The Immobilization of Laccase on Mixed Polymeric Microspheres for Methyl Red Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microsphere Preparation and Enzyme Immobilization
2.3. Characterization
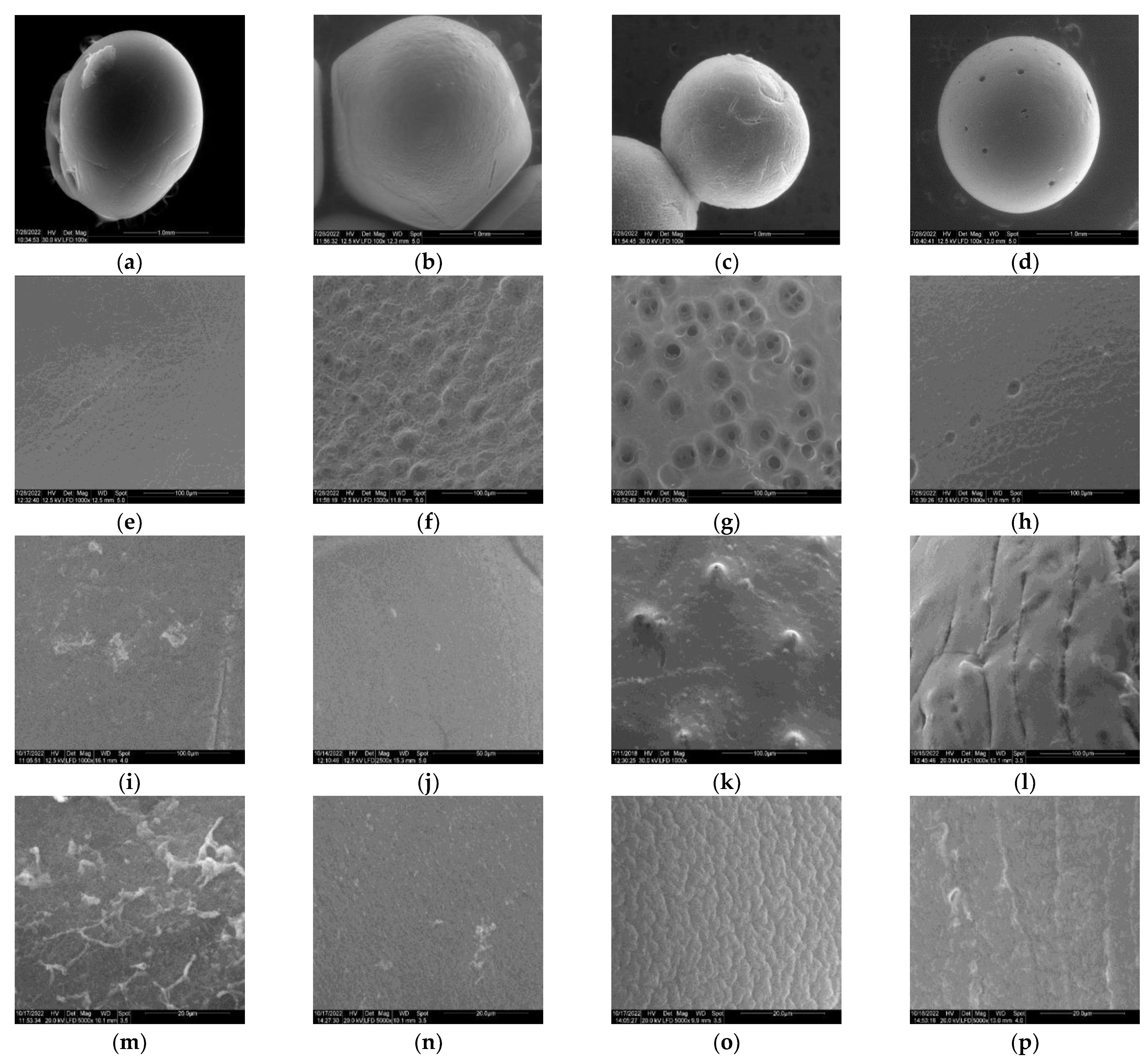
2.3.1. ESEM
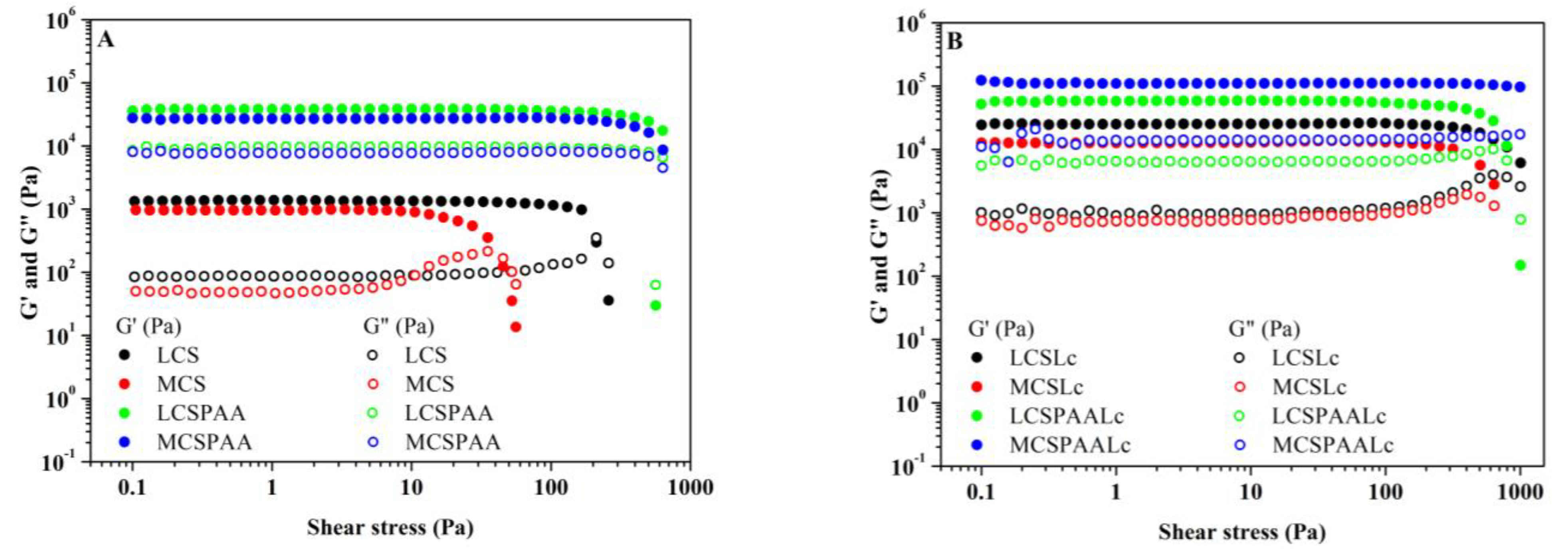
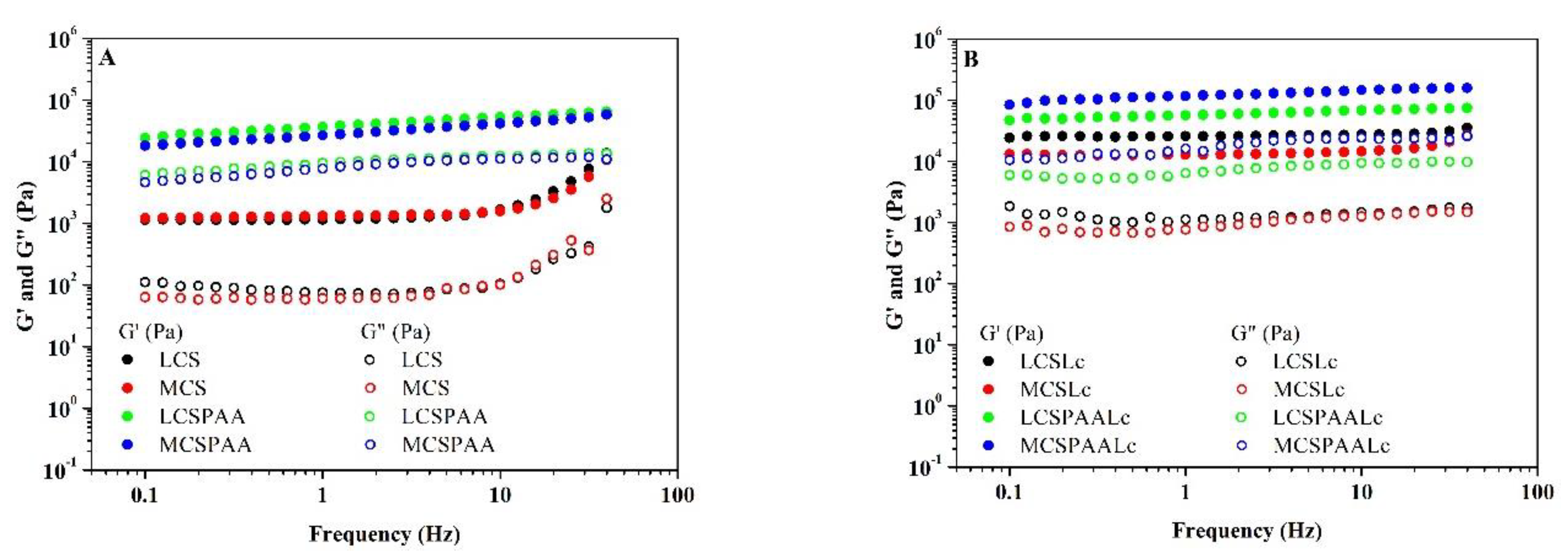
2.3.2. Rheology
2.4. UV–Vis Methodology
2.4.1. Laccase Assay
2.4.2. Active Laccase Content Estimation
2.4.3. Operational Stability
2.4.4. Michaelis–Menten kinetics
2.5. Molecular Docking
2.6. Practical Application
2.6.1. Discoloration of MR
2.6.2. Degradation Product Identification
2.6.3. Recyclability of Immobilized Laccase
3. Results and Discussion
3.1. Microsphere Characterization
3.1.1. ESEM Measurements
3.1.2. Rheology
3.2. Estimation of Active Immobilized Laccase Content on Immobilized Products
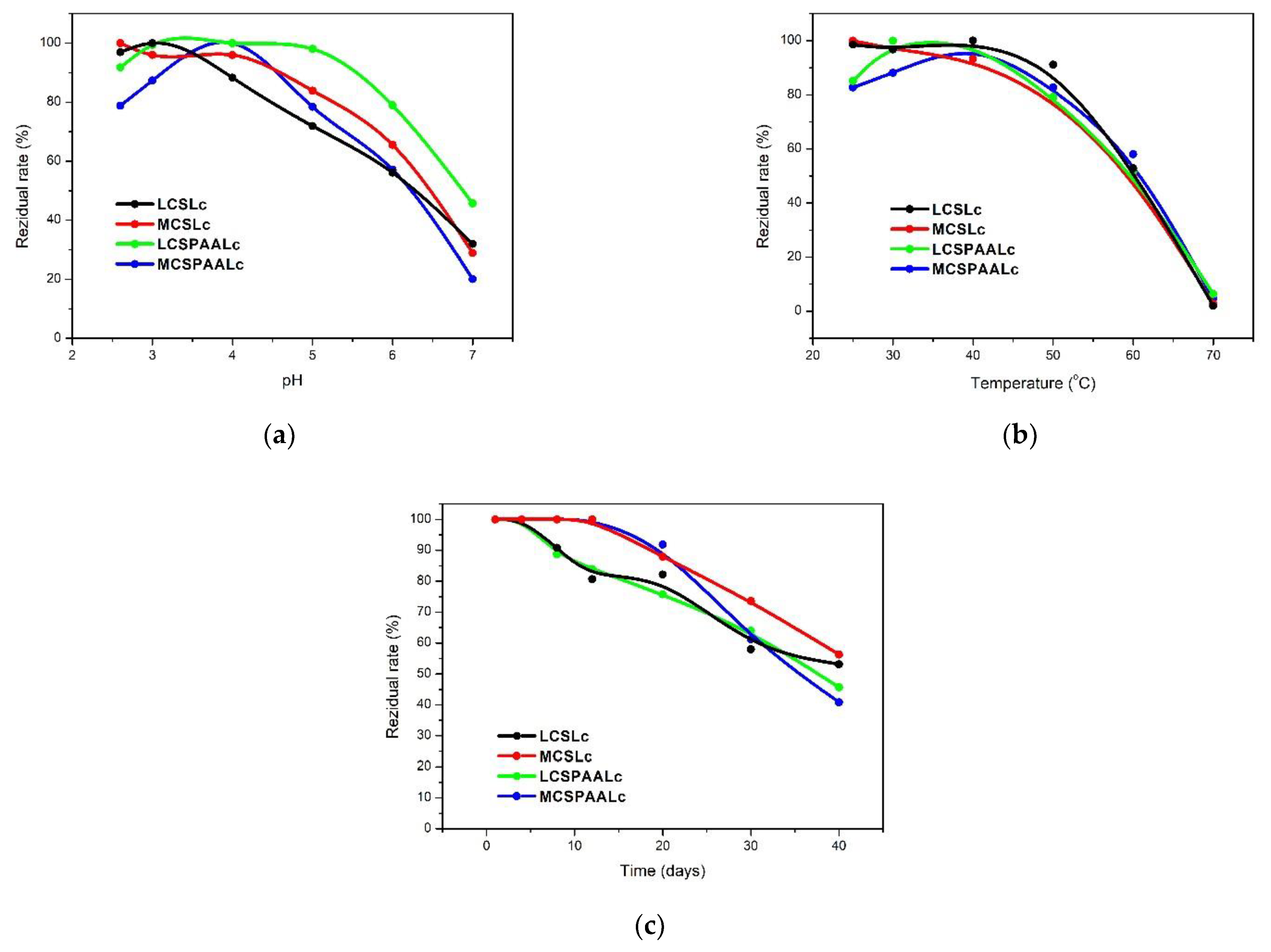
3.3. Operational Stability
3.4. Michaelis–Menten Kinetics
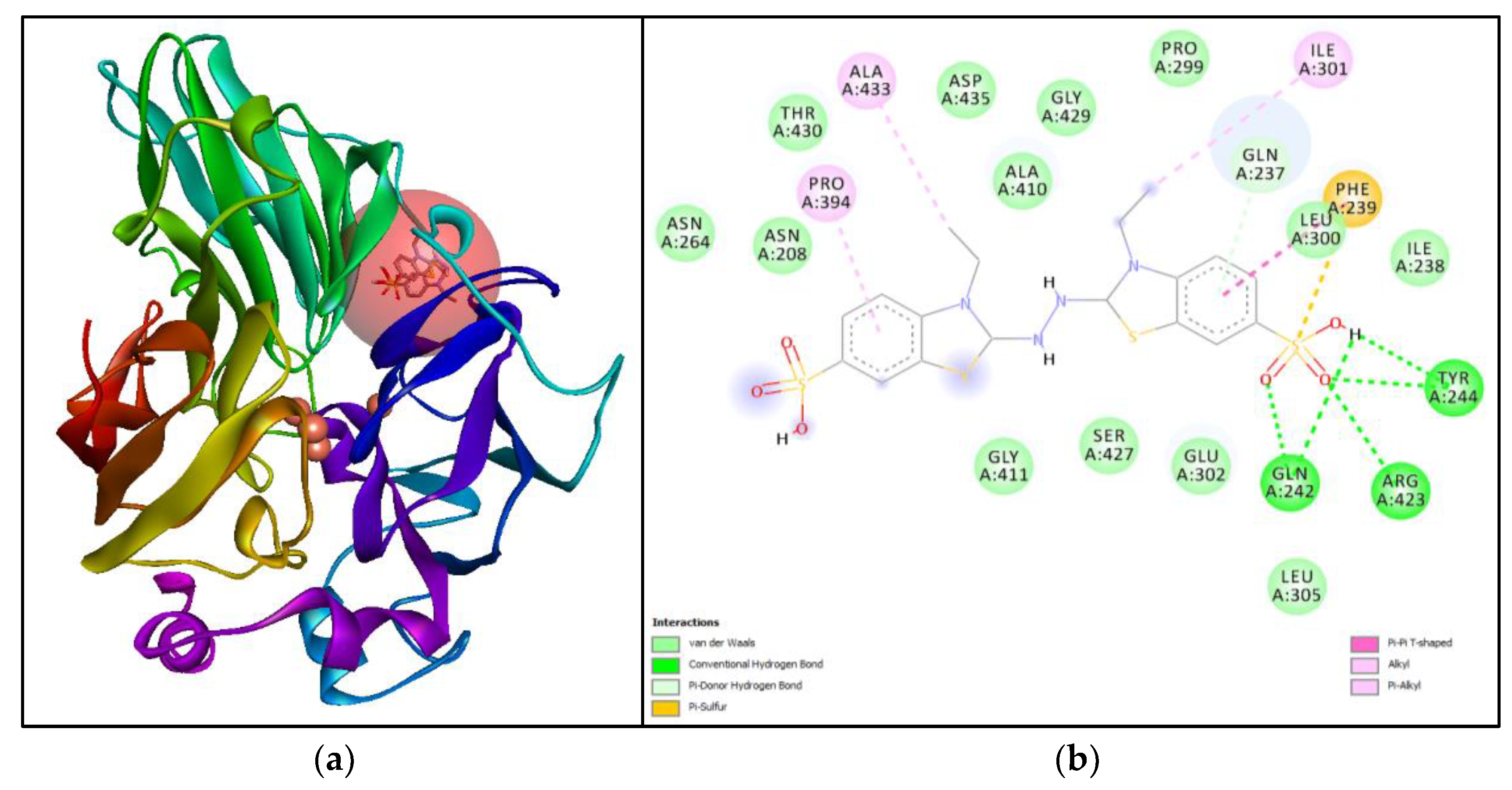
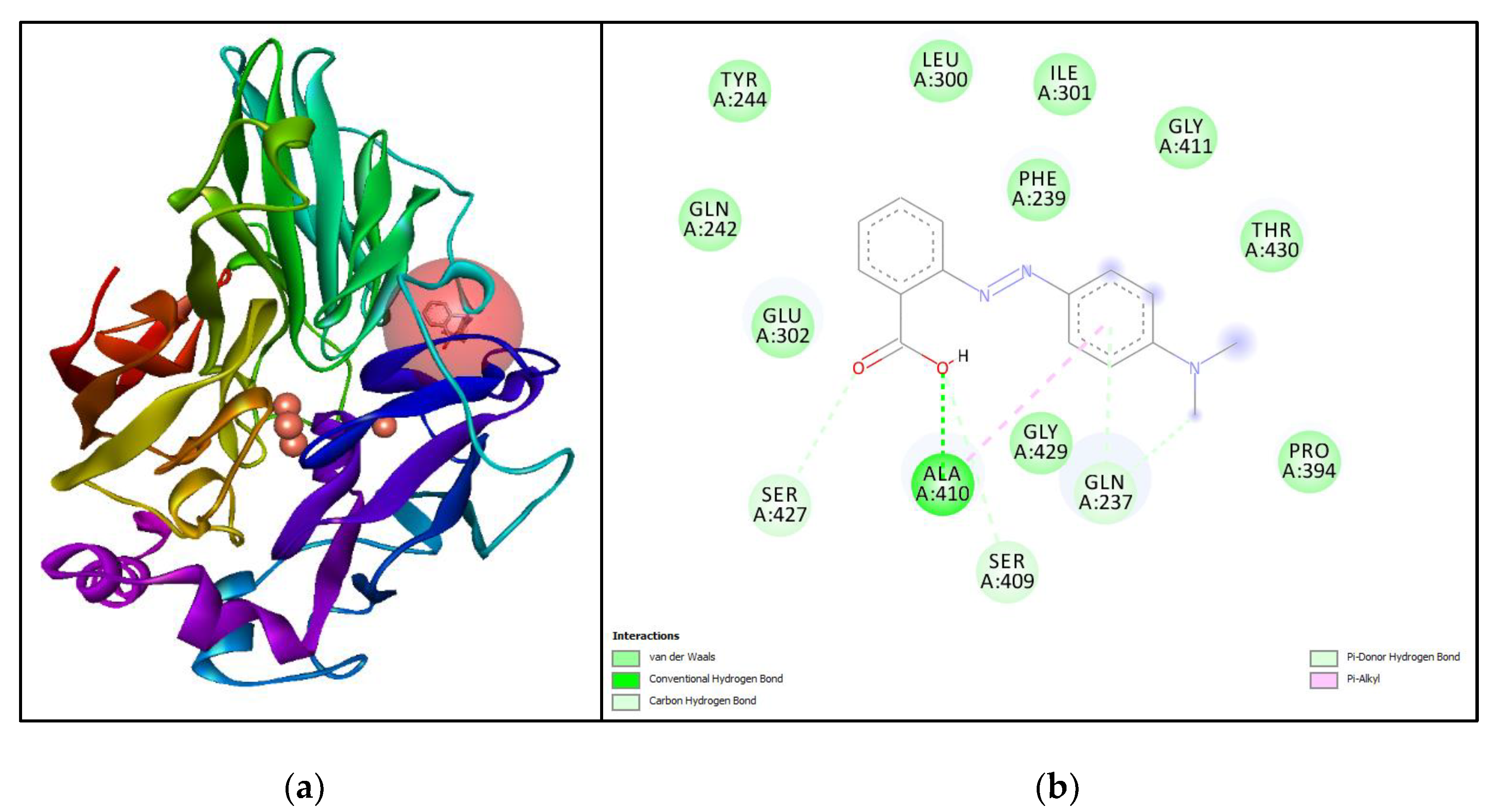
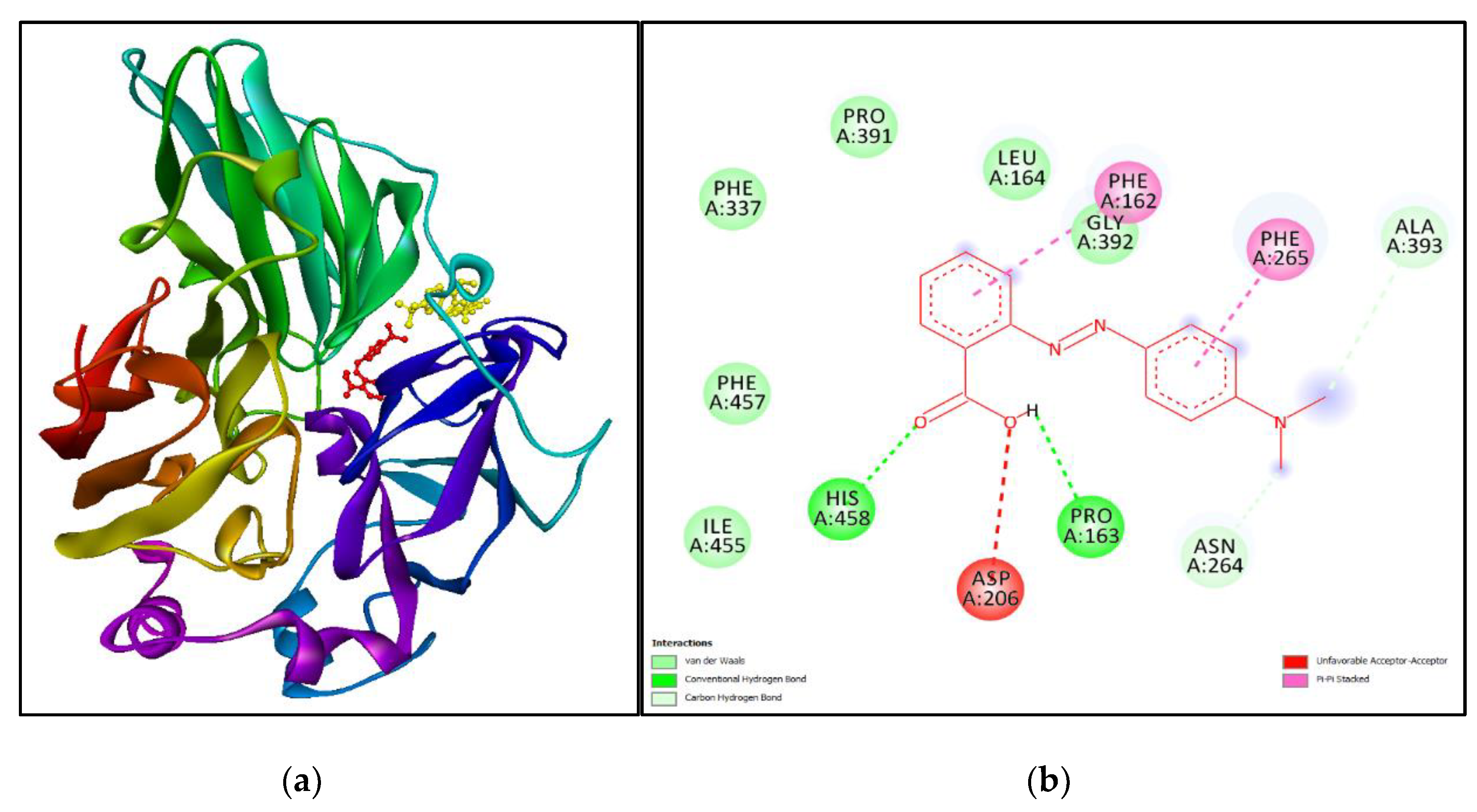
3.5. Molecular Simulation
3.6. Practical Application
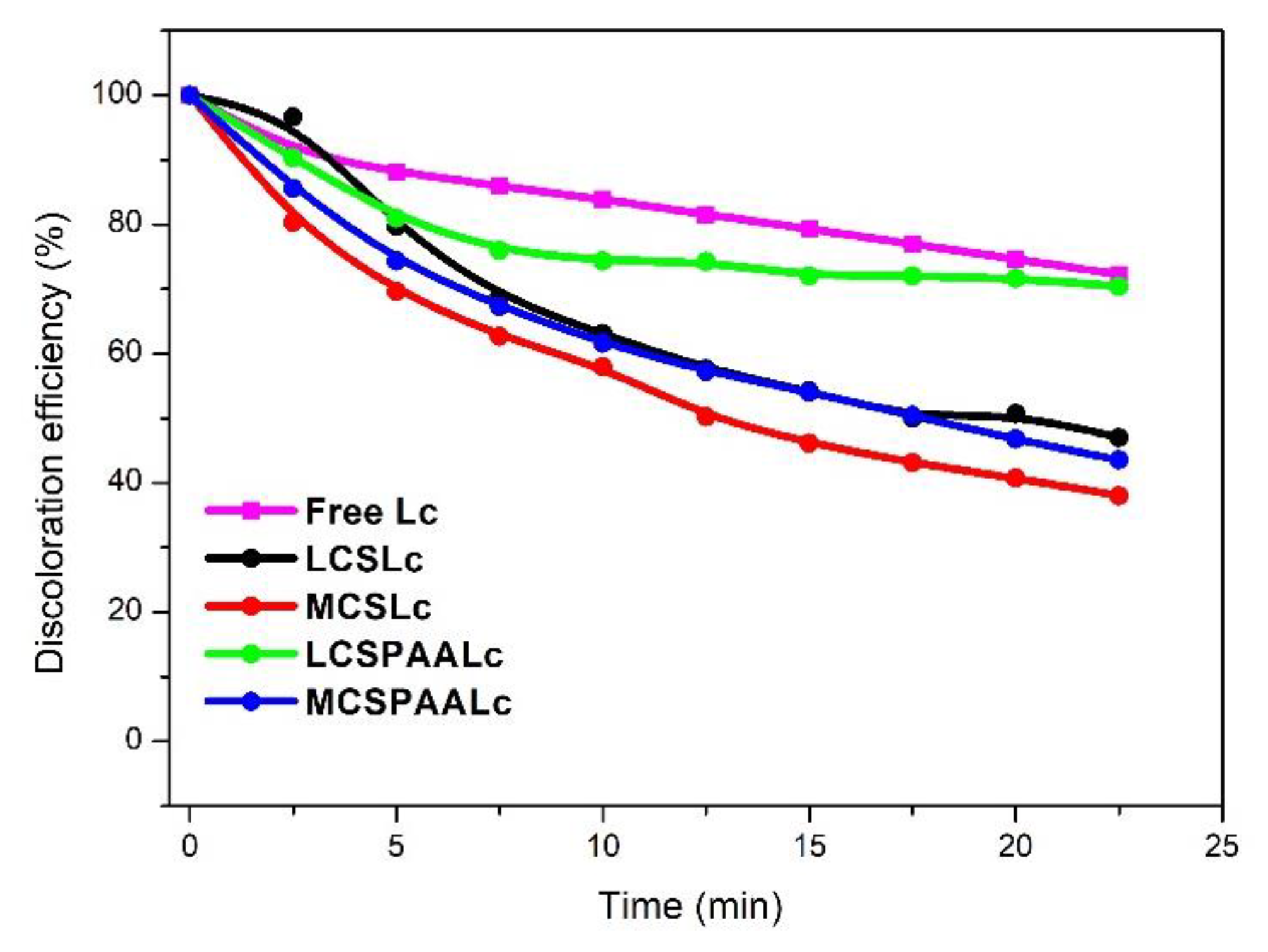
3.6.1. Discoloration of MR and Recyclability
3.6.2. Recyclability of Immobilized Laccase
3.6.3. Degradation Product Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marques, C.M.; Moniz, S.; de Sousa, J.P.; Barbosa-Póvoa, A.P. A simulation-optimization approach to integrate process design and planning decisions under technical and market uncertainties: A case from the chemical-pharmaceutical industry. Comput. Chem. Eng. 2017, 106, 796–813. [Google Scholar] [CrossRef] [Green Version]
- Guangorena Zarzosa, G.I.; Kobayashi, T. Sustainable Polymer Used as Renewable Source for Medical Industry. In Encyclopedia of Materials: Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 850–858. [Google Scholar] [CrossRef]
- Santos, A.S.; Raydan, D.; Cunha, J.C.; Viduedo, N.; Silva, A.M.S.; Marques, M.M.B. Advances in green catalysis for the synthesis of medicinally relevant N-heterocycles. Catalyst 2021, 11, 1108. [Google Scholar] [CrossRef]
- Islama, S.; Basumatary, B.; Rokhum, S.L.; Mochahari, P.; Basumatary, S. Advancement in utilization of nanomaterials as efficient and recyclable solid catalyst for biodiesel synthesis. Chem. Eng. Technol. 2022, 3, 10043. [Google Scholar] [CrossRef]
- Bilal, M.; Ashraf, S.S.; Cui, J.; Lou, W.Y.; Franco, M.; Mulla, S.I.; Iqbal, H.M.N. Harnessing the biocatalytic attributes and applied perspectives of nanoengineered laccases—A review. Int. J. Biol. Macromol. 2021, 166, 352–373. [Google Scholar] [CrossRef]
- Martínková, L.; Křístková, B.; Křen, V. Laccases and Tyrosinases in Organic Synthesis. Int. J. Mol. Sci. 2022, 23, 3462. [Google Scholar] [CrossRef]
- Dăscălescu, D.; Apetrei, C. Development of a Novel Electrochemical Biosensor Based on Organized Mesoporous Carbon and Laccase for the Detection of Serotonin in Food Supplements. Chemosensors 2022, 10, 365. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Sustainable Biosynthesis of Esterase Enzymes of Desired Characteristics of Catalysis for Pharmaceutical and Food Industry Employing Specific Strains of Microorganisms. Sustainability 2022, 14, 8673. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Tringali, C. Laccase-mediated synthesis of bioactive natural products and their analogues. RSC Chem. Biol. 2022, 3, 614–647. [Google Scholar] [CrossRef]
- Othman, A.M.; Sanroman, A.; Molde, D. Laccase-Oriented Immobilization Using Concanavalin A as an Approach for Efficient Glycoproteins Immobilization and Its Application to the Removal of Aqueous Phenolics. Sustainability 2022, 14, 13306. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Cost analysis in laccase production. J. Environ. Manag. 2011, 92, 2907–2912. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; De los Santos, M.H.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Fact. 2019, 18, 200. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Petrila, L.M.; Grădinaru, V.R.; Bucatariu, F.; Mihai, M. Polymer/Enzyme Composite Materials-Versatile Catalysts with Multiple Applications. Chemistry 2022, 4, 1312–1338. [Google Scholar] [CrossRef]
- Silva, A.R.M.; Alexandre, J.Y.N.H.; Souza, J.E.S.; Lima Neto, J.G.; de Sousa Júnior, P.G.; Rocha, M.V.P.; dos Santos, J.C.S. The Chemistry and Applications of Metal-Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties- Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [Green Version]
- Santa Cruz Matins de Queiroz, A.; Fook, B.R.P.L.; De Oliveira Lima, V.A.; De Farias Rached, R.I.; Lima, E.P.N.; Da Silva Lima, R.J.; Covas, C.A.P.; Fook, M.V.L. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Mane, S.; Pathan, E.; Tupe, S.; Deshmukh, S.; Kale, D.; Ghormade, V.; Chaudhari, B.; Deshpande, M. Isolation and Characterization of Chitosans from Different Fungi with Special Emphasis on Zygomycetous Dimorphic Fungus Benjaminiella poitrasii: Evaluation of Its Chitosan Nanoparticles for the Inhibition of Human Pathogenic Fungi. Biomacromolecules 2022, 23, 808–815. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Biró, E.; Németh, A.S.; Sisak, C.; Feczkó, T.; Gyenis, J. Preparation of chitosan particles suitable for enzyme immobilization. J. Biochem. Biophys. Methods 2008, 70, 1240–1246. [Google Scholar] [CrossRef]
- Degórska, O.; Zdarta, J.; Synoradzki, K.; Zgola-Grzeskowiak, A.; Ciesielczyk, F.; Jesionowski, T. From core-shell like structured zirconia/magnetite hybrid towards novel biocatalytic systems for tetracycline removal: Synthesis, enzyme immobilization, degradation and toxicity study. J. Environ. Chem. Eng. 2021, 9, 105701. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Yang, W.; Wang, C.; Fu, S. Preparation and characterization of chitosan-poly(acrylic acid) polymer magnetic microspheres. Polymer 2006, 47, 5287–5294. [Google Scholar] [CrossRef]
- Leontieș, A.R.; Răducan, A.; Culiță, D.C.; Alexandrescu, E.; Moroșan, A.; Mihaiescu, D.E.; Aricov, L. Laccase immobilized on chitosan-polyacrylic acid microspheres as highly efficient biocatalyst for naphthol green B and indigo carmine degradation. Chem. Eng. J. 2022, 439, 135654. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Zhao, G.; Sun, H.; Che, H.; Leng, X. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydr. Polym. 2020, 231, 115689. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Li, X.; Yuan, F. The stabilization and release performances of curcumin-loaded liposomes coated by high and low molecular weight chitosan. Food Hydrocoll. 2020, 99, 105355. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmed, N.B.; Adegoke, K.A.; Bello, O.S. Sorption studies of methyl red dye removal using lemon grass (Cymbopogon citratus). Chem. Data Collect. 2019, 22, 100249. [Google Scholar] [CrossRef] [Green Version]
- Takkar, S.; Tyagi, B.; Kumar, N.; Kumari, T.; Iqbal, K.; Varma, A.; Thakur, I.S.; Mishra, A. Biodegradation of methyl red dye by a novel actinobacterium Zhihengliuella sp. ISTPL4: Kinetic studies, isotherm and biodegradation pathway. Environ. Technol. Innov. 2022, 26, 102348. [Google Scholar] [CrossRef]
- Aricov, L.; Bǎran, A.; Simion, E.L.; Gîfu, I.C.; Anghel, D.F.; Jerca, V.; Vuluga, M. New insights into the self-assembling of some hydrophobically modified polyacrylates in aqueous solution. Colloid. Polym. Sci. 2016, 294, 667–679. [Google Scholar] [CrossRef]
- Höfer, C.; Schlosser, D. Novel enzymatic oxidation of Mn2+ to Mn3+ catalyzed by a fungal laccase. FEBS Lett. 1999, 451, 186–190. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, M.; Geng, Y.; Huang, J. ABTS-modified silica nanoparticles as laccase mediators for decolorization of indigo carmine dye. J. Chem. 2015, 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Aricov, L.; Leonties, A.R.; Gîfu, I.C.; Preda, D.; Raducan, A.; Anghel, D.F. Enhancement of laccase immobilization onto wet chitosan microspheres using an iterative protocol and its potential to remove micropollutants. J. Environ. Manag. 2020, 276, 111326. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Precupas, A.; Leonties, A.R.; Neacsu, A.; Angelescu, D.G.; Popa, V.T. Bovine hemoglobin thermal stability in the presence of naringenin: Calorimetric, spectroscopic and molecular modeling studies. J. Mol. Liq. 2022, 361, 119617. [Google Scholar] [CrossRef]
- Varlan, A. Hillebrand, Exploring the capabilities of TDDFT calculations to explain the induced chirality upon a binding process: A simple case, 3- M. carboxycoumarin. J. Mol. Struct. 2013, 1036, 341–349. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Precupas, A.; Ionescu, S. Exploring the interaction of 5,6-benzocoumarin-3-carboxylic acid with bovine serum albumin at the molecular level: A biophysical investigation using molecular dynamics. Rev. Roum. Chim. 2021, 66, 49–58. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Discovery Studio, San Diego: Dassault Systèmes. 2019. Available online: http://accelrys.com/products/discovery-studio (accessed on 3 August 2022).
- Cerón, A.A.; Nascife, L.; Norte, S.; Costa, S.A.; do Nascimento, J.H.O.; Dal Pont Morisso, F.; Baruque-Ramos, J.; Oliveira, R.C.; Costa, S.M. Synthesis of chitosan-lysozyme microspheres, physicochemical characterization, enzymatic and antimicrobial activity. Int. J. Biol. Macromol. 2021, 185, 572–581. [Google Scholar] [CrossRef]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M.N. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Spinelli, D.; Fatarella, E.; di Michele, A.; Pogni, R. Immobilization of fungal (Trametes versicolor) laccase onto Amberlite IR-120 H beads: Optimization and characterization. Process. Biochem. 2013, 48, 218–223. [Google Scholar] [CrossRef]
- Aydemir, T.; Güler, S. Characterization and immobilization of Trametes versicolor laccase on magnetic chitosan-clay composite beads for phenol removal. Artif. Cells Nanomed. Biotechnol. 2015, 43, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Christiano, C.S.; Fortes, A.L.; Daniel-da-Silva, A.M.R.B.; Xavier, A.; Tavares, P.M. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem. Eng. Process. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- De Mello, M.D.; Cordeiro, D.; Costa, L.T.; Follmer, C. Catalytic properties of lipases immobilized onto ultrasound-treated chitosan supports. Biotechnol. Bioprocess Eng. 2013, 18, 1090–1100. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Activities, stabilities, and reaction kinetics of three free and chitosan-clay composite immobilized enzymes. Enzyme Microb. Technol. 2005, 36, 75–82. [Google Scholar] [CrossRef]
- Christensen, N.J.; Kepp, K.P. Setting the stage for electron transfer: Molecular basis of ABTS-binding to four laccases from Trametes versicolor at variable pH and protein oxidation state. J. Mol. Catal. B Enzym. 2014, 100, 68–77. [Google Scholar] [CrossRef]
- Sharma, S.; Pathak, S.; Sharma, K.P. Toxicity of the azo dye methyl red to the organisms in microcosms, with special reference to the Guppy (Poecilia reticulata Peters). Bull. Environ. Contam. Toxicol. 2003, 70, 753–760. [Google Scholar] [CrossRef]
- Vršanská, M.; Voběrková, S.; Jiménez, A.M.; Strmiska, V.; Adam, V. Preparation and optimisation of cross-linked enzyme aggregates using native isolate white rot fungi Trametes versicolor and Fomes fomentarius for the decolourisation of synthetic dyes. Int. J. Environ. Res. Public Health 2018, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Skoronski, E.; Fernandes, M.; De Lourdes Borba Magalhães, M.; Da Silva, G.F.; João, J.J.; Soares, C.H.L.; Fúrigo Júnior, A. Substrate specificity and enzyme recycling using Chitosan immobilized laccase. Molecules 2014, 19, 16794–16809. [Google Scholar] [CrossRef]
- Zheng, F.; Cui, B.K.; Wu, X.J.; Meng, G.; Liu, H.X.; Si, J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodet. Biodegrad. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Matsumoto, M.; Yamaguchi, M.; Yoshida, Y.; Senuma, M.; Takashima, H.; Kawamura, T.; Kato, H.; Takahashi, M.; Hirata-Koizumi, M.; Ono, A.; et al. An antioxidant, N,N’-diphenyl-p-phenylenediamine (DPPD), affects labor and delivery in rats: A 28-day repeated dose test and reproduction/developmental toxicity test. Food Chem. Toxicol. 2013, 56, 290–296. [Google Scholar] [CrossRef]
- Available online: https://echa.europa.eu/documents/10162/81052d17-6bc1-972a-ebfd-7f9aa40644e7 (accessed on 3 November 2022).
- Sari, I.P.; Simarani, K. Comparative static and shaking culture of metabolite derived from methyl red degradation by Lysinibacillus fusiformis strain W1B6. R. Soc. Open Sci. 2019, 6, 190152. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.K.; Yuen, P.Y. Decolourization and biodegradation of N,N’-dimethyl-p-phenylenediamine by Klebsiella pneumoniae RS-13 and Acetobacter liquefaciens S-1. J. Appl. Microbiol. 1998, 85, 79–87. [Google Scholar] [CrossRef]
- Mittal, Y.; Dash, S.; Srivastava, P.; Mishra, P.M.; Aminabhavi, T.M.; Yadav, A.K. Azo dye containing wastewater treatment in earthen membrane based unplanted two chambered constructed wetlands-microbial fuel cells: A new design for enhanced performance. Chem. Eng. J. 2022, 427, 131856. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Wang, X.; Vekaria, A.; Jiang, M.; Zhang, H. Enhancing anthranilic acid biosynthesis using biosensor-assisted cell selection and in situ product removal. Biochem. Eng. J. 2020, 62, 107722. [Google Scholar] [CrossRef]

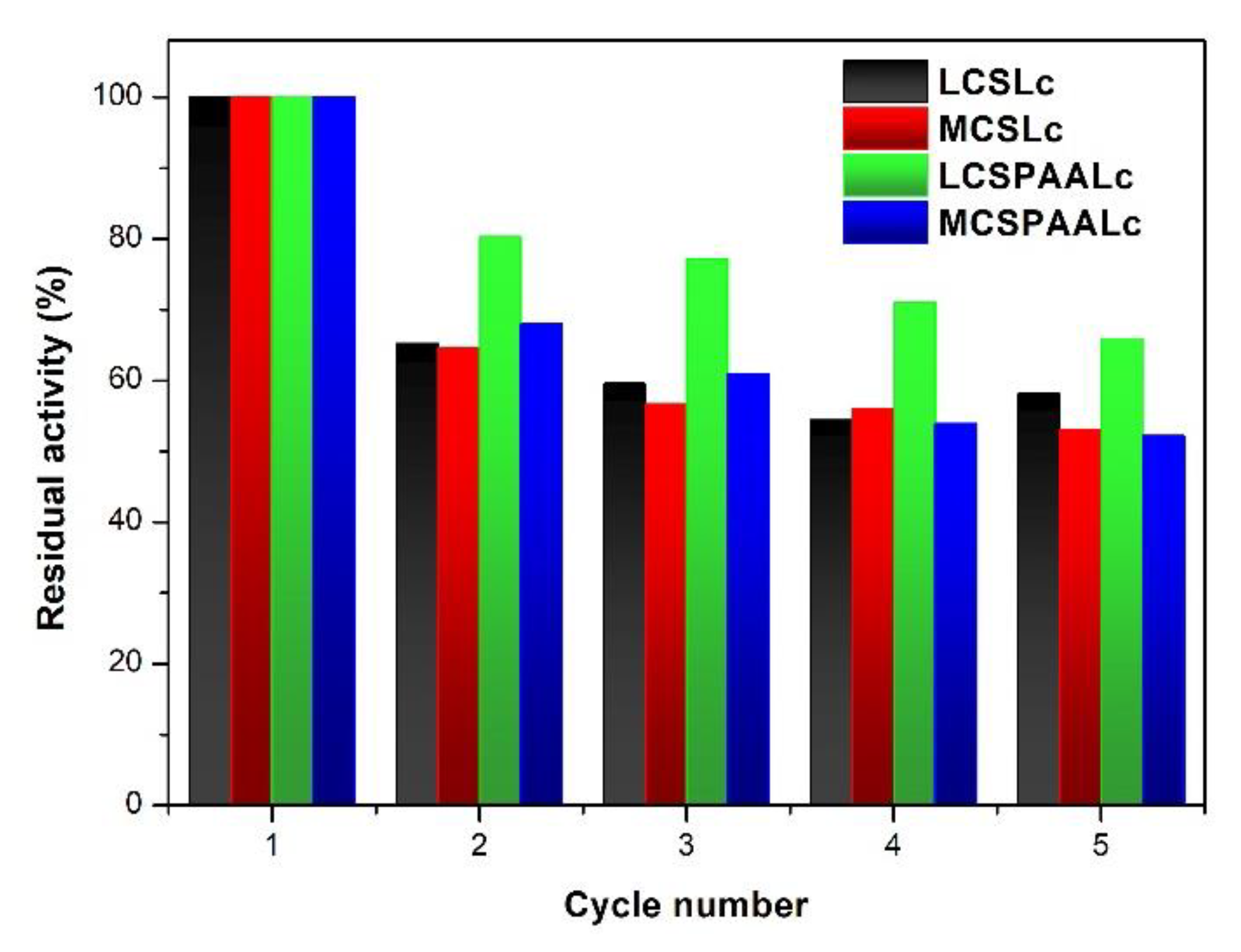
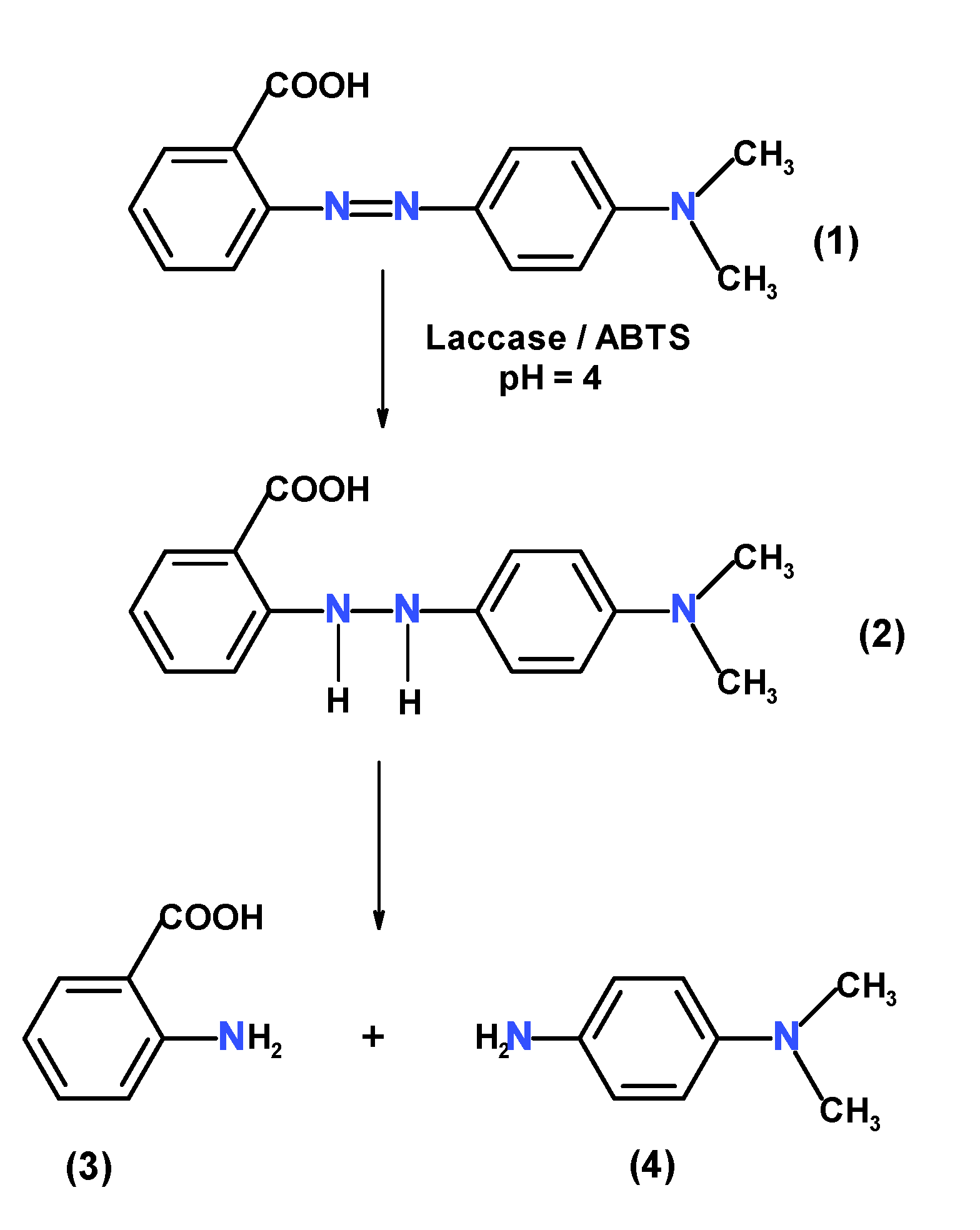
| System | Km (M) | Vmax (M·s−1) | kcat (s−1) | kcat/Km (M−1s−1) |
|---|---|---|---|---|
| LCSLc | 1.85 × 10−4 | 2.60 × 10−7 | 4.23 | 2.29 × 104 |
| MCSLc | 2.45 × 10−4 | 3.33 × 10−7 | 3.91 | 1.60 × 104 |
| LCSPAALc | 0.40 × 10−4 | 0.60 × 10−7 | 2.49 | 6.22 × 104 |
| MCSPAALc | 0.93 × 10−4 | 1.10 × 10−7 | 2.61 | 2.82 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aricov, L.; Raducan, A.; Gifu, I.C.; Alexandrescu, E.; Precupas, A.; Neculae, A.V.F.; Visan, R.M.; Morosan, A.; Leonties, A.R. The Immobilization of Laccase on Mixed Polymeric Microspheres for Methyl Red Decomposition. Coatings 2022, 12, 1965. https://doi.org/10.3390/coatings12121965
Aricov L, Raducan A, Gifu IC, Alexandrescu E, Precupas A, Neculae AVF, Visan RM, Morosan A, Leonties AR. The Immobilization of Laccase on Mixed Polymeric Microspheres for Methyl Red Decomposition. Coatings. 2022; 12(12):1965. https://doi.org/10.3390/coatings12121965
Chicago/Turabian StyleAricov, Ludmila, Adina Raducan, Ioana Catalina Gifu, Elvira Alexandrescu, Aurica Precupas, Alexandru Vincentiu Florian Neculae, Raluca Marieta Visan, Alina Morosan, and Anca Ruxandra Leonties. 2022. "The Immobilization of Laccase on Mixed Polymeric Microspheres for Methyl Red Decomposition" Coatings 12, no. 12: 1965. https://doi.org/10.3390/coatings12121965
APA StyleAricov, L., Raducan, A., Gifu, I. C., Alexandrescu, E., Precupas, A., Neculae, A. V. F., Visan, R. M., Morosan, A., & Leonties, A. R. (2022). The Immobilization of Laccase on Mixed Polymeric Microspheres for Methyl Red Decomposition. Coatings, 12(12), 1965. https://doi.org/10.3390/coatings12121965








