Abstract
In this study, novel magnetic poly(glycidyl methacrylate) (PGMA) microspheres with grafted polypyrrole chains (magnetic PGMA-g-PPy) were developed for the high-capacity adsorption of Congo red (CR) from aqueous solutions. The magnetic PGMA-g-PPy was synthesized by the typical dispersion polymerization method and the ring-opening reaction of epoxy groups, producing abundant hydroxyls for the grafting polymerization of pyrrole in the presence of FeCl3 as an oxidizing agent on the surface of the microspheres. The characterization results showed that magnetic PGMA-g-PPy was successfully fabricated. The adsorption equilibrium data of the adsorbents could be well fitted by the Langmuir isotherm model, showing a high maximum adsorption capacity of 502.5 mg/g for CR. The adsorption followed pseudo-second-order kinetics with a fast speed. The adsorbents had no leaching of Fe in the solution at pH 1.0–11.0 for 24 h. The adsorption process was strongly pH-dependent and weakly ionic-strength-dependent. Furthermore, the magnetic microspheres could be easily regenerated, rapidly separated from the solution, and reused for wastewater treatment. The results suggest that magnetic PGMA-g-PPy microspheres are a promising efficient adsorbent for the removal of CR from wastewater.
1. Introduction
Dyes are used in many industries, including food, paper and plastics, cosmetics, leather, textiles, dyestuffs, etc. A great volume of colored wastewater is produced by these industries. The release of this colored wastewater brings a great threat to the environment, because many of the dyes are toxic or strongly carcinogenic [1,2]. These dyes must be removed from their wastewater solutions before their discharge. Among the various techniques developed for dye removal from wastewater, such as flocculation [3], electrochemical treatment [4], photocatalytic oxidation [5,6,7], adsorption [8,9,10,11], and membrane separation [12], adsorption is reviewed as an effective, economical, and promising technology.
Some conventional adsorbents, including activated carbon [13], clay minerals [14], oxides [15], and bio-adsorbent [16,17], have been developed to remove these dyes from their wastewater solutions. However, the difficulty of separating dyes from aqueous solutions has limited their applications in real wastewater cleanup. In recent years, magnetic-loaded adsorbent particles based on Fe3O4 nanoparticles have obtained special attention in wastewater treatment [18,19,20,21,22,23,24,25], due to their easy recovery from the solutions by a simple, low-cost external magnetic field. Various magnetic sorbents [26,27], which are designed by making surface modifications to some special functional groups to target dyes, have been developed to treat the corresponding dyes’ wastewater. However, the majority of the magnetic adsorbents developed show relatively low adsorption capacities for objective dyes [28,29,30], and the stability of Fe3O4 nanoparticles against acidic erosion needs to be improved to increase their service time and applications in acidic conditions [31].
Congo red (CR) is a benzidine-based anionic disazo dye, with two azo groups as well as the benzidine group (a human carcinogen). It is toxic to many organisms due to a suspected carcinogen and mutagen. It is still widely used in several countries, although it is banned in many countries because of health concerns. The magnetic adsorbents developed for CR are few, and their adsorption capacity is not high.
In this work, one kind of novel magnetic microsphere with a high adsorption capacity and good acid resistance was developed for CR. Polypyrrole (PPy) chains have a high density of pyrrole functional groups, providing positively charged nitrogen atoms in the polymeric backbone available for anionic CR adsorption [32]. Therefore, this polymer chain was selected to increase the adsorption capacity by grafting it onto the surface of the magnetic microspheres. Poly (glycidyl methacrylate) (PGMA) with affluent high-reactivity epoxy groups were selected as protective substances. The developed adsorbents were characterized by XRD, VSM, FTIR, and SEM. The applicability of these particles to CR removal was evaluated based on the sorption capacity, acidic resistance, adsorption kinetic, effects of solution conditions, and renewability.
2. Experiments
2.1. Materials
FeCl2·4H2O and FeCl3·6H2O were procured from Xilong Chemical Co., Ltd. (Guangzhou, China). Polyethylene glycol 6000 (PEG 6000) was purchased from Its Group Chemical Reagent Co., Ltd. (Shanghai, China). Polyvinylpyrrolidone (PVP K-30), pyrrole, glycidyl methacrylate (GMA), ethylene glycol dimethacrylate (EGDMA), and 2,2′-azobisisobutyronitrile (AIBN) were obtained from TCI (Shanghai) Development Co., Ltd. (Shanghai, China). CR was purchased from Wenjiang Chemical Co., Ltd. (Shaoguan, China). All the reagents were analytical grade.
2.2. Preparation of Polypyrrole-Grafted Magnetic PGMA Microspheres
The synthetic process of creating magnetic PGMA microspheres with grafted polypyrrole (magnetic PGMA-g-PPy) is shown in Scheme 1.
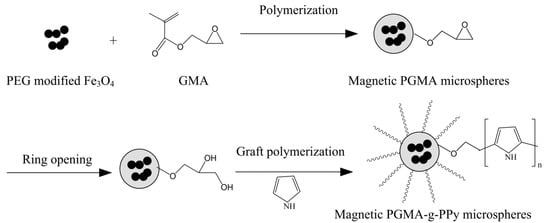
Scheme 1.
Preparation route of magnetic PGMA-g-PPy microspheres.
2.2.1. Preparation of Magnetic PGMA Microspheres
The magnetic PGMA microspheres were synthesized by our previous dispersion polymerization method with some modifications [33]. First, PEG-stabilized magnetic nanoparticles were prepared with a co-precipitation method. Amounts of 11.8 g of FeCl3·6H2O and 4.3 g of FeCl2·4H2O were dissolved in 200 mL water and heated to 80 °C under vigorous stirring with a continuous supply of nitrogen gas. An amount of 25 mL of ammonium hydroxide (25%) was quickly added into the above solution. After about 2 min, 50 mL of a solution with 15 g dissolved PEG6000 was rapidly added. The reaction was permitted to continue for 2 h, and the obtained black precipitates were washed several times with deionized water to remove unreacted PEG. Then, the magnetic PGMA microspheres were synthesized by the typical dispersion polymerization. In this method, GMA was selected as monomer, AIBN as initiator, PEG-stabilized magnetic nanoparticles as magnetic core, and PVP-K30 as stabilizer. Specifically, 2.5 g of PVP-K30 was fully mixed with an ethanol/water dispersion solution (80 mL of ethanol and 9 mL of water) in a 250 mL three-necked flask, then 2.5 g PEG-Fe3O4 was sonicated with the above solution for 20 min to be fully dispersed. Then, 12 mL of GMA, 100 uL of EGDMA, and 0.25 g of AIBN were successively added into the above dispersion medium. The mixing solution was purged under continuous nitrogen for 30 min to remove dissolved O2. The polymerization was maintained at 70 °C for 24 h under mechanical stirring (240 rpm) with good shaft seal and reflux condensation. The resulting magnetic PGMA microspheres were centrifuged (5000 r/min) and washed several times with hot deionized water and ethanol.
2.2.2. Grafting Polymerization of Pyrrole on the Magnetic PGMA Microspheres
Abundant hydroxyls on the surface of the magnetic PGMA microspheres were produced by the ring-opening reaction of epoxy groups. The above synthesized microspheres were dispersed in 200 mL H2SO4 (0.2 mol/L) and reacted at 40 °C for 48 h under mechanical stirring (200 rpm). The as-prepared product was washed several times with deionized water.
Then, grafting polymerization of pyrrole onto the microspheres was carried out using FeCl3 as oxidizer. An amount of 1.2 g obtained magnetic microspheres was dispersed in 200 mL deionized water in a conical flask by ultrasonic treatment for 15 min. Then, 18 g of FeCl3 and 2.4 mL of pyrrole were added and well dissolved. The polymerization reaction was permitted for 3 h at room temperature in the shaker with 200 r/min speed. The resulting product was collected with a magnetic field, then washed to neutral with deionized water. Finally, the product was dried in a vacuum freeze drier.
2.3. Characterization
The FT-IR spectra were measured on an FT-IR spectrophotometer (FT-IR, Thermo Nicolet 380, Madison, WI, USA) between 4000 and 500 cm−1 by the KBr pellet technique, and the resolution was 2 cm−1. The X-ray diffraction (XRD) characterizations were carried out on a D/max2550 18 KW rotating anode X-ray diffractometer (XRD, D/MAX-2500/PC, Tokyo, Japan). Their morphology and size were recorded using scanning electron microscopy (SEM, JSM-6610 LV, Tokyo, Japan). Their magnetic properties were determined by vibrating sampler magnetometer (VSM, Model 4 HF VSM, ADE Technologies, Westwood, MA, USA).
2.4. Dye-Removal Experiments
In a typical removal experiment, 28.5 mg of the magnetic PGMA-g-PPy microspheres were added into a centrifuge tube with a known concentration of CR solution; the mixture solution was vibrated under room temperature for 80 min in a reciprocating shaking-table with 200 r/min reaching the adsorption equilibrium. The phase separation was carried out by an external magnet. Then, the residual CR in the solution was determined with a UV-visible spectrophotometer (UV-4802s, Shanghai Instrument Co., Ltd., Shanghai, China). All experiments were repeated three times. The equilibrium adsorption capacity (qe, mg/g) was calculated according to the following equation:
where C0 (mg/L) and Ce (mg/L) are the initial and equilibrium CR concentrations in the aqueous solution after adsorption, respectively; V (L) represents the volume of the solution; and m (g) represents the mass of the adsorbent.
qe = (C0 − Ce) × V/m
2.5. Regeneration and Reusability Experiments
Regeneration experiments were performed for the dye-loaded magnetic PGMA-g-PPy recovered by magnetic separation, which were shaken in 6 mL of 500 mg/L CR solution at room temperature for 1 h. These microspheres were eluted twice using 6 mL of 0.01 mol/L NaOH solution for 1 h with a shaking speed of 200 rpm at every turn. After desorption, these magnetic microspheres were washed with distilled water three times and reused for the next run. In total, five sequential adsorption–desorption cycles were carried out with CR concentration determined for each cycle by UV-visible spectrophotometer.
3. Results and Discussions
3.1. Characterization and Synthesis Process of the Adsorbent
The magnetic PGMA-g-PPy was synthesized by the following three steps (shown in Scheme 1): firstly, the magnetic PGMA microspheres were prepared by the dispersion polymerization method, using GMA as the monomer, PEG-stabilized magnetic nanoparticles as the magnetic core, AIBN as the initiator, PVP-K30 as the stabilizer, and an ethanol aqueous solution as the dispersion medium; then, the abundant hydroxyls on the surface of the as-prepared microspheres were produced by the ring-opening reaction of epoxy groups under the H2SO4 (0.2 mol/L) solution, to provide enough for the reactive group grafting with pyrrole; finally, the polypyrrole chains were grafted onto the resultant microspheres by a polymerization reaction, using pyrrole as the monomer and FeCl3 as a common oxidizer in the aqueous solution.
The SEM image of magnetic PGMA-g-PPy is shown in Figure 1. The magnetic microspheres had a standard spherical form, and the particle diameter was approximately 36.5 µm.
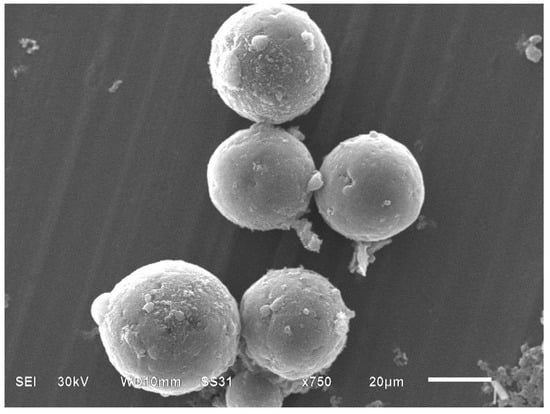
Figure 1.
SEM image of magnetic PGMA-g-PPy microspheres.
An FT-IR spectra characterization of the Fe3O4, magnetic PGMA, and magnetic PGMA-g-PPy was performed. As shown in Figure 2, the appearance of the same Fe-O adsorption band at 574 cm−1 in Figure 2B,C as in Fe3O4 (Figure 2A) indicated that the Fe3O4 nanoparticles were encapsulated into the PGMA microspheres and PGMA-g-PPy microspheres. The character band at 1726 cm−1 corresponded to the C = O vibration, and the bands at 842 and 904 cm−1 in Figure 2B were assigned to the epoxy groups. These results confirmed the presence of PGMA moieties on the magnetic PGMA microspheres. After the grafting reaction on the PGMA microspheres, the bands at 842 and 904 cm−1 disappeared, and new characteristic peaks appeared at 1537, 1443, 1038, and 948–855 cm−1 in Figure 2C. These variations were attributed to pyrrole ring stretching, conjugated C–N stretching, C–H stretching vibrations, and C–H deformation, respectively [34]. The above results proved that the PPy was successfully grafted onto the magnetic PGMA microspheres.
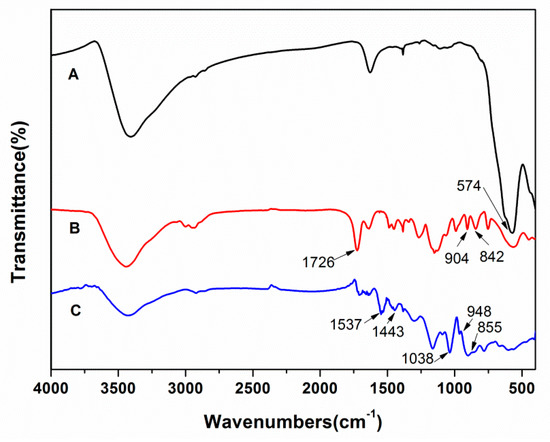
Figure 2.
FT-IR spectrum of Fe3O4 (A), magnetic PGMA (B), and magnetic PGMA-g-PPy (C).
The peaks’ positions and relative intensities in the XRD patterns of magnetic PGMA and PGMA-g-PPy are given in Figure 3. The diffraction peaks at 30.2°(220), 35.6°(311), 43.3°(400), 53.6°(422), 57.3°(511), and 62.8°(440) in both microspheres were consistent with the standard pattern for crystalline magnetic Fe3O4, indicating that Fe3O4 nanoparticles had been successfully encapsulated in these microspheres. The stronger broad diffraction peaks in magnetic PGMA-g-PPy than in magnetic PGMA also suggested that there were more amorphous polymer materials in the former than in the latter.
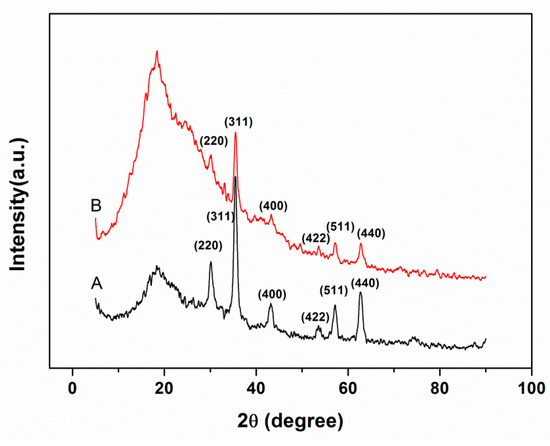
Figure 3.
XRD patterns of magnetic PGMA (A) and magnetic PGMA-g-PPy (B).
The magnetic property of the magnetic PGMA-g-PPy microspheres was analyzed by VSM at room temperature. The magnetization curve in Figure 4 showed that the saturated magnetization value of magnetic microspheres was 8.2 emu/g, which made these adsorbents become separated from the solution by a common permanent magnet in 30 s. Although the saturated magnetization value of these microspheres was obviously below that of the pure Fe3O4 nanoparticles due to the thick polymer coating, they could be usually be recovered from the solution more rapidly than Fe3O4 nanoparticles. This is because single magnetic microspheres with the encapsulation of many Fe3O4 nanoparticles received the resultant magnetic forces of these interior nanoparticles under an external magnetic field. The zero coercivity and reversible hysteresis behaviors also indicated that these microspheres were superparamagnetic. These magnetic features provided the easy re-dispersion and efficient recovery of the magnetic microspheres from the aqueous solution.
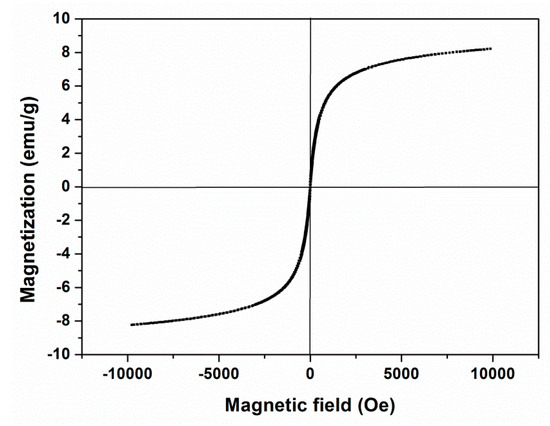
Figure 4.
Magnetization curves of magnetic PGMA-g-PPy microspheres.
3.2. Material Stability
In order to examine the stability of magnetic PGMA-g-PPy, the microspheres were dispersed into aqueous solutions within a pH range of 1.0–11.0 for 24 h with continuous vibration in a table concentrator, and the leaking of iron ions into the supernatant was quantified by atomic adsorption spectrometry. No iron in the supernatant was detected in the investigated pH range, suggesting that the developed adsorbents were highly stable in strongly acidic conditions for a long time. Their acidic resistance was obviously better than magnetic nanoparticles created by organic surface coating, with a leaching of 1.7–3.7% Fe at pH 4.0–1.0 for 6 h [35]; magnetite nanoparticles created by poly (glutamic acid) coating, with a leaching of 0.001–0.9% Fe at pH 3.0–1.0 for 2 h [31]; and magnetic particles created by thin-polystyrene-chain coating, with a leaching of 0.3–3% Fe in acidic conditions [36]. This is attributed to the thick, tight polymer-network protective coating provided by the PGMA polymer cross-linking with the cross-linking agent.
3.3. Adsorption Isotherms
Adsorption isotherm analysis was critical to assess the adsorption performance of the magnetic PGMA-g-PPy adsorbent. The equilibrium adsorption of CR on this adsorbent at room temperature and pH 2.0 shown in Figure 5 were described by the Langmuir and Freundlich adsorption isotherm models.
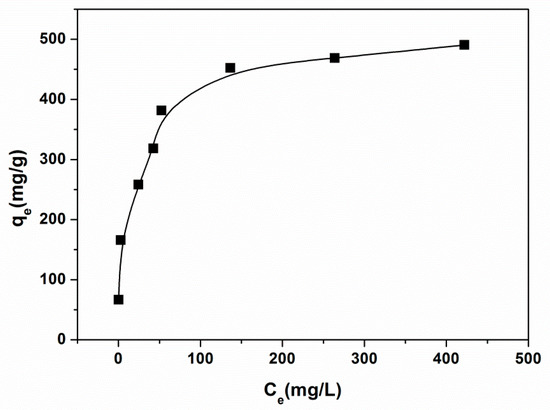
Figure 5.
Adsorption isotherms of CR onto magnetic PGMA-g-PPy at room temperature. (Adsorbent dose, 28.5 mg; volume, 10 mL; pH, 2.0; contact time, 2 h.)
The linear form of the Langmuir equation is represented as follows [37]:
where Ce represents the equilibrium concentration of CR (mg/L); qe (mg/g) is the equilibrium adsorption capacity; qm (mg/g) means the maximum saturated adsorption capacity of the adsorbent for CR; b (L/mg) is the Langmuir adsorption constant. The Freundlich isotherm equation in a linearized form is expressed as follows:
where KF (mg1−(1/n)L1/ng−1) and n correspond to the adsorption capacity and the intensity of the adsorbent, respectively. The values of KF and n are obtained based on the slope and intercept of linear plots of lnqe against lnCe by Equation (3).
Ce/qe = 1/bqm + Ce/qm
lnqe = lnKF + (1/n) ln Ce
The calculated theoretical parameters along with the regression coefficients (R2) by both adsorption models are shown in Table 1. The resulting regression coefficients showed that the adsorption isotherm data could be fitted better by the Langmuir model than by the Freundlich model. Based on the assumptions of the Langmuir model, the adsorption process of CR was mainly monolayer adsorption. It is also found that the maximum adsorption capacity for CR on the magnetic PGMA-g-PPy is 502.5 mg/g. A comparison of the maximum CR adsorption capacity (qm) of this adsorbent with those of other adsorbents in the reported literature is shown in Table 2. It is found that magnetic PGMA-g-PPy showed an apparently higher qm value for CR than the other adsorbents. In terms of available adsorption-specific surface area for CR, the large particle size of magnetic PGMA-g-PPy (36.5 µm) without porous structures was very low among the listed nanoparticles or porous microspheres. The higher adsorption capacity of CR should be a result of the higher density of the surface functional group obtained by grafting PPy chains.

Table 1.
Adsorption isotherm parameters for CR adsorption on magnetic PGMA-g-PPy by the Langmuir and Freundlich models.

Table 2.
Summary of the maximum adsorption capacities of CR onto various magnetic adsorbents.
The essential characteristics in the Langmuir isotherm model can be explained by the equilibrium parameter RL.
where b and C0 are defined as above. The adsorption process can be evaluated by the value of RL (favorable when 0 < RL < 1, linear when RL = 1, unfavorable when RL > 1) [38]. All the values of RL for different C0 in Table 1 were in the range of 0–1, indicating favorable adsorption.
RL = 1/(1 + bC0)
3.4. Adsorption Kinetics
The effect of contact time on the removal of CR by the magnetic PGMA-g-PPy is shown in Figure 6. About 80% of the CR was adsorbed on the adsorbent in 10 min, indicating that the adsorption process was fast. The adsorption equilibrium was reached at about 60 min. The pseudo-first-order equation and the pseudo-second-order equation were further applied to simulate the adsorption kinetics of CR on the adsorbent. The two linearized forms can be expressed as Equations (5) and (6), respectively:
where qt and qe represent the adsorption capacity of the adsorbent at equilibrium and at any time t (min), respectively; k1 (1/min) and k2 (g/mg/min) mean the kinetic rate constants for the pseudo-first-order and the pseudo-second-order models, respectively. The obtained kinetic parameters’ values in the pseudo-first-order model are 45.913 (qe, mg/g) and 0.00814 (k1, 1/min), with R2 of 0.9111, and those in the pseudo-second-order model are 106.9 (qe, mg/g) and 0.00433 (k2, g/mg/min), with R2 of 0.9986. It is obvious that the pseudo-second-order model is better than the pseudo-first-order model. Based on the assumptions of the pseudo-second-order model, it could be deduced that the rate-determining step may be a chemical sorption involving valence forces between the adsorbent and CR by the sharing of electrons [50].
Log (qe − qt) = log qe − (k1/2.303) t
t/qt = 1/k2qe2 + (1/qe) t
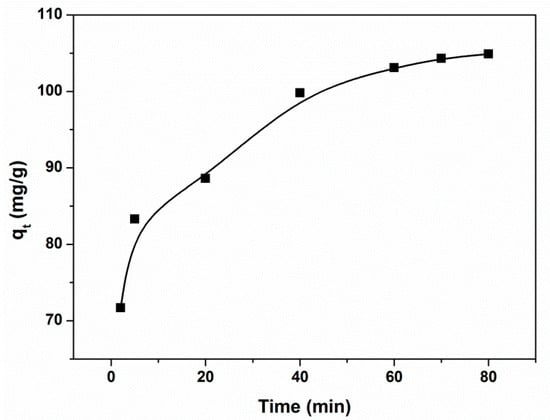
Figure 6.
Adsorption kinetics of CR in aqueous solution on the magnetic PGMA-g-PPy. (Adsorbent dose, 237 mg; volume, 50 mL; initial concentration, 500 mg/L; pH, 7.0.)
3.5. Effect of Initial pH
The effect of pH in the range 2.0–10.0 on the removal efficiency of CR was investigated by adjusting the pH using HCl and NaOH solutions. The results are shown in Figure 7. It is seen that the removal efficiency of CR remarkably increased by decreasing the pH and reached its maximum at pH 2.0, indicating that the removal of CR by this adsorbent was highly dependent on the solution’s pH. A CR molecule with two sulfonic groups generally exists in a soluble CR anion in an aqueous solution. However, the surface charge of magnetic PGMA-g-PPy will vary from positive electricity to negative electricity when the pH increases from 2.0 to 10.0. These variations lead to the electrostatic interaction between the adsorbent and the negatively charged CR changing from attraction to repulsion. Therefore, the removal efficiency of the CR increased with decreasing pH values.
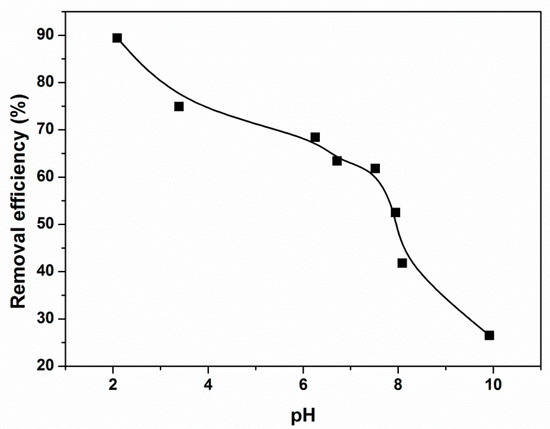
Figure 7.
Effect of pH on the removal of CR by PGMA-g-PPy. (Adsorbent dose, 15 mg; volume, 6 mL; initial concentration, 500 mg/L; contact time, 1 h.)
3.6. Effect of Salt
Co-existing salts influence the adsorption performance of the adsorbent because of their effect on the hydrophobicity, size, and solubility of CR. Thus, it is important to evaluate the effect of salts on the adsorption performance of the adsorbent. The results in Figure 8 show that the increase in the concentration of the salt NaCl had little influence on the CR removal. These results may be attributed to the multiple adsorption mechanisms of the magnetic adsorbent on CR, which make the various effects of the salt on the CR adsorption partially offset.
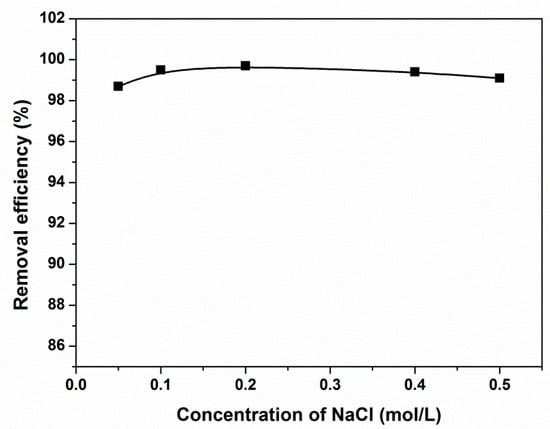
Figure 8.
Effect of ionic strength on the removal of CR by PGMA-g-PPy. (Adsorbent dose, 28.5 mg; volume, 6 mL; initial concentration, 500 mg/L; pH, 7.0; contact time, 1 h.)
3.7. Regeneration and Reusability of the Adsorbent
The reusability of the adsorbent is crucial for its practical application. The recycling experiments were carried out by using 0.01 mol/L NaOH solutions for 2 h with a shaking speed of 200 rpm, which led to a good desorption efficiency of above 95%. The results of five consecutive desorption–adsorptions are shown in Figure 9. After five cycles, above 90% of the adsorption capacity of CR on the adsorbent was retained, suggesting that magnetic PGMA-g-PPy adsorbent showed good regeneration and reusability performances.
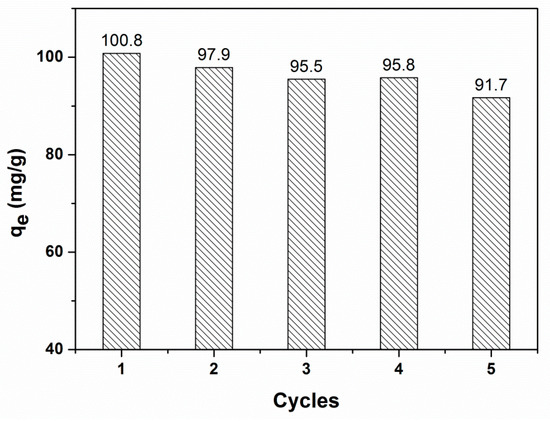
Figure 9.
The adsorption capacity of CR on magnetic PGMA-g-PPy in five successive cycles of desorption–adsorption.
4. Conclusions
In summary, magnetic PGMA-g-PPy microspheres with high adsorption capacity and good acidic resistance were successfully developed. Magnetic microspheres created by grafting PPy showed a high maximum adsorption capacity of 502.5 mg/g for CR. The thick PGMA polymer coating could effectively prevent the Fe3O4 nanoparticles from dissoluting in acidic solutions. The adsorption equilibrium data of the microspheres accorded well with the Langmuir isotherm model. The adsorption followed pseudo-second-order kinetics with an equilibrium adsorption time of about 60 min. The adsorption process was easily affected by the solution’s pH, but little affected by the presence of NaCl. About 90% of the adsorption capacity of the magnetic microspheres was retained after 5 reuses. Furthermore, the magnetic microspheres could be rapidly separated from the solution and reused for wastewater treatment. The results suggest that magnetic PGMA-g-PPy microspheres are an efficient prospective adsorbent for CR removal from wastewater. It is worth mentioning that this adsorption material suffers from some limitations, which would affect its full-scale practical application for CR removal from wastewater, such as acid wastewater, how to lower its cost, and how to make its large-scale synthesis feasible.
Author Contributions
Data curation, J.L.; Formal analysis, Y.Y.; Funding acquisition, J.S. and W.L.; Methodology, J.S.; Project administration, W.L.; Software, Y.Y.; Visualization, J.L.; Writing—original draft, Y.Y. and J.L.; Writing—review & editing, J.S. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2021 Wuhan City College Key Scientific Research Project (2021CYZDKY005), the 2021 Department of Education of Hubei Province Science and Technology Research Project (B2021418), the start-up and incubation funding from Zhongshan Pioneering Park for Overseas Chinese Scholar (Project on Synthesis, Modification and Application of Biopolymeric Ultra-microparticle).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fonovich, T.M. Sudan dyes: Are they dangerous for human health? Drug Chem. Toxicol. 2013, 36, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Gita, S.; Hussan, A.; Choudhury, T. Impact of textile dyes waste on aquatic environments and its treatment. Environ. Ecol. 2017, 35, 2349–2353. [Google Scholar]
- Wang, Y.-F.; Gao, B.-Y.; Yue, Q.-Y.; Wang, Y.; Yang, Z.-L. Removal of acid and direct dye by epichlorohydrin–dimethylamine: Flocculation performance and floc aggregation properties. Bioresour. Technol. 2012, 113, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Srivastava, V.C.; Mall, I.D. Mechanism of dye degradation during electrochemical treatment. J. Phys. Chem. C 2013, 117, 15229–15240. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, C.; Wang, J.; Xiao, G.; Luo, G. Green synthesis of Ag–TiO2 supported on porous glass with enhanced photocatalytic performance for oxidative desulfurization and removal of dyes under visible light. ACS Sustain. Chem. Eng. 2018, 6, 13276–13286. [Google Scholar] [CrossRef]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Değermenci, G.D.; Değermenci, N.; Ayvaoğlu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gu, J.; Meng, H.; Knebel, A.; Caro, J. High-flux membranes based on the covalent organic framework COF-LZU1 for selective dye separation by nanofiltration. Angew. Chem. Int. Ed. 2018, 57, 4083–4087. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Agricultural based activated carbons for the removal of dyes from aqueous solutions: A review. J. Hazard. Mater. 2009, 167, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Potter, N.; Rasmussen, J.; Weng, J.; Lv, G. Removal of rhodamine 6G with different types of clay minerals. Chemosphere 2018, 202, 127–135. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef]
- Chuah, T.G.; Jumasiah, A.; Azni, I.; Katayon, S.; Choong, S.Y.T. Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: An overview. Desalination 2005, 175, 305–316. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, J.; Liu, Z.; Liang, X.; Shang, C. Adsorption of acid dyes from aqueous solutions by the ethylenediamine-modified magnetic chitosan nanoparticles. J. Hazard. Mater. 2011, 185, 1045–1052. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, J.; Pan, B. Fabrication of novel magnetic nanoparticles of multifunctionality for water decontamination. Environ. Sci. Technol. 2016, 50, 881–889. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.-R.; Wang, X.; Li, X.-S.; Wu, M.-X.; Liang, F.; Yang, Y.-W. One-pot solvothermal synthesis of Carboxylatopillar[5]arene-modified Fe3O4 magnetic nanoparticles for ultrafast separation of cationic dyes. Dye Pigment 2019, 162, 512–516. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Reshadi, M.A.M.; Bazargan, A.; McKay, G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci. Total Environ. 2020, 731, 138863. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wang, L.; Zhao, Y.; Bao, W. A review of amino-functionalized magnetic nanoparticles for water treatment: Features and prospects. J. Clean. Prod. 2021, 281, 124668. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Baloch, H.A.; Nizamuddin, S.; Kashi, S.; Tanjung, F.A.; Hossain, N.; Mazari, S.A.; Mubarak, N.; Griffin, G.; Srinivasan, M. Thermal, mechanical, rheological, electrical and electromagnetic interference shielding performance of polypropylene/magnetic carbon nanocomposites. J. Environ. Chem. Eng. 2021, 9, 105447. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Amirian, J.; Rezaei, S.; Sharma, G. Polyamine-modified magnetic graphene oxide surface: Feasible adsorbent for removal of dyes. J. Mol. Liq. 2019, 289, 111118. [Google Scholar] [CrossRef]
- Huo, Y.; Wu, H.; Wang, Z.; Wang, F.; Liu, Y.; Feng, Y.; Zhao, Y. Preparation of core/shell nanocomposite adsorbents based on amine polymer-modified magnetic materials for the efficient adsorption of anionic dyes. Colloid. Surface A 2018, 549, 174–183. [Google Scholar] [CrossRef]
- Hu, L.; Guang, C.; Liu, Y.; Su, Z.; Gong, S.; Yao, Y.; Wang, Y. Adsorption behavior of dyes from an aqueous solution onto composite magnetic lignin adsorbent. Chemosphere 2020, 246, 125757. [Google Scholar] [CrossRef]
- Mittal, H.; Alhassan, S.M.; Ray, S.S. Efficient organic dye removal from wastewater by magnetic carbonaceous adsorbent prepared from corn starch. J. Environ. Chem. Eng. 2018, 6, 7119–7131. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Qin, D.; Da, W.; Hou, B.; Hao, C.; Wu, J. Construction of magnetic lignin-based adsorbent and its adsorption properties for dyes. J. Hazard. Mater. 2019, 369, 50–61. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chen, B.H. Dye adsorption characteristics of magnetite nanoparticles coated with a biopolymer poly(γ-glutamic acid). Bioresour. Technol. 2011, 102, 8868–8876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, R. Surface electric properties of polypyrrole in aqueous solutions. Langmuir 2003, 19, 10703–10709. [Google Scholar] [CrossRef]
- Li, W.; Yang, L.; Zhou, H.; Li, X.; Wang, F.; Yang, X.; Liu, H. Gas-Assisted Superparamagnetic Extraction for Selective Separation of Binary Mixed Proteins. Ind. Eng. Chem. Res. 2013, 52, 16314–16320. [Google Scholar] [CrossRef]
- Bhaumik, M.; McCrindle, R.; Maity, A. Efficient removal of Congo red from aqueous solutions by adsorption onto interconnected polypyrrole–polyaniline nanofibers. Chem. Eng. J. 2013, 228, 506–515. [Google Scholar] [CrossRef]
- Liu, J.-F.; Zhao, Z.-S.; Jiang, G.-B. Coating Fe3O4 Magnetic Nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef]
- Jainae, K.; Sanuwong, K.; Nuangjamnong, J.; Sukpirom, N.; Unob, F. Extraction and recovery of precious metal ions in wastewater by polystyrene-coated magnetic particles functionalized with 2-(3-(2-aminoethylthio)propylthio)ethanamine. Chem. Eng. J. 2010, 160, 586–593. [Google Scholar] [CrossRef]
- Lanowix, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 30, 1361. [Google Scholar]
- Liu, S.; Ding, Y.; Li, P.; Diao, K.; Tan, X.; Lei, F.; Zhan, Y.; Li, Q.; Huang, B.; Huang, Z. Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N,N-dimethyl dehydroabietylamine oxide. Chem. Eng. J. 2014, 248, 135–144. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, A.; Zhong, L.; Wen, X.; Yan, P.; Wang, J. Amino-modified γ-Fe2O3/sepiolite composite with rod-like morphology for magnetic separation removal of Congo red dye from aqueous solution. Powder Technol. 2018, 339, 872–881. [Google Scholar] [CrossRef]
- Afkhami, A.; Moosavi, R. Adsorptive removal of Congo red, a carcinogenic textile dye, from aqueous solutions by maghemite nanoparticles. J. Hazard. Mater. 2010, 174, 398–403. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Jiang, J.H.; Xiao, L.; Zeng, G.M.; Zhao, S.L.; Wang, Y. Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 173, 494–502. [Google Scholar] [CrossRef]
- Guan, Y.; Rao, J.; Wu, Y.; Gao, H.; Liu, S.; Chen, G.; Peng, F. Hemicelluloses-based magnetic aerogel as an efficient adsorbent for Congo red. Int. J. Biol. Macromol. 2020, 155, 369–375. [Google Scholar] [CrossRef]
- Han, L.-J.; Ge, F.-Y.; Sun, G.-H.; Gao, X.-J.; Zheng, H.-G. Effective adsorption of Congo red by a MOF-based magnetic material. Dalton Trans. 2019, 48, 4650–4656. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhao, X.; Zhang, Y.-X.; Liu, Z.-H. Feasible synthesis of hierarchical porous MgAl-borate LDHs functionalized Fe3O4@SiO2 magnetic microspheres with excellent adsorption performance toward congo red and Cr(VI) pollutants. J. Alloy. Compd. 2021, 861, 157974. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Yao, J.; Xiao, L.; Zeng, G.M. Novel magnetic chitosan/poly(vinyl alcohol) hydrogel beads: Preparation, characterization and application for adsorption of dye from aqueous solution. Bioresour. Technol. 2012, 105, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Duan, H.; Wang, X.; Luo, C. Removal of Congo Red by magnetic mesoporous titanium dioxide-graphene oxide core-shell microspheres for water purification. Dalton Trans. 2014, 43, 8431–8438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, R.; Li, J.; Fu, Y.; Jiang, S.; Yao, J. Magnetically recyclable Fe3O4/Bi2S3 microspheres for effective removal of Congo red dye by simultaneous adsorption and photocatalytic regeneration. Sep. Purif. Technol. 2017, 179, 184–193. [Google Scholar] [CrossRef]
- Lu, L.; Li, J.; Yu, J.; Song, P.; Ng, D.H.L. A hierarchically porous MgFe2O4/γ-Fe2O3 magnetic microspheres for efficient removals of dye and pharmaceutical from water. Chem. Eng. J. 2016, 283, 524–534. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Yang, H.; Tang, A.; Zuo, X. Magnetic carbon-coated palygorskite loaded with cobalt nanoparticles for Congo Red removal from waters. Appl. Clay Sci. 2020, 198, 105856. [Google Scholar] [CrossRef]
- Ho, Y.-S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).