Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy Dental Meshes
Abstract
:1. Introduction
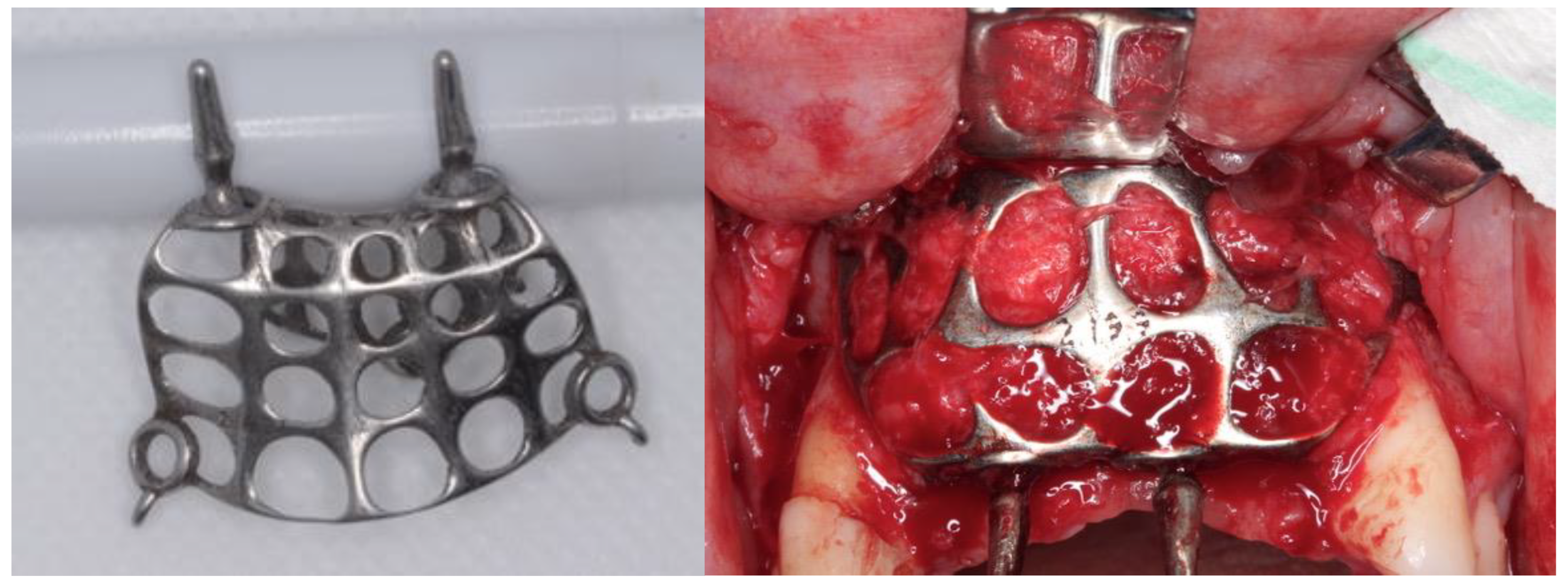
2. Materials and Methods
2.1. Samples
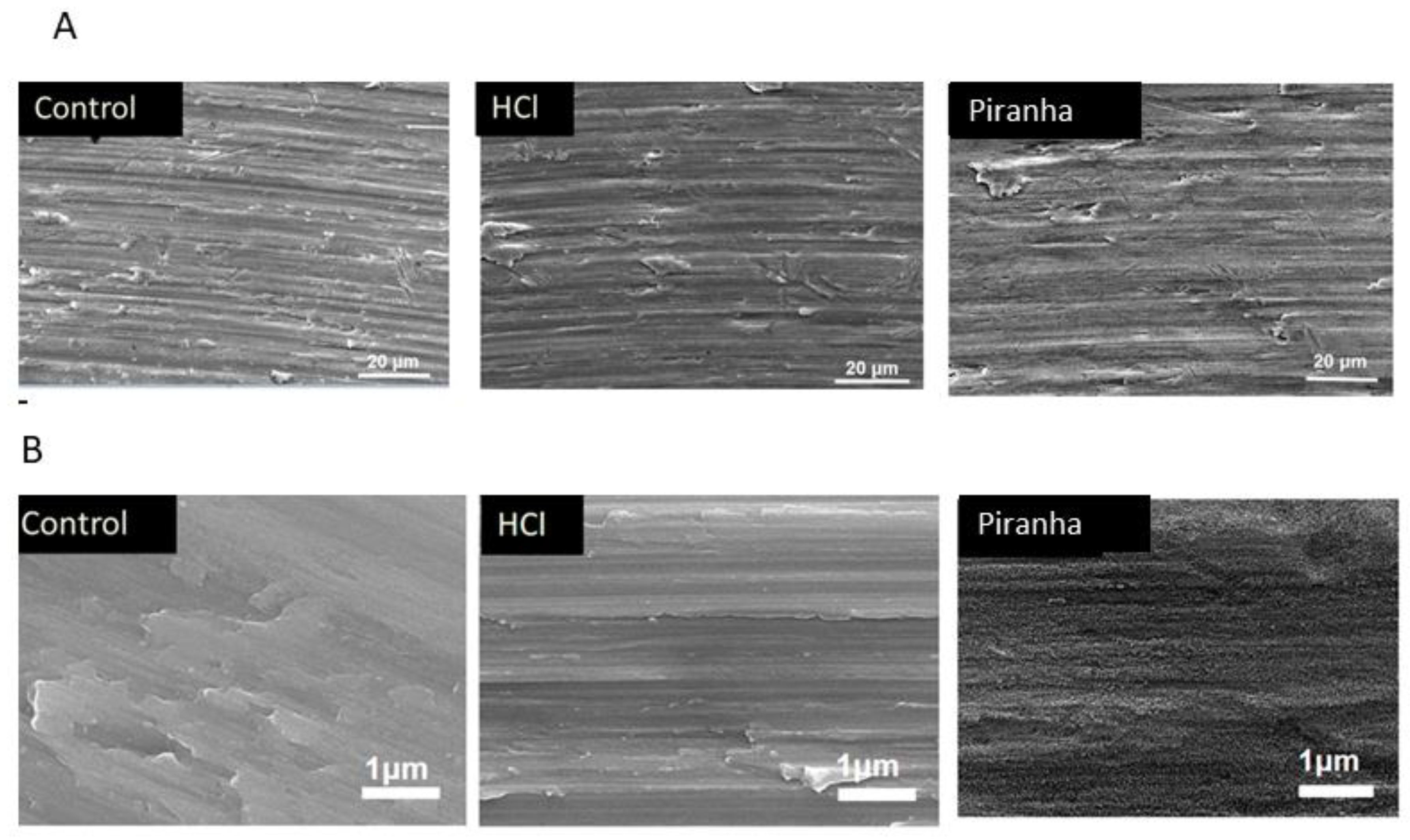
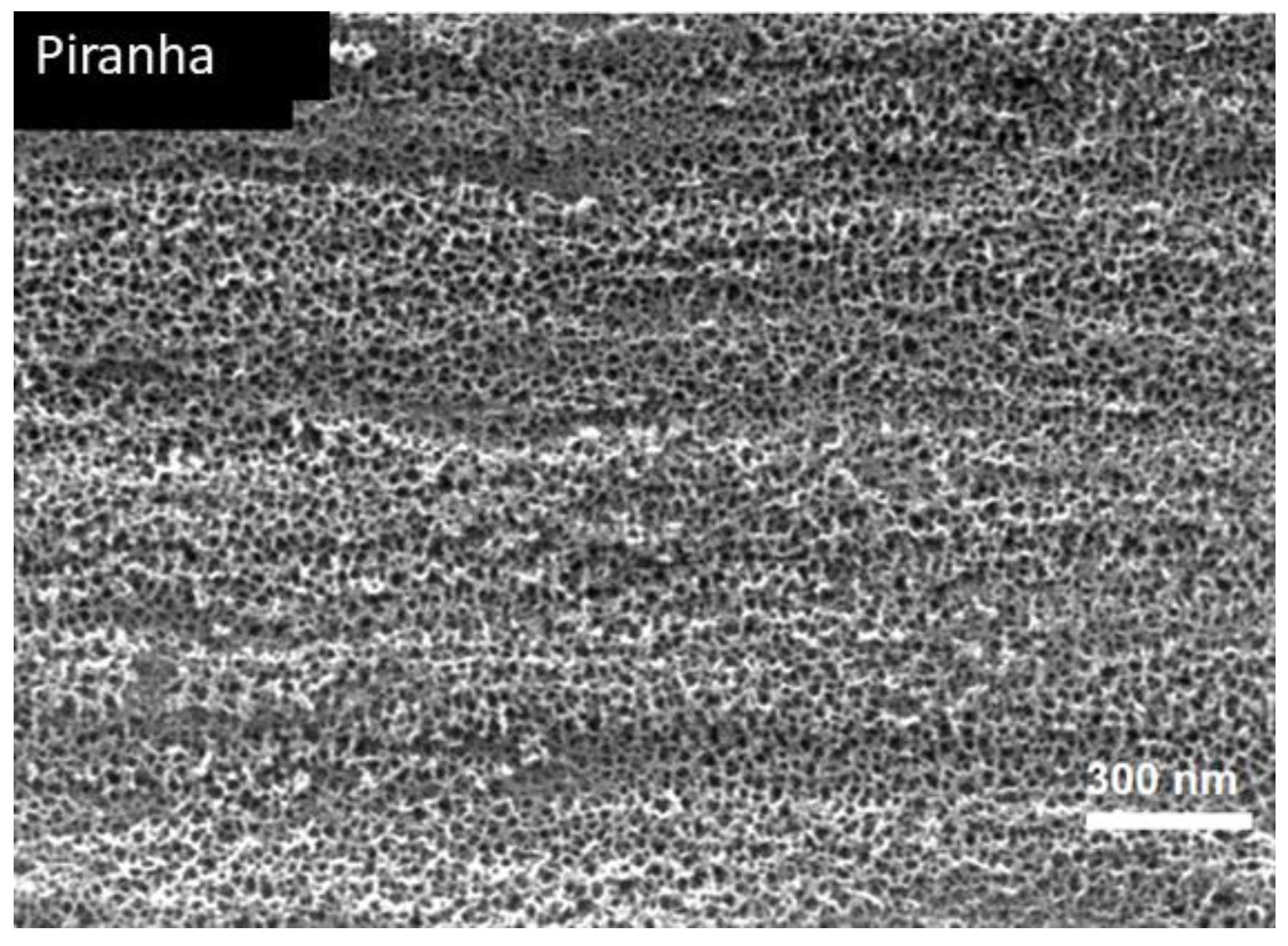
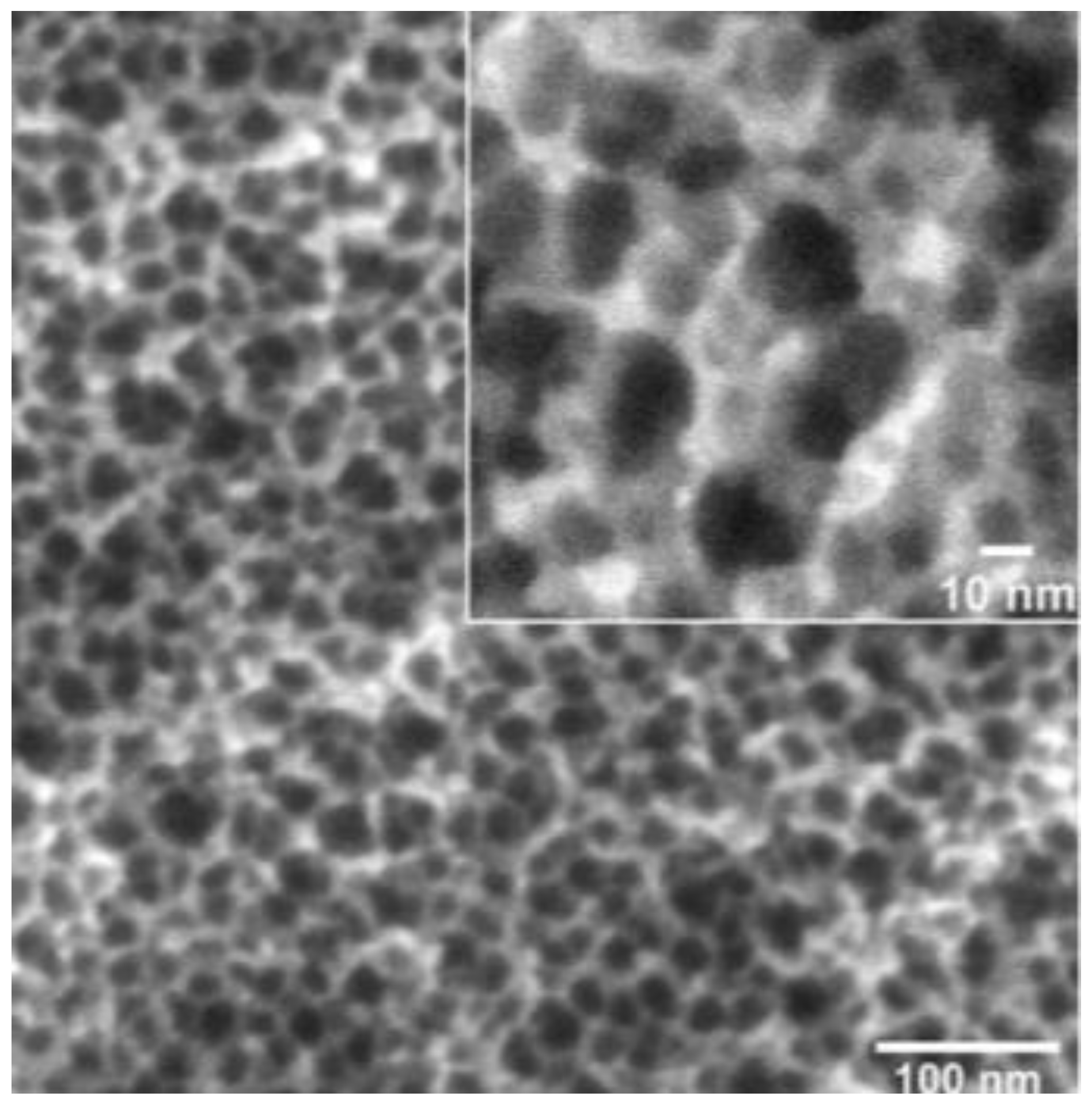
2.2. Surface Characterization
2.3. Corrosion Behavior
- icorr (μA/cm2)—corrosion current density;
- Ecorr (mV)—corrosion potential: value at which the current density changes from cathodic to anodic;
- Erep (mV)—repassivation potential: potential at which the passive layer regenerates;
- Ep (mV)—pitting potential: value at which pitting corrosion may occur;
- ip (μA/cm2)—passivation current density;
- ip (μA/cm2)—repassivation current density.
2.4. Ion Release
2.5. Bacteria Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Toledano-Serrabona, J.; Sanchez-Garces, M.; Sánchez-Torres, A.; Escoda, C.G. Alveolar distraction osteogenesis for dental implant treatments of the vertical bone atrophy: A systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2018, 24, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Tonetti, M.S.; Suvan, J.E.; Bernard, J.P.; Botticelli, D.; Fourmousis, I.; Hallund, M.; Jung, R.; Laurell, L.; Salvi, G.E.; et al. Immediate implant placement with transmucosal healing in areas of aesthetic priority: A multicentre randomized-controlled clinical trial I. Surgical outcomes. Clin. Oral Imp. Res. 2007, 18, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Martín, I.S.; Figuero, E.; Sanz, M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension. J. Dent. Res. 2015, 94, 128–142. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, N.; Solimei, L.; Pasquale, C.; Alvito, L.; Lagazzo, A.; Barberis, F. Mechanical Properties and Corrosion Resistance of TiAl6V4 Alloy Produced with SLM Technique and Used for Customized Mesh in Bone Augmentations. Appl. Sci. 2021, 11, 5622. [Google Scholar] [CrossRef]
- Wang, H.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant. Dent 2006, 15, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.; McAllister, B. Implant Site Development Using Ti-Mesh and Cellular Allograft in the Esthetic Zone for Restorative-Driven Implant Placement: A Case Report. Int. J. Periodontics Restor. Dent 2016, 36, 373–381. [Google Scholar] [CrossRef]
- Tan, X.; Tan, Y.J.; Chow, C.; Tor, S.B.; Yeong, W.Y. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Mater. Sci. Eng. C 2017, 76, 1328–1343. [Google Scholar] [CrossRef]
- Cruz, N.; Martins, M.I.; Santos, J.D.; Gil Mur, J.; Tondela, J.P. Surface Comparison of Three Different Commercial Custom-Made Titanium Meshes Produced by SLM for Dental Applications. Materials 2020, 13, 2177. [Google Scholar] [CrossRef]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the Titanium Implant Surface Treatment on the Surface Roughness and Chemical Composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.; Valderrama, P.; Wilson, T.; Palmer, K.; Thomas, A.; Sridhar, S.; Sadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy-Gallardo, M.; Manzanares-Céspedes, M.C.; Sevilla, P.; Nart, J.; Manzanares, N.; Manero, J.M.; Gil, F.J.; Boyd, S.K.; Rodríguez, D. Evaluation of bone loss in antibacterial coated dental implants: An experimental study in dogs. Mater. Sci. Eng. C 2016, 69, 538–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, F.J.; Rodriguez, A.; Espinar, E.; Llamas, J.M.; Padulles, E.; Juarez, A. Effect of the oral bacteria on the mechanical behavior of titanium dental implants. Int. J. Oral. Maxillofac. Implants 2012, 27, 64–68. [Google Scholar] [PubMed]
- Mombelli, A.; van Oosten, M.A.; Schurch, E.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Punset, M.; Villarrasa, J.; Nart, J.; Manero, J.M.; Bosch, B.; Padrós, R.; Perez, R.A.; Gil, F.J. Citric Acid Passivation of Titanium Dental Implants for Minimizing Bacterial Colonization Impact. Coatings 2021, 11, 214. [Google Scholar] [CrossRef]
- Duncan, W.J.; Lee, M.H.; Bae, T.S.; Lee, S.J.; Gay, J.; Loch, C. Anodisation increases integration of unloaded titanium implants in sheep mandible. Biomed. Res. Int. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kasemo, B.; Gold, J. Implant Surfaces and Interface Processes. Adv. Dent. Res. 1999, 13, 8–20. [Google Scholar] [CrossRef]
- Variola, F.; Lauria, A.; Nanci, A.; Rosei, F. Influence of Treatment Conditions on the Chemical Oxidative Activity of H2SO4/H2O2Mixtures for Modulating the Topography of Titanium. Adv. Eng. Mater. 2009, 11, 227–234. [Google Scholar] [CrossRef]
- Variola, F.; Francis-Zalzal, S.; Leduc, A.; Barbeau, J.; Nanci, A. Oxidative nanopatterning of titanium generates mesoporous surfaces with antimicrobial properties. Int. J. Nanomed. 2014, 9, 2319–2325. [Google Scholar] [CrossRef] [Green Version]
- Brunette, D.M.; Chehroudi, B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J. Biomech. Eng. 1999, 121, 49–57. [Google Scholar] [CrossRef]
- Jones, C.W. Applications of Hydrogen Peroxide and Derivatives. In RSC Clean Technology, Monographs; Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Bagno, A.; Di Bello, C. Surface treatments and roughness properties of Ti-based biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–46. [Google Scholar] [CrossRef] [PubMed]
- ASTM-E3-11; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM G5-14e1; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneve, Switzerland, 2009.
- ASTM G-102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2010.
- Gil, F.J.; Rodríguez, D.; Planell, J.A.; Cortada, M.; Giner, L.; Costa, S. Galvanic corrosion behaviour of Titanium implants coupled to dental alloys. J. Mat. Sci. Mat. Med. 2000, 11, 287–293. [Google Scholar]
- Gil, F.J.; Sánchez, L.A.; Espias, A.; Planell, J.A. In vitro corrosion behaviour and metallic ion release of different prosthodontic alloys. Int. Dent. J. 1999, 49, 347–351. [Google Scholar] [CrossRef]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent. Mater. 2007, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Wang, Z.; Shen, Y.; Manero, J.M.; Gil, F.J.; Rodriguez, D.; Haapasalo, M. Antibacterial coatings on titanium surfaces: A comparison study between in vitro single-species and multispecies biofilm. ACS Appl. Mater. Interfaces 2015, 7, 599–601. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.-H.; Bernard, C.; Variola, F.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Characterization of a bioactive nanotextured surface created by controlled chemical oxidation of titanium. Surf. Sci. 2006, 600, 4613–4621. [Google Scholar] [CrossRef]
- Castner, D.G.; Ratner, B.D. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Wheelis, S.E.; Gindri, I.M.; Valderrama, P.; Wilson, T.G., Jr.; Huang, J.; Rodrigues, D.C. Effects of decontamination solutions on the surface of titanium: Investigation of surface morphology, composition, and roughness. Clin. Oral Implants Res. 2015, 27, 329–340. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000 2010, 531, 67. [Google Scholar] [CrossRef] [PubMed]
- Michiardi, A.; Aparicio, C.; Ratner, B.D.; Planell, J.A.; Gil, J. The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces. Biomaterials 2007, 28, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef]
- Muhonen, V.; Heikkinen, R.; Danilov, A.; Jamsa, T.; Tuukkanen, J. The effects of oxide thickness on osteoblast attachment and survival on NiTi alloy. J. Mater. Sci. Mater. Med. 2007, 18, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.B.; Baud, G.; Besse, J.P.; Jacquet, M. Structural and optical properties of sputtered titania films. Mater. Sci. Eng. B 1997, 47, 110–118. [Google Scholar] [CrossRef]
- Velten, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W.; Breme, J. Preparation of TiO2 layers on cp-Ti and Ti6Al4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization. J. Biomed. Mater. Res. 2002, 59, 18–28. [Google Scholar] [CrossRef]
- Amor, S.B.; Guedri, L.; Baud, G.; Jacquet, M.; Ghedira, M. Influence of the temperature on the properties of sputtered titanium oxide films. Mater. Chem. Phys. 2002, 77, 903–911. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. An X-ray photoelectron spectroscopy sputter profile study of the native air-formed oxide film on titanium. Appl. Surf. Sci. 1999, 143, 92–100. [Google Scholar] [CrossRef]
- Arys, A.; Philippart, C.; Dourov, N.; He, Y.; Le, Q.T.; Pireaux, J.J. Analysis of titanium dental implants after failure of osseointegration: Combined histological, electron microscopy, and X-ray photoelectron spectroscopy approach. J. Biomed. Mater. Res. 1998, 43, 300–312. [Google Scholar] [CrossRef]
- Lee, T.M.; Chang, E.; Yang, C.Y. Surface characteristics of Ti6Al4V alloy: Effect of materials, passivation and autoclaving. J. Mater. Sci. Mater. Med. 1998, 9, 439–448. [Google Scholar] [CrossRef]
- Pouilleau, J.; Devilliers, D.; Garrido, F.; Durand-Vidal, S.; Mahe, E. Structure and composition of passive titanium oxide films. Mater. Sci. Eng. B 1997, 47, 235–243. [Google Scholar] [CrossRef]
- Lisowski, W.; van den Berg, A.H.J.; Smithers, M. Characterization of titanium hydride film after long-term air interaction: SEM, ARXPS and AES depth profile studies. Surf. Interface Anal. 1998, 26, 213–219. [Google Scholar] [CrossRef]
- Pegueroles, M.; Aparicio, C.; Bosio, M.; Engel, E.; Gil, F.J.; Planell, J.A.; Altankov, G. Spatial organization of osteoblast fibronectin matrix on titanium surfaces: Effects of roughness, chemical heterogeneity and surface energy. Acta Biomater. 2010, 6, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mukaddam, K.; Astasov-Frauenhoffer, M.; Fasler-Kan, E.; Marot, L.; Kisiel, M.; Meyer, E.; Köser, J.; Waser, M.; Bornstein, M.M.; Kühl, S. Effect of a Nanostructured Titanium Surface on Gingival Cell Adhesion, Viability and Properties against P. gingivalis. Materials 2021, 14, 7686. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural Bactericidal Surfaces: Mechanical Rupture of Pseudomonas Aeruginosa Cells by Cicada Wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; García-Fernández, L.; Fernández-Blázquez, J.P.; Barbeck, M.; Ghanaati, S.; Unger, R.; Kirkpatrick, J.; Arzt, E.; Funk, L.; Turón, P.; et al. Nanostructured Medical Sutures with Antibacterial Properties. Biomaterials 2015, 52, 291–300. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial Effects of Nanopillar Surfaces Are Mediated by Cell Impedance, Penetration and Induction of Oxidative Stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Xu, Z.; He, Y.; Zeng, X.; Zeng, X.; Huang, J.; Lin, X.; Chen, J. Enhanced Human Gingival Fibroblast Response and Reduced Porphyromonas Gingivalis Adhesion with Titania Nanotubes. Biomed. Res. Int. 2020, 2020, 5651780. [Google Scholar] [CrossRef]
- Kiran, A.S.; Kumar, T.S.; Perumal, G.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Dual nanofibrous bioactive coating and antimicrobial surface treatment for infection resistant titanium implants. Progress in Organic Coatings 2018, 121, 112–119. [Google Scholar] [CrossRef]
- Seddiki, O.; Harnagea, C.; Levesque, L.; Mantovani, D.; Rosei, F. Evidence of antibacterial activity on titaniumsurfaces through nanotextures. Appl. Surf. Sci. 2014, 308, 275–284. [Google Scholar] [CrossRef]
- Skindersoe, M.E.; Krogfelt, K.A.; Blom, A.; Jiang, G.; Prestwich, G.D.; Mansell, J.P. Dual Action of Lysophosphatidate-Functionalised Titanium:Interactions with Human (MG63) Osteoblasts and Methicillin Resistant Staphylococcus aureus. PLoS ONE 2015, 10, e0143509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, N.; Werden, J.; Keeler, W.J.; Nandakumar, K.; Leung, K.T. The Bactericidal Effect of Ultraviolet and Visible Light on Escherichia Coli. Biotechnol. Bioeng. 2008, 99, 550–556. [Google Scholar] [CrossRef] [PubMed]



| Chemical Product | Composition (mM) |
|---|---|
| K2HPO4 | 0.44 |
| KCl | 5.4 |
| CaCl2 | 1.3 |
| Na2HPO4 | 0.25 |
| NaCl | 137 |
| NaHCO3 | 4.2 |
| MgSO4 | 1.0 |
| C6H12O6 | 5.5 |
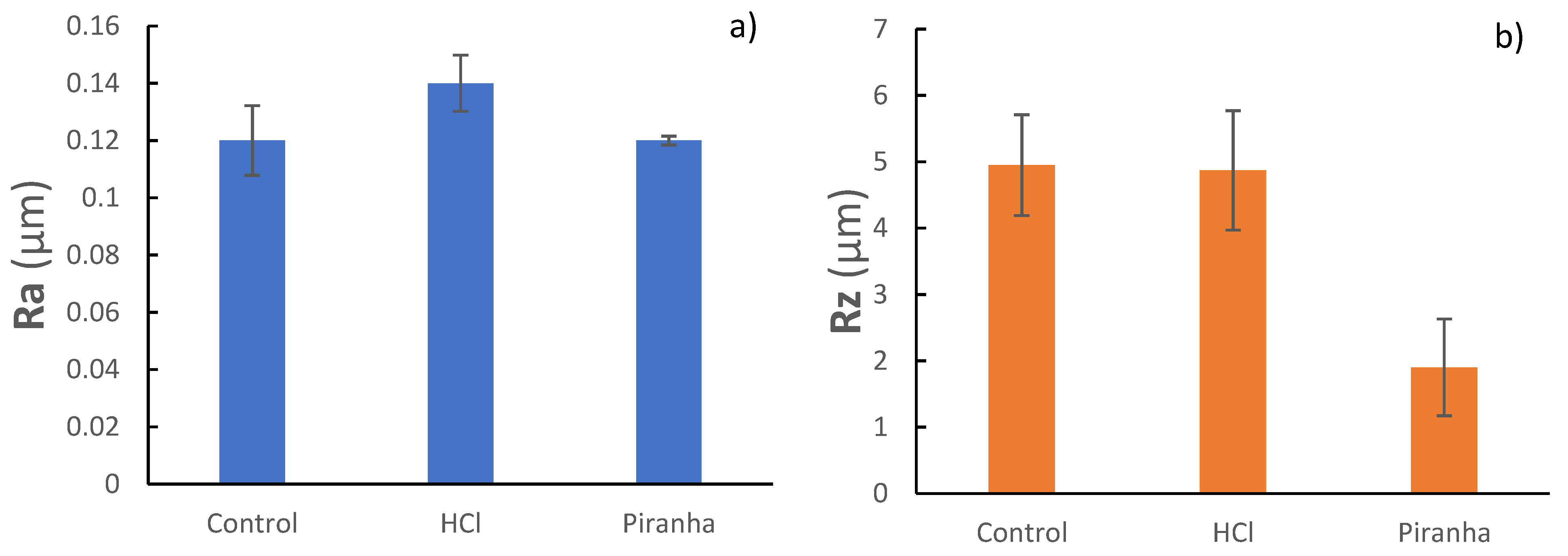
| Mesh | Ra (μm) | Rz (μm) |
|---|---|---|
| Control | 0.12 ± 0.03 (a) | 4.95 ± 0.76 (A) |
| HCl | 0.14 ± 0.08 (a) | 4.87 ± 0.90 (A) |
| Piranha | 0.12 ± 0.05 (a) | 1.90 ± 0.73 (B) |
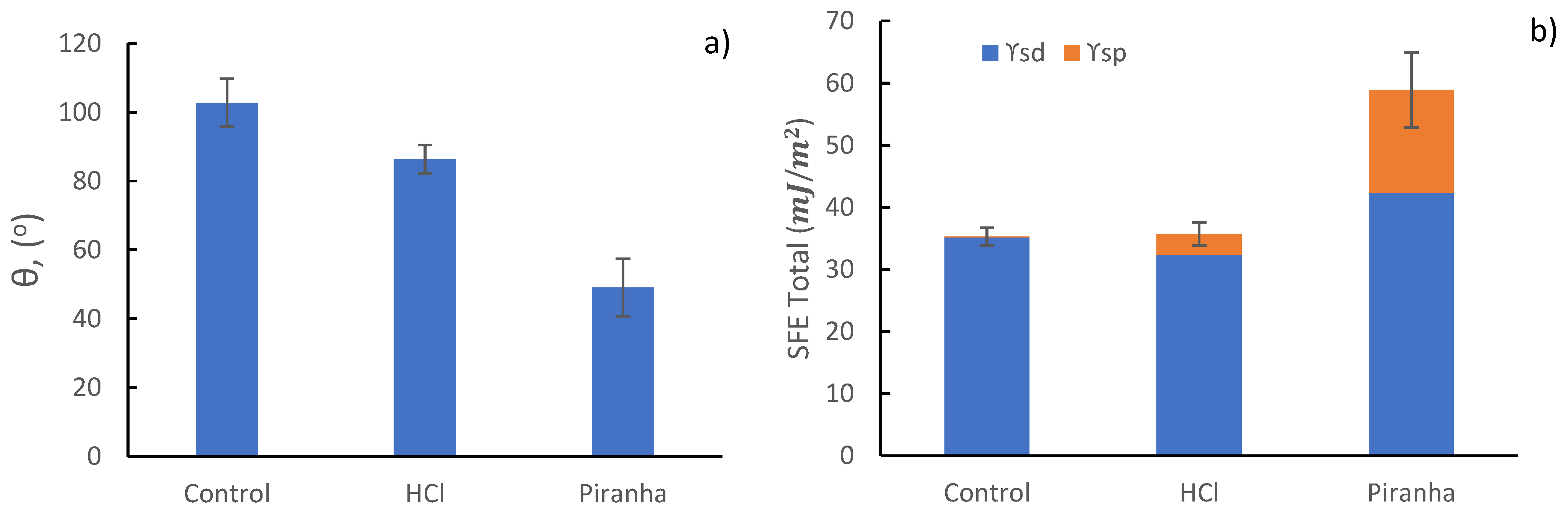
| Mesh | Θ Water (°) | Θ Diidomethane (°) | γd (mJ/m2) | γp (mJ/m2) | SFE (mJ/m2) |
|---|---|---|---|---|---|
| Control | 102.76 ± 7.00 | 48.40 ± 2.32 | 35.15 ± 1.28 | 0.12 ± 0.10 | 35.28 ± 1.35 |
| HCl | 86.37 ± 4.12 | 53.54 ± 0.92 | 32.39 ± 0.52 | 3.31 ± 1.28 | 35.70 ± 1.60 |
| Piranha | 49.05 ± 7.67 | 34.12 ± 3.94 | 42.37 ± 1.79 | 16.52 ± 4.22 | 58.90 ± 4.11 |
| Mesh | EOCP (mV) | icorr (μA/cm2) | Rp (MΩ/cm2) | Ecorr (V) | Vc (μm/Year) |
|---|---|---|---|---|---|
| Control | −196 ± 01 | 0.027 ± 0.008 | 2.428 ± 0.390 | −361 ± 14 | 0.233 ± 0.066 |
| HCl | −145 ± 11 | 0.018 ± 0.005 | 2.479 ± 0.083 | −536 ± 39 | 0.176 ± 0.048 |
| Piranha | −206 ± 27 | 0.056 ± 0.006 | 1.102 ± 0.149 | −447 ± 26 | 0.488 ±0.047 |
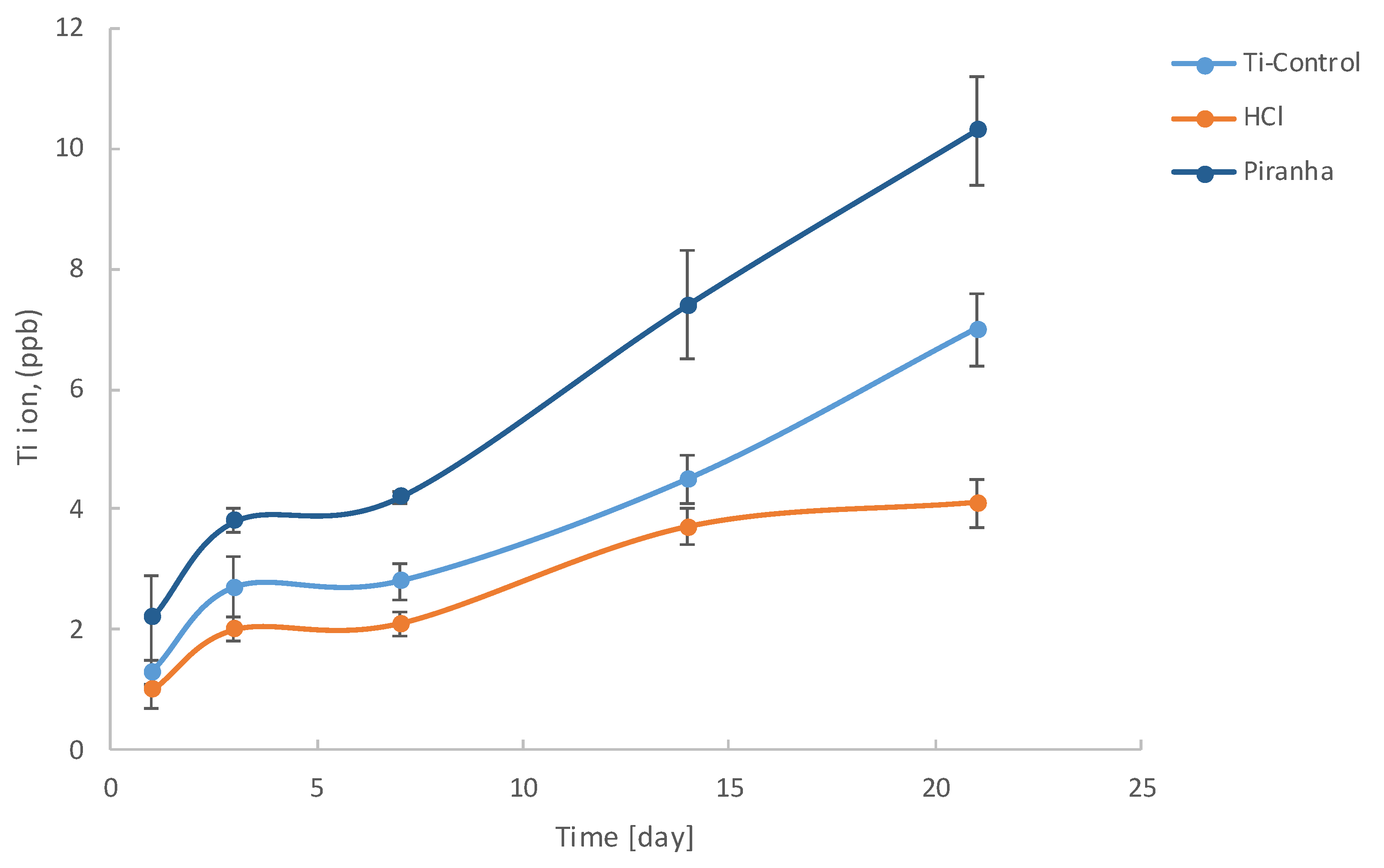
| Mesh | 1 Day | 3 Days | 7 Days | 14 Days | 21 Days |
|---|---|---|---|---|---|
| Control | 1.3 ± 0.2 | 2.7 ± 0.5 | 2.8 ± 0.3 | 4.5 ± 0.4 | 7.0 ± 0.6 |
| HCl | 1.0 ± 0.3 | 2.0 ± 0.2 | 2.1 ± 0.2 | 3.7 ± 0.3 | 4.1 ± 0.4 |
| Piranha | 2.2 ± 0.7 | 3.8 ± 0.2 | 4.2 ± 0.1 | 7.4 ± 0.9 | 10.3 ± 0.9 |
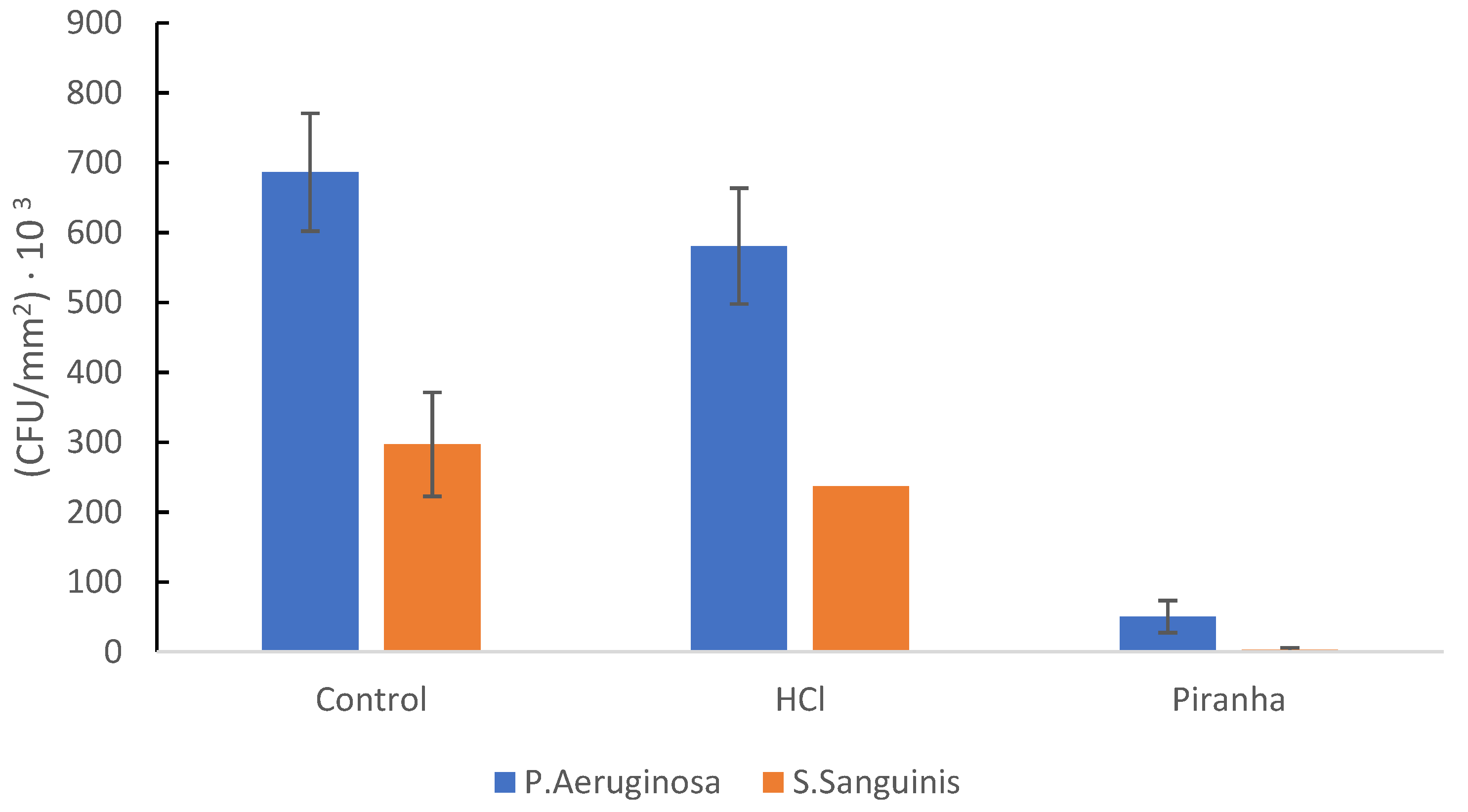
| Mesh | P. aeruginosa (Number of Bacteria/mm2) | S. sanguinis (Number of Bacteria/mm2) |
|---|---|---|
| Control | 7.02 × 105 ± 0.52 × 105 | 3.52 × 105 ± 0.48 × 105 |
| HCl | 5.75 × 105 ± 0.33 × 105 | 2.25 × 105 ± 0.13 × 105 |
| Piranha | 1.23 × 104 ± 0.02 × 104 | 5.03 × 103 ± 0.10 × 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, N.; Gil, J.; Punset, M.; Manero, J.M.; Tondela, J.P.; Verdeguer, P.; Aparicio, C.; Rúperez, E. Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy Dental Meshes. Coatings 2022, 12, 154. https://doi.org/10.3390/coatings12020154
Cruz N, Gil J, Punset M, Manero JM, Tondela JP, Verdeguer P, Aparicio C, Rúperez E. Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy Dental Meshes. Coatings. 2022; 12(2):154. https://doi.org/10.3390/coatings12020154
Chicago/Turabian StyleCruz, Nuno, Javier Gil, Miquel Punset, José María Manero, João Paulo Tondela, Pablo Verdeguer, Conrado Aparicio, and Elisa Rúperez. 2022. "Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy Dental Meshes" Coatings 12, no. 2: 154. https://doi.org/10.3390/coatings12020154
APA StyleCruz, N., Gil, J., Punset, M., Manero, J. M., Tondela, J. P., Verdeguer, P., Aparicio, C., & Rúperez, E. (2022). Relevant Aspects of Piranha Passivation in Ti6Al4V Alloy Dental Meshes. Coatings, 12(2), 154. https://doi.org/10.3390/coatings12020154









