The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Chitosan/Phenolic Acid Films
2.3. Mechanical Testing
2.4. Roughness
2.5. Differential Scanning Calorimetry (DSC)
2.6. Water Vapor Permeation Rate (WVPR)
2.7. DPPH Radical Scavenging Assay
2.8. Film Color
2.9. Statistics
3. Results
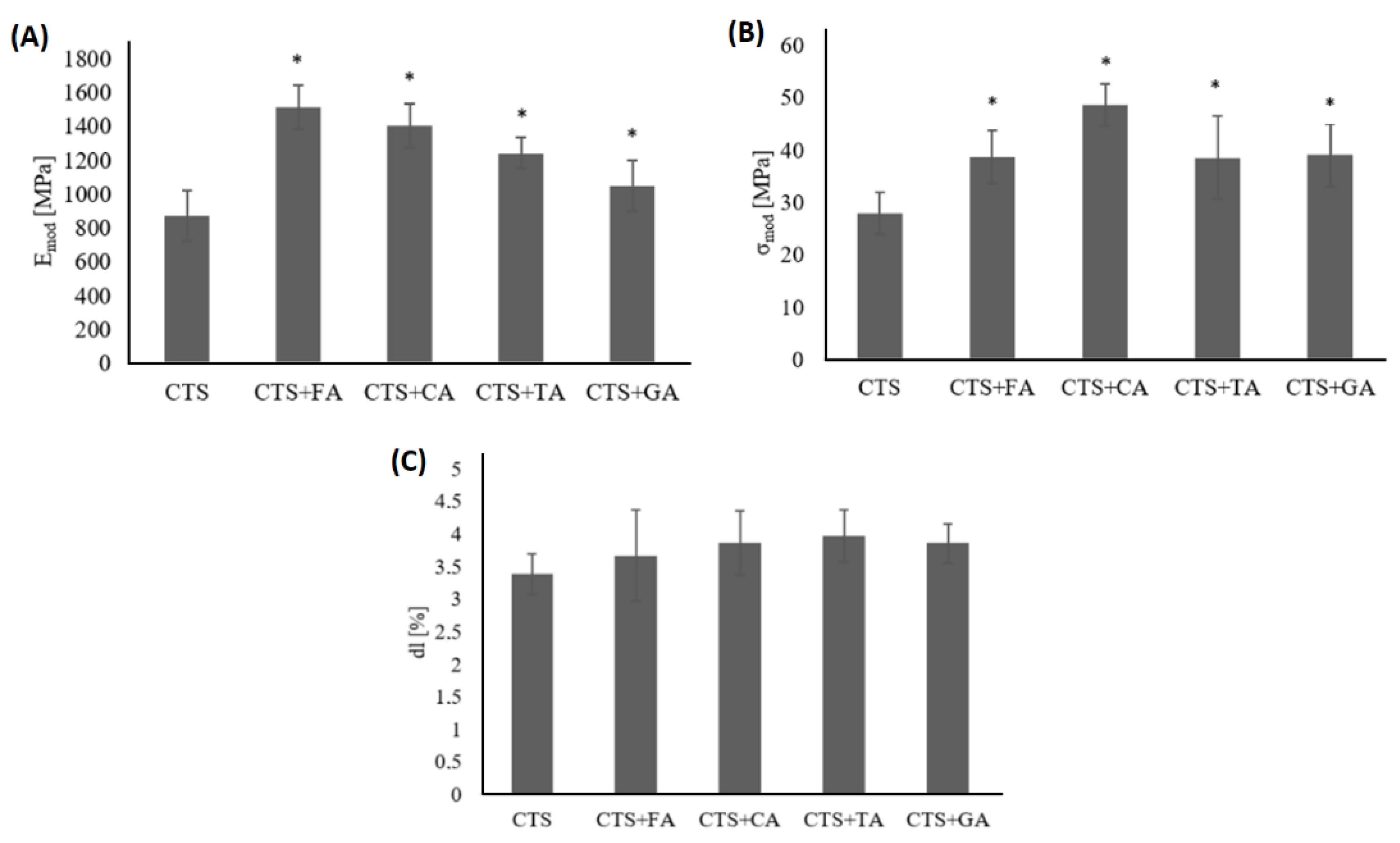
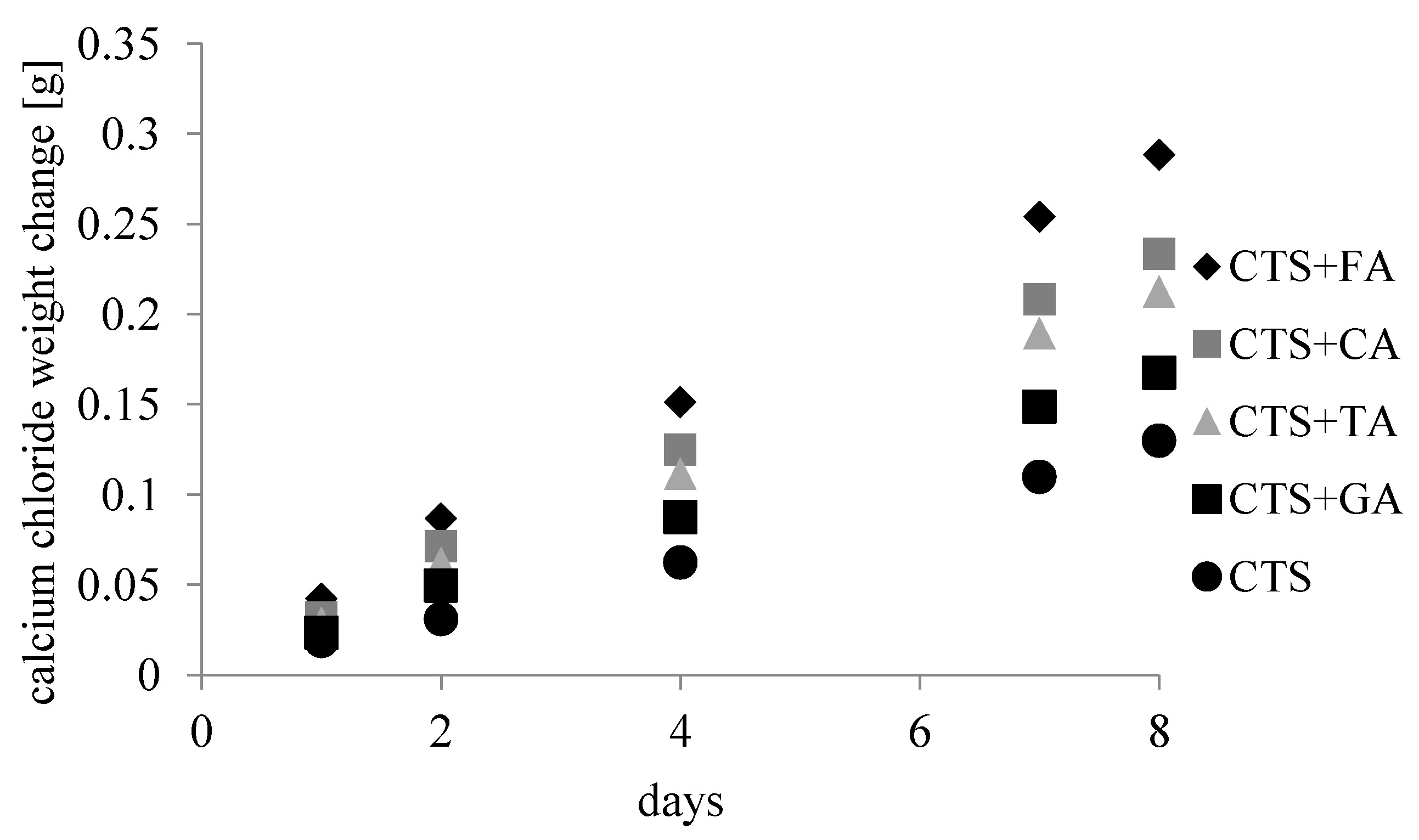
3.1. Mechanical Testing
3.2. Roughness
3.3. Differential Scanning Calorimetry (DSC)
3.4. Water Vapor Permeation Rate (WVPR)
3.5. DPPH Radical Scavenging Assay
3.6. Film Color
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2011, 13, 1105. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 2, 27–35. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2008, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folino, A.; Karageorgiou, A.; Calabro, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Bahrami, R.; Zibaei, R.; Hashami, Z.; Hasanvand, S.; Garavand, F.; Rouhi, M.; Jafari, S.M.; Mohammadi, R. Modification and improvement of biodegradable packaging films by cold plasma; a critical review. Crit. Rev. Food Sci. Nutr. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Baisthakur, P.; Yemul, O.S. Synthesis, characterization and application of crosslinked alginate as green packaging material. Heliyon 2020, 6, e03026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Cruz, M.D.L.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef]
- Ortega-Ortiz, H.; Gutierrez-Rodriguez, B.; Cadenas-Pliego, G.; Jimenez, L.I. Antibacterial Activity of Chitosan and the Interpolyelectrolyte Complexes of Poly(acrylic acid)-Chitosan, Braz. Arch. Biol. Technol. 2010, 53, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2020, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant protein-based food packaging films; recent advances in fabrication, characterization, and applications. Trends Food Sci. Technol. 2020, 20, 154–173. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Kurasova, M.N.; Volkova, O.V.; Meledina, T.V.; Lipkan, N.A.; Tskhovrebov, A.G.; Kurliuk, A.V.; Shakola, T.V.; Dysin, A.P.; et al. Novel non-toxic high efficient antibacterial azido chitosan derivatives with potential application in food coatings. Food Chem. 2019, 301, 125247. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A.; Michalska-Sionkowska, M.; Sionkowska, A. The characterization of thin films based on chitosan and tannic acid mixture for potential applications as wound dressings. Polym. Test. 2019, 78, 106007. [Google Scholar] [CrossRef]
- Kaczmarek, B. Improving sodium alginate films properties by phenolic acid addition. Materials 2020, 13, 2895. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Lewandowska, K.; Sionkowska, A. Modification of collagen properties with ferulic acid. Materials 2020, 13, 3419. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Grabska-Zielińska, S.; Skopińska-Wiśniewska, J.; Jakubowska, E. Examining the Impact of Squaric Acid as a Crosslinking Agent on the Properties of Chitosan-Based Films. Int. J. Mol. Sci. 2021, 22, 3329. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sosik, A.; Małkowska, A.; Zasada, L.; Michalska-Sionkowska, M. The Study of Physicochemical Properties and Blood Compatibility of Sodium Alginate-Based Materials via Tannic Acid Addition. Materials 2021, 14, 4905. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernandez-Lopez, J.; Viuda-Martos, M. Evaluation of the antibacterial and antioxidant activities of chitosan edible films incorporated with organic essential oils obtained from four Thymus species. J. Food Sci. Technol. 2016, 53, 3374–3379. [Google Scholar] [CrossRef] [Green Version]
- Prus-Walendziak, W.; Kozłowska, J. Design of sodium alginate/gelatin-based emulsion film fused with polylactide microparticles charged with plant extract. Materials 2021, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Pushparaj, C.; Subramani, R. Recent development in preparation of food packaging films using biopolymers. Food Res. 2021, 5, 12–22. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Bouaziz, M. Polylactide Films with the Addition of Olive Leaf Extract—Physico-Chemical Characterization. Materials 2021, 14, 7623. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, J.; Li, L.; Xue, B.; Li, X.; Gan, J.; Shao, Z.; Sun, T. Properties of phenolic acid-chitosan composite films and preservative effect on Penaeus vannamei. J. Mol. Str. 2021, 1239, 130531. [Google Scholar] [CrossRef]
- Yong, H.; Liu, Y.; Yun, D.; Zong, S.; Jin, C.; Liu, J. Chitosan Films Functionalized with Different Hydroxycinnamic Acids: Preparation, Characterization and Application for Pork Preservation. Foods 2021, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Zarandona, I.; Puertas, A.I.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Assessment of active chitosan films incorporated with gallic acid. Food Hydrocoll. 2020, 101, 105486. [Google Scholar] [CrossRef]
- Ringus, D.L.; Mararu, C.I. Pulsed Light inactivation of Listeria innocua on food packaging materials of different surface roughness and reflectivity. J. Food Eng. 2013, 114, 331–337. [Google Scholar] [CrossRef]
- Suganthi, S.; Vignesh, S.; Sundar, J.K.; Raj, V. Fabrication of PVA polymer flms with improved antibacterial activity by fne-tuning via organic acids for food packaging applications. Appl. Water Sci. 2020, 10, 100. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek-Szczepańska, B.; Wekwejt, M.; Mazur, O.; Zasada, L.; Pałubicka, A.; Olewnik-Kruszkowska, E. The Physicochemical and Antibacterial Properties of Chitosan- Based Materials Modified with Phenolic Acids Irradiated by UVC Light. Int. J. Mol. Sci. 2021, 22, 6472. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohyd. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Kosaraju, S.L.; D’ath, L.; Lawrence, A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohyd. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Prearation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Tseng, H.H.; Wahab, R.A.; Ismail, A.F. Progress of interfacial polymerization techniques for polyamide thin film (nano)composite membrane fabrication: A comprehensive review. Polymers 2020, 12, 2817. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.B.; Bozell, J.J.; Hayes, D.G.; Zivanovic, S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agriculture Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 225, 115328. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, B.; Weng, Y.M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 7, 176–186. [Google Scholar] [CrossRef]
| Specimen | Ra [nm] | Rq [nm] |
|---|---|---|
| CTS | 72.4 ± 0.30 | 81.5 ± 0.52 |
| CTS + FA | 57.9 ± 0.40 * | 71.9 ± 0.37 * |
| CTS + CA | 24.1 ± 0.34 * | 29.7 ± 0.31 * |
| CTS + TA | 21.7 ± 0.42 * | 26.7 ± 0.42 * |
| CTS + GA | 34.1 ± 0.37 * | 43.2 ± 0.52 * |
| Specimen | T1 [°C] | ΔH1 [mW/mg] | T2 [°C] | ΔH2 [mW/mg] |
|---|---|---|---|---|
| CTS | 77.9 | 1.342 | 196.0 | 0.3357 |
| CTS + FA | 68.6 * | 1.238 | 204.7 * | 0.2747 * |
| CTS + CA | 71.2 * | 0.993 * | 200.8 | 0.2188 * |
| CTS + TA | 68.3 * | 1.050 * | 200.4 | 0.1376 * |
| CTS + GA | 69.3 * | 1.174 * | 201.8 * | 0.3104 * |
| Specimen | WVPR [mg/cm2/h] |
|---|---|
| CTS | 4.04 |
| CTS + FA | 9.06 * |
| CTS + CA | 6.82 * |
| CTS + TA | 6.18 * |
| CTS + GA | 5.04 * |
| Specimen | RSA [%] |
|---|---|
| CTS | 17.21 ± 1.03 |
| CTS + FA | 89.83 ± 2.10 * |
| CTS + CA | 30.84 ± 2.20 * |
| CTS + TA | 17.21 ± 1.03 |
| CTS + GA | 56.03 ± 2.15 * |
| Specimen | L* | a* | b* | ΔE | WI |
|---|---|---|---|---|---|
| CTS | 94.09 ± 0.37 | −1.65 ± 0.23 | 2.03 ± 0.45 | 3.91 ± 0.04 | 93.54 ± 0.09 |
| CTS + FA | 92.74 ± 0.10 * | −1.66 ± 0.21 | 3.79 ± 0.09 * | 4.34 ± 0.08 * | 91.64 ± 0.11 * |
| CTS + CA | 93.06 ± 1.18 | −1.05 ± 0.16 * | 3.23 ± 2.16 * | 4.02 ± 0.11 * | 92.27 ± 0.15 * |
| CTS + TA | 91.76 ± 2.05 | −1.81 ± 0.40 * | 4.56 ± 0.60 * | 4.77 ± 0.07 * | 90.41 ± 0.08 * |
| CTS + GA | 89.98 ± 1.40 * | −1.98 ± 0.13 * | 10.40 ± 0.40 * | 10.54 ± 0.13 * | 85.43 ± 0.10 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek-Szczepańska, B.; Zasada, L.; Grabska-Zielińska, S. The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids. Coatings 2022, 12, 126. https://doi.org/10.3390/coatings12020126
Kaczmarek-Szczepańska B, Zasada L, Grabska-Zielińska S. The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids. Coatings. 2022; 12(2):126. https://doi.org/10.3390/coatings12020126
Chicago/Turabian StyleKaczmarek-Szczepańska, Beata, Lidia Zasada, and Sylwia Grabska-Zielińska. 2022. "The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids" Coatings 12, no. 2: 126. https://doi.org/10.3390/coatings12020126
APA StyleKaczmarek-Szczepańska, B., Zasada, L., & Grabska-Zielińska, S. (2022). The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids. Coatings, 12(2), 126. https://doi.org/10.3390/coatings12020126








