Plasma-Induced Graft Polymerization of Polyethylenimine onto Chitosan/Polycaprolactone Composite Membrane for Heavy Metal Pollutants Treatment in Industrial Wastewater
Abstract
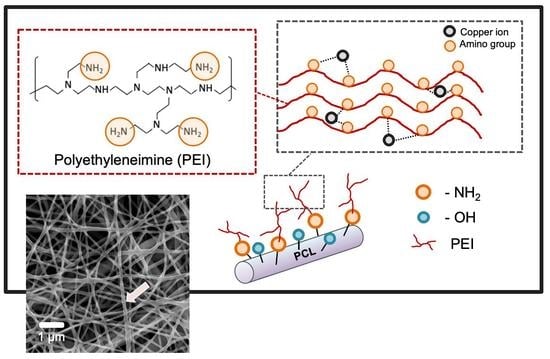
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membranes Fabrication
2.3. Scanning Electron Microscopy (SEM)
2.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.5. X-ray Photoelectron Spectroscopy (XPS)
3. Results and Discussion
3.1. Chacterizations of CS/PCL Nanofibers
3.2. Chacterizations of CS/PCL Nanofibers after APNP
3.3. Chacterizations of PEI Grafted CS/PCL Nanofibers
3.4. Adsorption of PEI Grafted CS/PCL Nanofibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Kempf, T.; Bobek, V.; Horvat, T. The Impacts of the American-Chinese Trade War and COVID-19 Pandemic on Taiwan’s Sales in Semiconductor Industry. Int. J. Econ. Financ. 2021, 13, 62–72. [Google Scholar] [CrossRef]
- Kumar, P. Ukraine War to Extend Chip Supply Crunch: Semiconductor Group. Business, Nikkei Asia. 2022. Available online: https://asia.nikkei.com/Business/Tech/Semiconductors/Ukraine-war-to-extend-chip-supply-crunch-semiconductor-group (accessed on 15 September 2022).
- Wu, S. Analysis: Chip Industry Rethinks Taiwan Risk after Pelosi Visit but Options Limited. Technology, Reuters. 2022. Available online: https://www.reuters.com/technology/chip-industry-rethinks-taiwan-risk-after-pelosi-visit-options-limited-2022-10-07 (accessed on 15 September 2022).
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabii, M.; Rezauem, A.; Ghaedi, M. Simultaneous extraction and preconcentration of some metal ions using eucalyptus-wood based activated carbon modified with silver hydroxide nanoparticles and a chelating agent: Optimization by an experimental design. RSC Adv. 2015, 5, 89204–89217. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A. The performance of nanorods material as adsorbent for removal of azo dyes and heavy metal ions: Application of ultrasound wave, optimization and modeling. Ultrason. Sonochem. 2017, 34, 792–802. [Google Scholar] [CrossRef]
- Cheng, W.; Ding, C.; Wu, Q.; Wang, X.; Sun, Y.; Shi, W.; Hayat, T.; Alsaedi, A.; Chai, Z.; Wang, X. Mutual effect of U(VI) and Sr(II) on graphene oxides: Evidence from EXAFS and theoretical calculations. Environ. Sci. Nano 2017, 4, 1124–1131. [Google Scholar] [CrossRef]
- Trakal, L.; Šigut, R.; Šillerová, H.; Faturíková, D.; Komárek, M. Copper removal from aqueous solution using biochar: Effect of chemical activation. Arab. J. Chem. 2014, 7, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Zafar, S.; Khan, M.I.; Lashari, M.H.; Khraisheh, M.; Almomani, F.; Mirza, M.L.; Khalid, N. Removal of copper ions from aqueous solution using NaOH-treated rice husk. Emergent Mater. 2022, 3, 857–870. [Google Scholar] [CrossRef]
- Das, P.; Sharma, M.; Purkait, M. Recent progress on electrocoagulation process for wastewater treatment: A review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Fedje, K.K.; Strömvall, A.M. Enhanced soil washing with copper recovery using chemical precipitation. J. Environ. Manag. 2019, 236, 68–74. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, W.; Liu, J.; Ye, W.; Lin, J.; Xie, J.; Huang, X.; Gao, S.; Xie, J.; Liu, S.; et al. An integrated process of chemical precipitation and sulfate reduction for treatment of flue gas desulphurization wastewater from coal-fired power plant. J. Clean. Prod. 2019, 228, 63–72. [Google Scholar] [CrossRef]
- Li, Y.; Fu, F.; Cai, W.; Tang, B. Synergistic effect of mesoporous feroxyhyte nanoparticles and Fe(II) on phosphate immobilization: Adsorption and chemical precipitation. Powder Technol. 2019, 345, 786–795. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Q.; Huang, G.; Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 2020, 27, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Bezzina, J.; Ruder, L.; Dawson, R.; Ogden, M. Ion exchange removal of Cu(II), Fe(II), Pb(II) and Zn(II) from acid extracted sewage sludge—Resin screening in weak acid media. Water Res. 2019, 158, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Hoslett, J.; Ghazal, H.; Ahmad, D.; Jouhara, H. Removal of copper ions from aqueous solution using low temperature biochar derived from the pyrolysis of municipal solid waste. Sci. Total Environ. 2019, 673, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Niu, X.; Li, X.; He, T. Selective separation of copper and nickel by membrane extraction using hydrophilic nanoporous ion-exchange barrier membranes. Process Saf. Environ. Prot. 2018, 113, 1–9. [Google Scholar] [CrossRef]
- Mokhtar, M.; Dickson, S.; Kim, Y.; Mekky, W. Preparation and characterization of ion selective membrane and its application for Cu2+ removal. J. Ind. Eng. Chem. 2018, 60, 475–484. [Google Scholar] [CrossRef]
- Khan, J.; Lin, S.; Nizeyimana, J.; Wu, Y.; Wang, Q.; Liu, X. Removal of copper ions from wastewater via adsorption on modified hematite (α-Fe2O3) iron oxide coated sand. J. Clean. Prod. 2021, 319, 128687. [Google Scholar] [CrossRef]
- Shahin, S.; Mossad, M.; Fouad, M. Evaluation of copper removal efficiency using water treatment sludge. Water Sci. Eng. 2019, 12, 37–44. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Eleryan, A.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Eldeeb, T.M.; El-Nemr, M.A.; Hassaan, M.A.; Ragab, S.; Osibote, O.A.; Kusuma, H.S.; et al. Copper(II) ion removal by chemically and physically modified sawdust biochar. In Biomass Conversion and Biorefinery; Springer: Dordrecht, The Netherlands, 2022. [Google Scholar]
- Meringer, A.; Liffourrena, A.; Heredia, M.; Lucchesi, G.; Boeris, P. Removal of copper and/or zinc ions from synthetic solutions by immobilized, non-viable bacterial biomass: Batch and fixed-bed column lab-scale study. J. Biotechnol. 2021, 328, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Al-Homaidan, A.; Al-Houri, H.; Al-Hazzani, A.; Elgaaly, G.; Moubayed, N. Biosorption of copper ions from aqueous solutions by Spirulina platensis biomass. Arab. J. Chem. 2014, 7, 57–62. [Google Scholar] [CrossRef] [Green Version]
- SenthilKumar, P.; Ramalingam, S.; Sathyaselvabala, V.; Kirupha, S.D.; Sivanesan, S. Removal of copper (II) ions from aqueous solution by adsorption using cashew nut shell. Desalination 2011, 266, 63–71. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Chong, L.; Zarith, N.; Sultana, N. Poly(Caprolactone)/chitosan-based scaffold using freeze drying technique for bone tissue engineering application. In Proceedings of the 2015 10th Asian Control Conference (ASCC), Kota Kinabalu, Malaysia, 31 May–3 June 2015; IEEE Xplore: Piscataway, NJ, USA, 2006. [Google Scholar]
- Khandaker, M.; Progri, H.; Arasu, D.T.; Nikfarjam, S.; Shamim, N. Use of Polycaprolactone Electrospun Nanofiber Mesh in a Face Mask. Materials 2021, 14, 4272. [Google Scholar] [CrossRef]
- Tian, G.; Huang, Z.; Wang, H.; Cui, C.; Zhang, Y. Polycaprolactone nanofiber membrane modified with halloysite and ZnO for anti-bacterial and air filtration. Appl. Clay Sci. 2022, 223, 106512. [Google Scholar] [CrossRef]
- Mourabit, F.; Boulaid, M. Pesticide removal in drinking water treatment using biodegradable polymers. Mater. Today Proc. 2019, 13, 1033–1038. [Google Scholar] [CrossRef]
- Boley, A.; Mergaert, J.; Muller, C.; Lebrenz, H.; Cnockaert, M.C.; Müller, W.-R.; Swings, J. Denitrification and Pesticide Elimination in Drinking Water Treatment with the Biodegradable Polymer Poly(ϵ-caprolactone) (PCL). Acta Hydrochim. Et Hydrobiol. 2003, 31, 195–203. [Google Scholar] [CrossRef]
- Maio, A.; Gammino, M.; Gulino, E.; Megna, B.; Fara, P.; Scaffaro, R. Rapid One-Step Fabrication of Graphene Oxide-Decorated Polycaprolactone Three-Dimensional Templates for Water Treatment. ACS Appl. Polym. Mater. 2020, 2, 4993–5005. [Google Scholar] [CrossRef]
- Nivedita, S.; Joseph, S. Performance of polycaprolactone/TiO2 composite membrane for the effective treatment of dairy effluents. Water Sci. Technol. 2021, 83, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Oldinski, R.; Ma, H.; Bryers, J.; Zhang, M. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohydr. Polym. 2013, 92, 254–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakib, M.; Mallik, A.; Rahman, M. Update on chitosan-based electrospun nanofibers for wastewater treatment: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible core-shell/bead-like alginate@PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr(VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Elmas, S.; Gedefaw, D.; Larsson, M.; Ying, Y.; Cavallaro, A.; Andersson, G.; Nydén, M.; Andersson, M. Porous PEI Coating for Copper Ion Storage and Its Controlled Electrochemical Release. Adv. Sustain. Syst. 2020, 4, 1900123. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, B.; Li, H.; Zhang, W.; Lv, L.; Wu, J. Selective Removal of Cu(II) Ions by Using Cation-exchange Resin-Supported Polyethyleneimine (PEI) Nanoclusters. Environ. Sci. Technol. 2010, 44, 3508–3513. [Google Scholar] [CrossRef]
- Mangindaan, D.; Chen, C.T.; Wang, M.J. Integrating sol–gel with cold plasmas modified porous polycaprolactone membranes for the drug-release of silver-sulfadiazine and ketoprofen. Appl. Surf. Sci. 2012, 262, 114–119. [Google Scholar] [CrossRef]
- Mangindaan, D.; Kuo, W.H.; Wang, M.J. Two-dimensional amine-functionality gradient by plasma polymerization. Biochem. Eng. J. 2013, 78, 198–204. [Google Scholar] [CrossRef]
- Schueren, L.; Steyaert, I.; Schoenmaker, B.; Clerck, K. Polycaprolactone/chitosan blend nanofibres electrospun from an acetic acid/formic acid solvent system. Carbohydr. Polym. 2012, 88, 1221–1226. [Google Scholar] [CrossRef]
- Stevens, J.; Schroeder, S. Quantitative analysis of saccharides by X-ray photoelectron spectroscopy. Surf. Interface Anal. 2009, 41, 453–462. [Google Scholar] [CrossRef]
- Roozbahani, R.; Sultana, N.; Almasi, D.; Naghizadeh, F. Effects of Chitosan Concentration on the Protein Release Behaviour of Electrospun Poly(-caprolactone)/Chitosan Nanofibers. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Abdelrazek, E.; Hezma, A.; El-khodary, A.; Elzayat, A. Spectroscopic studies and thermal properties of PCL/PMMA biopolymer blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F.; Czerkies, M.; Kołbuk, D. Structure and properties of polycaprolactone/chitosan nonwovens tailored by solvent systems. Biomed. Mater. 2017, 12, 015020. [Google Scholar] [CrossRef]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Manea, L.R.; Lupu, I.G.; Cramariuc, O. Fiber diameter in electrospinning process. J. Electrost. 2013, 71, 189–198. [Google Scholar] [CrossRef]
- Yarin, A.L.; Kataphinan, W.; Reneker, D.H. Branching in electrospinning of nanofibers. J. Appl. Phys. 2005, 98, 064501. [Google Scholar] [CrossRef] [Green Version]
- Dvoryaninova, O.; Sokolov, A.; Peregonchaya, O.; Solovyeva, E.; Syanov, E. Identification of composition and structure of functional groups of ferment lysates based on IR spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 032062. [Google Scholar] [CrossRef]
- Khelifa, F.; Ershov, S.; Habibi, Y.; Snyders, R.; Dubois, P. Free-Radical-Induced Grafting from Plasma Polymer Surfaces. Chem. Rev. 2016, 116, 3975–4005. [Google Scholar] [CrossRef]
- Hu, T.-L.; Chen, G.-Y.; Shi, S.-C.; Yang, J.H.C. Plasma-Initiated Grafting of Bioactive Peptide onto Nano-CuO/Tencel Membrane. Polymers 2022, 14, 4497. [Google Scholar] [CrossRef]
- Yasuda, H. New Insights into Aging Phenomena from Plasma Chemistry. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 515, 15–30. [Google Scholar] [CrossRef]
- Can-Herrera, L.A.; Ávila-Ortega, A.; de la Rosa-García, S.; Oliva, A.I.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Surface modification of electrospun polycaprolactone microfibers by air plasma treatment: Effect of plasma power and treatment time. Eur. Polym. J. 2016, 84, 502–513. [Google Scholar] [CrossRef]
- Sadeghi-avalshahr, A.R.; Nokhasteh, S.; Molavi, A.M.; Mohammad-pour, N.; Sadeghi, M. Tailored PCL Scaffolds as Skin Substitutes Using Sacrificial PVP Fibers and Collagen/Chitosan Blends. Int. J. Mol. Sci. 2020, 21, 2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Li, L.; Chen, B.; Shi, S.; Nie, J.; Ma, G. Functionalized chitosan electrospun nanofiber membranes for heavy-metal removal. Polymer 2019, 163, 74–85. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Yang, X.; Peng, P.; Li, Z.; Jin, C. Enhanced Transformation of Cr(VI) by Heterocyclic-N within Nitrogen-Doped Biochar: Impact of Surface Modulatory Persistent Free Radicals (PFRs). Environ. Sci. Technol. 2020, 54, 8123–8132. [Google Scholar] [CrossRef]
- Pinilla-Torres, A.M.; Carrión-García, P.Y.; Sánchez-Domínguez, C.N.; Gallardo-Blanco, H.; Sánchez-Domínguez, M. Modification of Branched Polyethyleneimine Using Mesquite Gum for Its Improved Hemocompatibility. Polymers 2021, 13, 2766. [Google Scholar] [CrossRef]
- Islam, M.S.; Choi, W.S.; Lee, H. Controlled Etching of Internal and External Structures of SiO2 Nanoparticles Using Hydrogen Bond of Polyelectrolytes. ACS Appl. Mater. Interfaces 2014, 6, 9563–9571. [Google Scholar] [CrossRef]
- Mangindaan, D.; Kuo, W.H.; Kurniawan, H.; Wang, M.J. Creation of biofunctionalized plasma polymerized allylamine gradients. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1361–1367. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Mi, H.; Salick, M.; Cordie, T.; McNulty, J.; Peng, X.; Turng, L. In vitro evaluations of electrospun nanofiber scaffolds composed of poly(ɛ-caprolactone) and polyethylenimine. J. Mater. Res. 2015, 30, 1808–1819. [Google Scholar] [CrossRef]
- Kislenko, V.; Oliynyk, L. Complex formation of polyethyleneimine with copper(II), nickel(II), and cobalt(II) ions. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 914–922. [Google Scholar] [CrossRef]







| C1s | N1s | O1s | |
|---|---|---|---|
| CS/PCL | 74.4 | 1.5 | 24.2 |
| PEI-CS/PCL_0s | 72.2 | 1.8 | 26.0 |
| PEI-CS/PCL_15s | 70.5 | 2.0 | 27.5 |
| PEI-CS/PCL_30s | 70.3 | 2.1 | 27.7 |
| PEI-CS/PCL_60s | 70.1 | 3.3 | 26.6 |
| Contact Time (min) | Copper Ion Concentration (ppm) | Adsorption (%) |
|---|---|---|
| 0 | 100 | -- |
| 10 | 88.13 | 7.76 |
| 20 | 86.40 | 11.7 |
| 30 | 78.80 | 15.9 |
| 60 | 77.06 | 17.7 |
| 120 | 75.80 | 19.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, S.-L.; Chen, C.-K.; Shi, S.-C.; Yang, J.H.C. Plasma-Induced Graft Polymerization of Polyethylenimine onto Chitosan/Polycaprolactone Composite Membrane for Heavy Metal Pollutants Treatment in Industrial Wastewater. Coatings 2022, 12, 1966. https://doi.org/10.3390/coatings12121966
Tu S-L, Chen C-K, Shi S-C, Yang JHC. Plasma-Induced Graft Polymerization of Polyethylenimine onto Chitosan/Polycaprolactone Composite Membrane for Heavy Metal Pollutants Treatment in Industrial Wastewater. Coatings. 2022; 12(12):1966. https://doi.org/10.3390/coatings12121966
Chicago/Turabian StyleTu, Sung-Lin, Chih-Kuang Chen, Shih-Chen Shi, and Jason Hsiao Chun Yang. 2022. "Plasma-Induced Graft Polymerization of Polyethylenimine onto Chitosan/Polycaprolactone Composite Membrane for Heavy Metal Pollutants Treatment in Industrial Wastewater" Coatings 12, no. 12: 1966. https://doi.org/10.3390/coatings12121966
APA StyleTu, S.-L., Chen, C.-K., Shi, S.-C., & Yang, J. H. C. (2022). Plasma-Induced Graft Polymerization of Polyethylenimine onto Chitosan/Polycaprolactone Composite Membrane for Heavy Metal Pollutants Treatment in Industrial Wastewater. Coatings, 12(12), 1966. https://doi.org/10.3390/coatings12121966