonia | ||
|---|---|---|---|---|
| 0.1 mol/L | 0.2 mol/L | 0.5 mol/L | ||
| 8 h | 0.021 | 0.42 | 0.061 | 0.15 |
| 16 h | 0.095 | 0.43 | 0.11 | 0.11 |
| 24 h | 0.068 | 0.14 | 0.11 | 0.084 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itano, N.; Lee, S.Y.; Serizawa, A. Effect of Ammonia Addition on the Growth of an AlO(OH) Film during Steam Coating Process. Coatings 2022, 12, 262. https://doi.org/10.3390/coatings12020262
Itano N, Lee SY, Serizawa A. Effect of Ammonia Addition on the Growth of an AlO(OH) Film during Steam Coating Process. Coatings. 2022; 12(2):262. https://doi.org/10.3390/coatings12020262
Chicago/Turabian StyleItano, Naotaka, So Yoon Lee, and Ai Serizawa. 2022. "Effect of Ammonia Addition on the Growth of an AlO(OH) Film during Steam Coating Process" Coatings 12, no. 2: 262. https://doi.org/10.3390/coatings12020262
APA StyleItano, N., Lee, S. Y., & Serizawa, A. (2022). Effect of Ammonia Addition on the Growth of an AlO(OH) Film during Steam Coating Process. Coatings, 12(2), 262. https://doi.org/10.3390/coatings12020262







