Effective Fluorescence Detection of Hydrazine and the Photocatalytic Degradation of Rhodamine B Dye Using CdO-ZnO Nanocomposites
Abstract
:1. Introduction
2. Materials and Experimental Details
2.1. Materials
2.2. Synthesis of CdO-ZnO Nanocomposites
2.3. Characterization Techniques for CdO-ZnO Nanocomposites
2.4. Photocatalytic Dye Degradation
2.5. Fluorescence-Based Hydrazine Chemical Sensor
3. Results and Discussion
3.1. Characterization of CdO-ZnO Nanocomposites
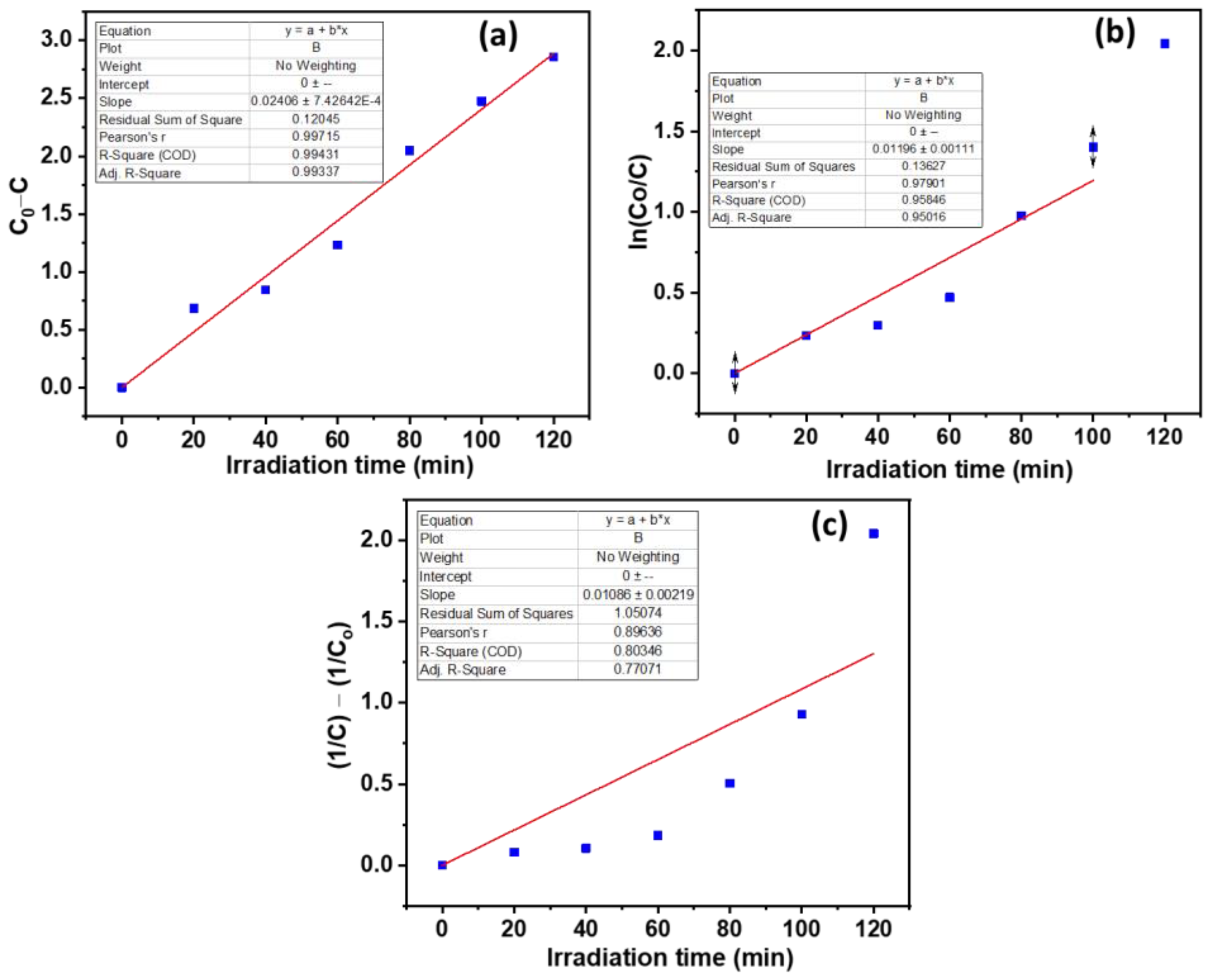
3.2. Photocatalytic Degradation Applications of CdO-ZnO Nanocomposites
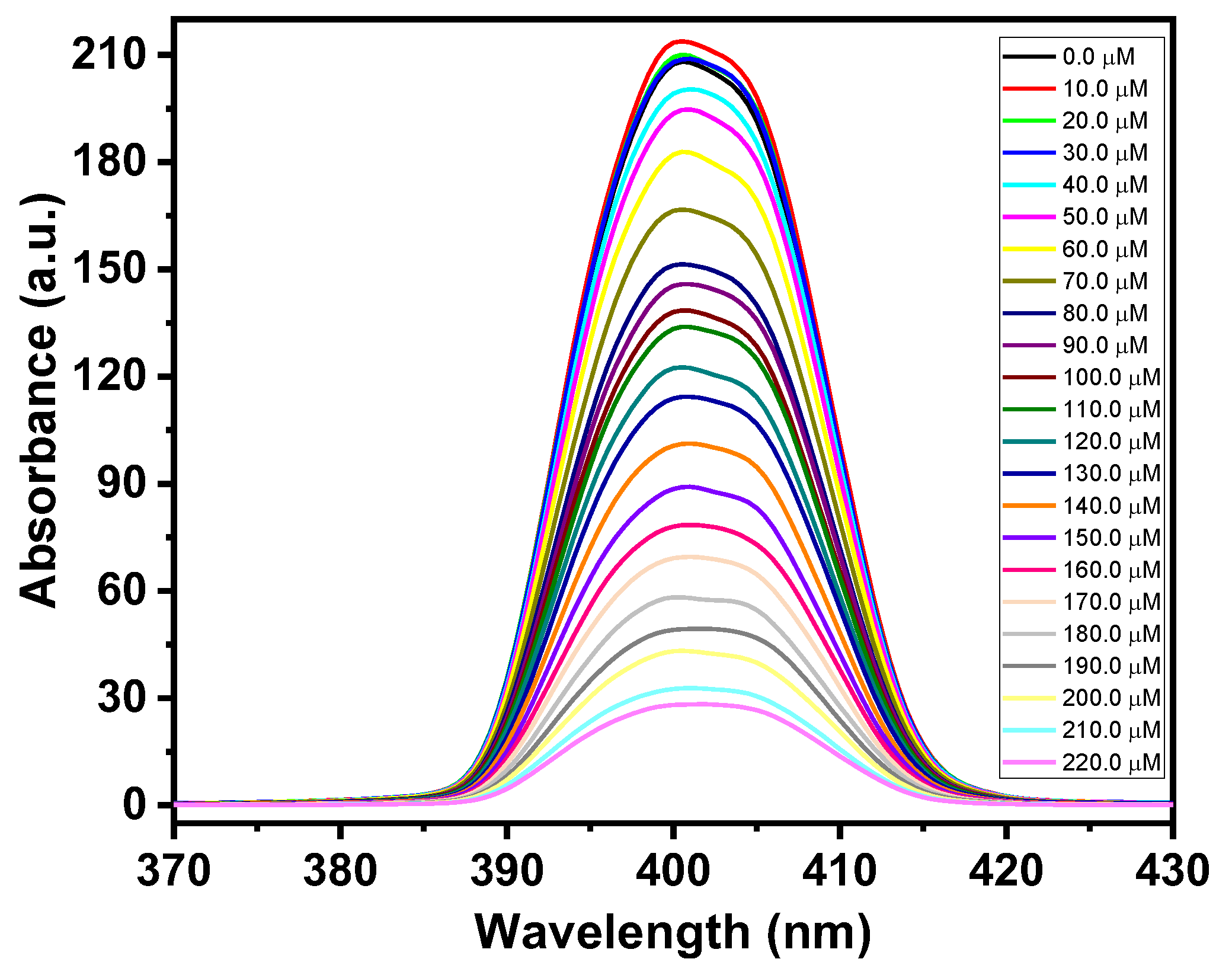
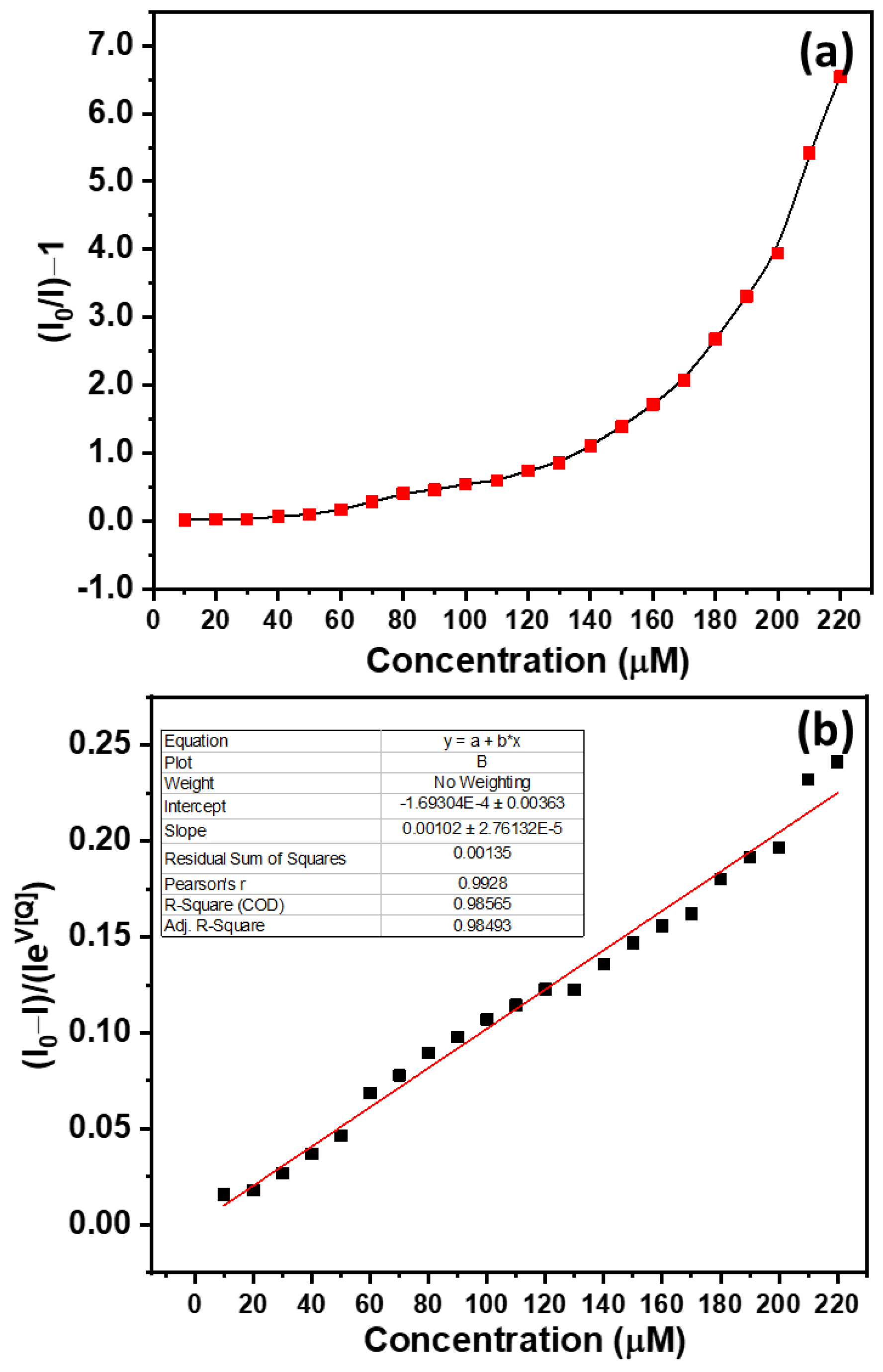
3.3. Sensing Applications of CdO-ZnO Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Li, Y.; Cheng, Q.; Wang, X.; Wang, J.; Zhang, G. Sb-based photocatalysts for degradation of organic pollutants: A review. J. Clean. Prod. 2022, 367, 133060. [Google Scholar] [CrossRef]
- Abdul Mutalib, A.A.; Jaafar, N.F. ZnO photocatalysts applications in abating the organic pollutant contamination: A mini review. Total Environ. Res. Themes 2022, 3–4, 100013. [Google Scholar] [CrossRef]
- Feng, X.; Shang, Y.; Zhang, K.; Hong, M.; Li, J.; Xu, H.; Wang, L.; Li, Z. In situ ligand-induced Ln-MOFs based on a chromophore moiety: White light emission and turn-on detection of trace antibiotics. CrystEngComm 2022, 24, 4187–4200. [Google Scholar] [CrossRef]
- Tufail, M.A.; Iltaf, J.; Zaheer, T.; Tariq, L.; Amir, M.B.; Fatima, R.; Asbat, A.; Kabeer, T.; Fahad, M.; Naeem, H.; et al. Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Sci. Total Environ. 2022, 850, 157961. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L. Ti3C2Tx MXene as Electrocatalyst for Designing Robust Glucose Biosensors. Adv. Mater. Technol. 2022, 1, 2200151. [Google Scholar] [CrossRef]
- Feng, X.; Shang, Y.; Zhang, H.; Liu, X.; Wang, X.; Chen, N.; Wang, L.; Li, Z. Multi-functional lanthanide-CPs based on tricarboxylphenyl terpyridyl ligand as ratiometric luminescent thermometer and highly sensitive ion sensor with turn on/off effect. Dalt. Trans. 2020, 49, 4741–4750. [Google Scholar] [CrossRef]
- Zuo, K.; Zhang, J.; Zeng, L. A smartphone-adaptable chromogenic and fluorogenic sensor for rapid visual detection of toxic hydrazine in the environment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 283, 121765. [Google Scholar] [CrossRef]
- Umar, A.; Harraz, F.A.; Ibrahim, A.A.; Almas, T.; Kumar, R.; Al-Assiri, M.S.; Baskoutas, S. Iron-Doped Titanium Dioxide Nanoparticles As Potential Scaffold for Hydrazine Chemical Sensor Applications. Coatings 2020, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Wu, Z.; Rokni, H.; Yin, Z.; Lin, J.; Zhang, H.; Cheraghi, S. Electrochemical sensor amplified with cellulose nanofibers/Fe3O4 composite to the monitoring of hydrazine as a pollutant in water and wastewater samples. Chemosphere 2022, 309, 136568. [Google Scholar] [CrossRef]
- Muthusamy, S.; Yin, S.; Rajalakshmi, K.; Meng, S.; Zhu, D.; Xie, M.; Xie, J.; Lodi, R.S.; Xu, Y. Development of a quinoline-derived turn-on fluorescent probe for real time detection of hydrazine and its applications in environment and bioimaging. Dye. Pigment. 2022, 206, 110618. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, R.; Chauhan, M.S.; Al-Hadeethi, Y. ZnO–SnO2 nanocubes for fluorescence sensing and dye degradation applications. Ceram. Int. 2021, 47, 6201–6210. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. ZnO nano-mushrooms for photocatalytic degradation of methyl orange. Mater. Lett. 2013, 97, 100–103. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Akhtar, M.S.; Umar, A. Sonophotocatalytic degradation of methyl orange using ZnO nano-aggregates. J. Alloys Compd. 2015, 629, 167–172. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A review of eco-sustainable techniques for the removal of Rhodamine B dye utilizing biomass residue adsorbents. Phys. Chem. Earth Parts A/B/C 2022, 128, 103267. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Bhatt, U.; Soni, V. Toxic effects of Rhodamine B on antioxidant system and photosynthesis of Hydrilla verticillata. J. Hazard. Mater. Lett. 2022, 3, 100069. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, R.; Rana, D.; Chauhan, M.S. Low-temperature synthesis of cadmium-doped zinc oxide nanosheets for enhanced sensing and environmental remediation applications. J. Alloys Compd. 2021, 863, 158649. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Z.; Cheng, X.; Sui, L.; Gao, S.; Zhang, X.; Xu, Y.; Zhao, H.; Huo, L. Ionic liquid-assisted synthesis of α-Fe2O3 mesoporous nanorod arrays and their excellent trimethylamine gas-sensing properties for monitoring fish freshness. J. Mater. Chem. A 2017, 5, 19846–19856. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, Y.; Hua, Q.; Zhang, J.; Zhang, Y.; Xu, X.; Long, Y.; Tang, J.; Wang, F. Controlled Synthesis of Hollow α-Fe2O3 Microspheres Assembled With Ionic Liquid for Enhanced Visible-Light Photocatalytic Activity. Front. Chem. 2019, 7, 58. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Yang, Q.-D.; Xu, G.; Lei, N.-Y.; Tsang, Y.K.; Wong, N.-B.; Ho, J.C. Highly active and enhanced photocatalytic silicon nanowire arrays. Nanoscale 2011, 3, 3269–3276. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Xu, D.; Ma, H. Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis. J. Clean. Prod. 2021, 313, 127758. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.-H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230, 115877. [Google Scholar] [CrossRef]
- Kati, N. Controlling of optical band gap of the CdO films by zinc oxide. Mater. Sci. 2019, 37, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Mosquera, E.; del Pozo, I.; Morel, M. Structure and red shift of optical band gap in CdO–ZnO nanocomposite synthesized by the sol gel method. J. Solid State Chem. 2013, 206, 265–271. [Google Scholar] [CrossRef]
- Ziabari, A.A.; Ghodsi, F.E. Optoelectronic studies of sol–gel derived nanostructured CdO–ZnO composite films. J. Alloys Compd. 2011, 509, 8748–8755. [Google Scholar] [CrossRef]
- Saravanan, R.; Shankar, H.; Prakash, T.; Narayanan, V.; Stephen, A. ZnO/CdO composite nanorods for photocatalytic degradation of methylene blue under visible light. Mater. Chem. Phys. 2011, 125, 277–280. [Google Scholar] [CrossRef]
- Zheng, Z.; Gan, L.; Li, H.; Ma, Y.; Bando, Y.; Golberg, D.; Zhai, T. A Fully Transparent and Flexible Ultraviolet–Visible Photodetector Based on Controlled Electrospun ZnO-CdO Heterojunction Nanofiber Arrays. Adv. Funct. Mater. 2015, 25, 5885–5894. [Google Scholar] [CrossRef]
- Bai, S.; Sun, X.; Han, N.; Shu, X.; Pan, J.; Guo, H.; Liu, S.; Feng, Y.; Luo, R.; Li, D.; et al. rGO modified nanoplate-assembled ZnO/CdO junction for detection of NO2. J. Hazard. Mater. 2020, 394, 121832. [Google Scholar] [CrossRef]
- Naderi, H.; Hajati, S.; Ghaedi, M.; Espinos, J.P. Highly selective few-ppm NO gas-sensing based on necklace-like nanofibers of ZnO/CdO n-n type I heterojunction. Sens. Actuators B Chem. 2019, 297, 126774. [Google Scholar] [CrossRef]
- Cai, X.; Hu, D.; Deng, S.; Han, B.; Wang, Y.; Wu, J.; Wang, Y. Isopropanol sensing properties of coral-like ZnO–CdO composites by flash preparation via self-sustained decomposition of metal–organic complexes. Sens. Actuators B Chem. 2014, 198, 402–410. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M.; Alamry, K.A.; Rub, M.A.; Khan, A.; Khan, A.A.P.; Azum, N. Facile synthesis of doped ZnO-CdO nanoblocks as solid-phase adsorbent and efficient solar photo-catalyst applications. J. Ind. Eng. Chem. 2014, 20, 2278–2286. [Google Scholar] [CrossRef]
- Umar, A.; Akhtar, M.S.; Al-Assiri, M.S.; Al-Salami, A.E.; Kim, S.H. Composite CdO-ZnO hexagonal nanocones: Efficient materials for photovoltaic and sensing applications. Ceram. Int. 2018, 44, 5017–5024. [Google Scholar] [CrossRef]
- Choudhary, K.; Saini, R.; Upadhyay, G.K.; Purohit, L.P. Sustainable behavior of cauliflower like morphology of Y-doped ZnO:CdO nanocomposite thin films for CO2 gas sensing application at low operating temperature. J. Alloys Compd. 2021, 879, 160479. [Google Scholar] [CrossRef]
- Hu, G.; Zhang, Y. Quantum piezotronic devices based on ZnO/CdO quantum well topological insulator. Nano Energy 2020, 77, 105154. [Google Scholar] [CrossRef]
- Wang, T.; Kou, X.; Zhao, L.; Sun, P.; Liu, C.; Wang, Y.; Shimanoe, K.; Yamazoe, N.; Lu, G. Flower-like ZnO hollow microspheres loaded with CdO nanoparticles as high performance sensing material for gas sensors. Sens. Actuators B Chem. 2017, 250, 692–702. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Deshmukh, P.R.; Osuji, R.U.; Maaza, M.; Lokhande, C.D.; Ezema, F.I. Synthesis, characterization and gas-sensing properties of SILAR deposited ZnO-CdO nano-composite thin film. Sens. Actuators B Chem. 2015, 206, 671–678. [Google Scholar] [CrossRef]
- Maafa, I.M.; Al-Enizi, A.M.; Abutaleb, A.; Zouli, N.I.; Ubaidullah, M.; Shaikh, S.F.; Al-Abdrabalnabi, M.A.; Yousef, A. One-pot preparation of CdO/ZnO core/shell nanofibers: An efficient photocatalyst. Alexandria Eng. J. 2021, 60, 1819–1826. [Google Scholar] [CrossRef]
- Warshagha, M.Z.A.; Muneer, M. Facile synthesis of CdO-ZnO heterojunction photocatalyst for rapid removal of organic contaminants from water using visible light. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100728. [Google Scholar] [CrossRef]
- Saravanan, R.; Gracia, F.; Khan, M.M.; Poornima, V.; Gupta, V.K.; Narayanan, V.; Stephen, A. ZnO/CdO nanocomposites for textile effluent degradation and electrochemical detection. J. Mol. Liq. 2015, 209, 374–380. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, R.D.; Purohit, L.P. ZnO-CdO nanocomposites incorporated with graphene oxide nanosheets for efficient photocatalytic degradation of bisphenol A, thymol blue and ciprofloxacin. J. Hazard. Mater. 2022, 424, 127332. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-K.; Shan, C.-X.; He, G.-H.; Wang, R.-Q.; Sun, Z.-P.; Liu, Q.; Dong, L.; Shen, D.-Z. Advanced encryption based on fluorescence quenching of ZnO nanoparticles. J. Mater. Chem. C 2017, 5, 7167–7173. [Google Scholar] [CrossRef]
- Irimpan, L.; Krishnan, B.; Deepthy, A.; Nampoori, V.P.N.; Radhakrishnan, P. Excitation wavelength dependent fluorescence behaviour of nano colloids of ZnO. J. Phys. D. Appl. Phys. 2007, 40, 5670. [Google Scholar] [CrossRef]
- Umm-e-Kulsoom; Gursal, S.A.; Khurshid, M.S.; Saif-ur-Rehman, M.; Minhas, A.S.; Yasin, T.; Mehboob, N.; Mehmood, M.S. Investigating the effect of adding CdO nano particles on neutron shielding efficacy of HDPE. Radiat. Phys. Chem. 2020, 177, 109145. [Google Scholar] [CrossRef]
- Chen, X.-X.; Chen, L.; Li, G.; Cai, L.-X.; Miao, G.-Y.; Guo, Z.; Meng, F.-L. Selectively enhanced gas-sensing performance to n-butanol based on uniform CdO-decorated porous ZnO nanobelts. Sens. Actuators B Chem. 2021, 334, 129667. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Al-Heniti, S.H.; Kumar, R.; Kim, S.H.; Zhang, X.; Raffah, B.M. 2D Sn-doped ZnO ultrathin nanosheet networks for enhanced acetone gas sensing application. Ceram. Int. 2017, 43, 2418–2423. [Google Scholar] [CrossRef]
- Zhou, Q.; Umar, A.; Sodki, E.M.; Amine, A.; Xu, L.; Gui, Y.; Ibrahim, A.A.; Kumar, R.; Baskoutas, S. Fabrication and characterization of highly sensitive and selective sensors based on porous NiO nanodisks. Sens. Actuators B 2018, 259, 604–615. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Zhang, Z.; Wang, X.; Tang, J.; Liu, H.; Shao, Q.; Ding, T.; Umar, A.; Guo, Z. Solvent-free graphene liquids: Promising candidates for lubricants without the base oil. J. Colloid Interface Sci. 2019, 542, 159–167. [Google Scholar] [CrossRef]
- Umar, A.; Ammar, H.Y.; Kumar, R.; Ibrahim, A.A.; Al-Assiri, M.S. Square disks-based crossed architectures of SnO2 for ethanol gas sensing applications—An experimental and theoretical investigation. Sens. Actuators B Chem. 2020, 304, 127352. [Google Scholar] [CrossRef]
- Umar, A.; Kumar, S.A.; Inbanathan, S.S.R.; Modarres, M.; Kumar, R.; Algadi, H.; Ibrahim, A.A.; Wendelbo, R.; Packiaraj, R.; Alhamami, M.A.M.; et al. Enhanced sunlight-driven photocatalytic, supercapacitor and antibacterial applications based on graphene oxide and magnetite-graphene oxide nanocomposites. Ceram. Int. 2022, 48, 29349–29358. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Kumar, R.; Algadi, H.; Albargi, H.; Alsairi, M.A.; Alhmami, M.A.M.; Zeng, W.; Ahmed, F.; Akbar, S. CdO–ZnO nanorices for enhanced and selective formaldehyde gas sensing applications. Environ. Res. 2021, 200, 111377. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Ahmad, R.; Kumar, R.; Ibrahim, A.A.; Baskoutas, S. Bi2O2CO3 nanoplates: Fabrication and characterization of highly sensitive and selective cholesterol biosensor. J. Alloys Compd. 2016, 683, 433–438. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Ramesh, P.; Saravanan, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Bio-Sensing Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Umar, A.; Ibrahim, A.A.; Kumar, R.; Almas, T.; Al-Assiri, M.S.; Baskoutas, S. Nitroaniline chemi-sensor based on bitter gourd shaped ytterbium oxide (Yb2O3) doped zinc oxide (ZnO) nanostructures. Ceram. Int. 2019, 45, 13825–13831. [Google Scholar] [CrossRef]
- Umar, A.; Kumar, R.; Kumar, G.; Algarni, H.; Kim, S.H. Effect of annealing temperature on the properties and photocatalytic efficiencies of ZnO nanoparticles. J. Alloys Compd. 2015, 648, 46–52. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Kumar, R.; Umar, A.; Kim, S.H.; Bumajdad, A.; Ansari, Z.A.; Baskoutas, S. Cauliflower-shaped ZnO nanomaterials for electrochemical sensing and photocatalytic applications. Electrochim. Acta 2016, 222, 463–472. [Google Scholar] [CrossRef]
- Somasundaram, G.; Rajan, J.; Sangaiya, P.; Dilip, R. Hydrothermal synthesis of CdO nanoparticles for photocatalytic and antimicrobial activities. Results Mater. 2019, 4, 100044. [Google Scholar] [CrossRef]
- Sarker, M.A.R.; Ahn, Y.-H. Strategic insight into enhanced photocatalytic remediation of pharmaceutical contaminants using spherical CdO nanoparticles in visible light region. Chemosphere 2023, 311, 137040. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Verma, N.; Chundawat, T.S.; Chandra, H.; Vaya, D. An efficient time reductive photocatalytic degradation of carcinogenic dyes by TiO2-GO nanocomposite. Mater. Res. Bull. 2023, 158, 112043. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Shim, J. Synthesis, optical properties and efficient photocatalytic activity of CdO/ZnO hybrid nanocomposite. J. Phys. Chem. Solids 2018, 112, 20–28. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P. V Green Emission to Probe Photoinduced Charging Events in ZnO−Au Nanoparticles. Charge Distribution and Fermi-Level Equilibration. J. Phys. Chem. B 2003, 107, 7479–7485. [Google Scholar] [CrossRef]
- Sudheer Khan, S. Enhancement of visible light photocatalytic activity of CdO modified ZnO nanohybrid particles. J. Photochem. Photobiol. B Biol. 2015, 142, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Aslam, M.; Jabeen, U.; Zafar, M.N.; Malghani, M.N.K.; Alwadai, N.; Alshammari, F.H.; Almuslem, A.S.; Ullah, Z. ZnO and Ni-doped ZnO photocatalysts: Synthesis, characterization and improved visible light driven photocatalytic degradation of methylene blue. Inorg. Chim. Acta 2022, 543, 121167. [Google Scholar] [CrossRef]
- Amir, M.; Fazal, T.; Iqbal, J.; Din, A.A.; Ahmed, A.; Ali, A.; Razzaq, A.; Ali, Z.; Rehman, M.S.U.; Park, Y.-K. Integrated adsorptive and photocatalytic degradation of pharmaceutical micropollutant, ciprofloxacin employing biochar-ZnO composite photocatalysts. J. Ind. Eng. Chem. 2022, 115, 171–182. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. ZnO-CuxO heterostructure photocatalyst for efficient dye degradation. J. Phys. Chem. Solids 2020, 143, 109463. [Google Scholar] [CrossRef]
- Mukherjee, N.; Show, B.; Maji, S.K.; Madhu, U.; Bhar, S.K.; Mitra, B.C.; Khan, G.G.; Mondal, A. CuO nano-whiskers: Electrodeposition, Raman analysis, photoluminescence study and photocatalytic activity. Mater. Lett. 2011, 65, 3248–3250. [Google Scholar] [CrossRef]
- Pradeev Raj, K.; Sadaiyandi, K.; Kennedy, A.; Sagadevan, S. Photocatalytic and antibacterial studies of indium-doped ZnO nanoparticles synthesized by co-precipitation technique. J. Mater. Sci. Mater. Electron. 2017, 28, 19025–19037. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B Biol. 2019, 199, 111621. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumar, R.; Ravi, G.; Pavithra, S.; Thambidurai, M.; Dang, C.; Velauthapillai, D. Pure and Ce-doped spinel CuFe2O4 photocatalysts for efficient rhodamine B degradation. Environ. Res. 2021, 200, 111528. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shao, Q.; Zhao, J.; Pan, D.; Dong, M.; Jia, C.; Ding, T.; Wu, T.; Guo, Z. Microwave solvothermal carboxymethyl chitosan templated synthesis of TiO2/ZrO2 composites toward enhanced photocatalytic degradation of Rhodamine B. J. Colloid Interface Sci. 2019, 541, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Rajendrachari, S.; Taslimi, P.; Karaoglanli, A.C.; Uzun, O.; Alp, E.; Jayaprakash, G.K. Photocatalytic degradation of Rhodamine B (RhB) dye in waste water and enzymatic inhibition study using cauliflower shaped ZnO nanoparticles synthesized by a novel One-pot green synthesis method. Arab. J. Chem. 2021, 14, 103180. [Google Scholar] [CrossRef]
- Toal, S.J.; Trogler, W.C. Polymer sensors for nitroaromatic explosives detection. J. Mater. Chem. 2006, 16, 2871–2883. [Google Scholar] [CrossRef]
- Pritchard, G.O.; Mattinen, W.A.; Dacey, J.R. Stern–volmer plots in the photolysis of perfluoroazoethane. Int. J. Chem. Kinet. 1970, 2, 191–197. [Google Scholar] [CrossRef]
- Green, N.J.B.; Pimblott, S.M.; Tachiya, M. Generalizations of the Stern-Volmer relation. J. Phys. Chem. 1993, 97, 196–202. [Google Scholar] [CrossRef]
- Varlan, A.; Hillebrand, M. Bovine and Human Serum Albumin interactions with 3-carboxyphenoxathiin studied by fluorescence and circular dichroism spectroscopy. Molecules 2010, 15, 3905–3919. [Google Scholar] [CrossRef] [Green Version]
- Airinei, A.; Tigoianu, R.I.; Rusu, E.; Dorohoi, D.O. Fluorescence quenching of anthracene by nitroaromatic compounds. Dig. J. Nanomater. Biostruct. 2011, 6, 1265–1272. [Google Scholar]
- Hanagodimath, S.M.; Evale, B.G.; Manohara, S.R. Nonlinear fluorescence quenching of newly synthesized coumarin derivative by aniline in binary mixtures. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2009, 74, 943–948. [Google Scholar] [CrossRef]
- Xue, C.; Luo, F.T.; Liu, H. Post-polymerization functionalization approach for highly water-soluble well-defined regioregular head-to-tail glycopolythiophenes. Macromolecules 2007, 40, 6863–6870. [Google Scholar] [CrossRef]

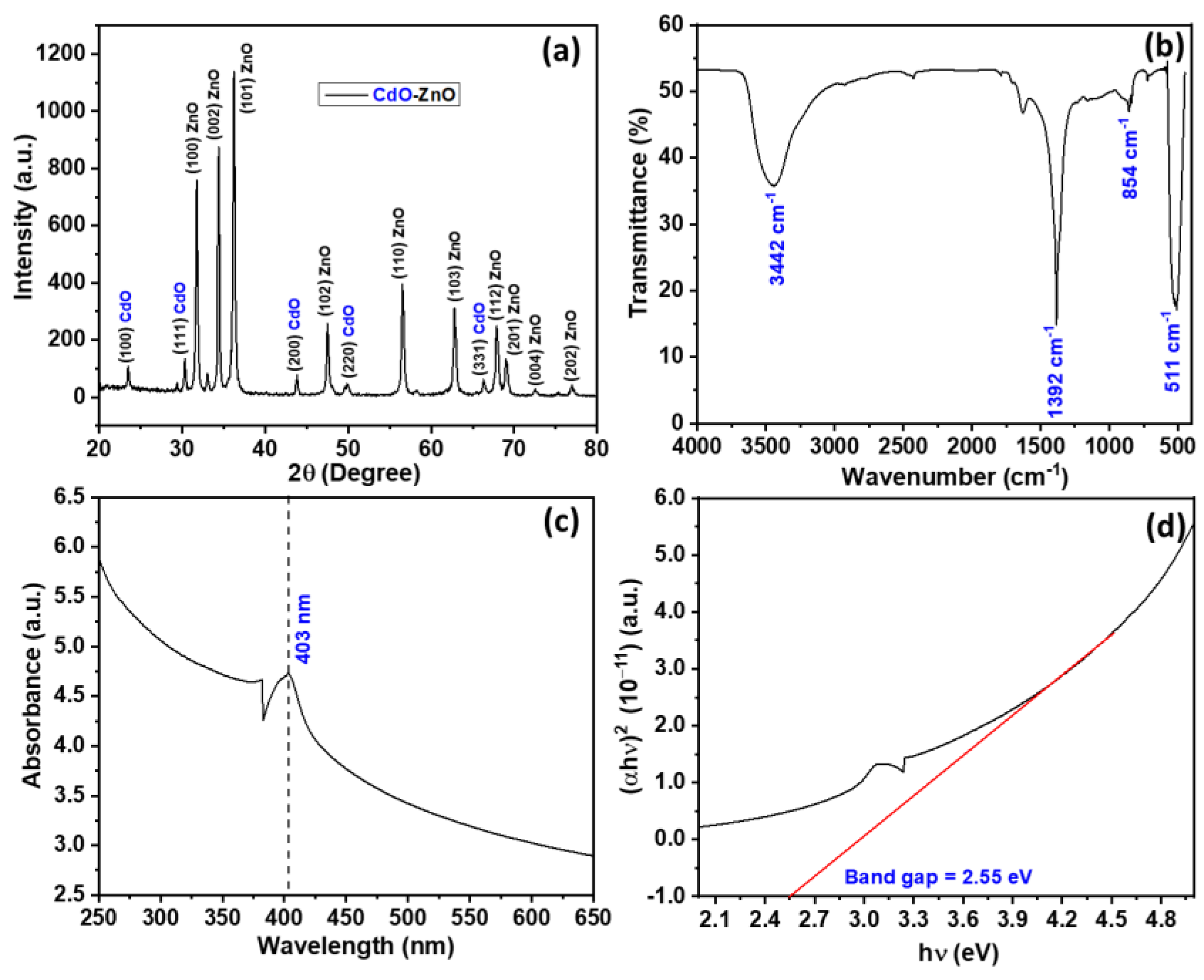


| Diffraction Planes (h k l) | Diffraction Angles (°) | FWHM (β) | Crystal Size (nm) |
|---|---|---|---|
| (1 0 0) | 31.75 | 0.25494 | 32.06 |
| (0 0 2) | 34.40 | 0.26447 | 31.12 |
| (1 0 1) | 36.25 | 0.27482 | 30.10 |
| (1 0 2) | 47.50 | 0.30312 | 28.34 |
| (1 1 0) | 56.55 | 0.33747 | 26.45 |
| (1 0 3) | 62.80 | 0.37188 | 24.77 |
| (1 1 2) | 67.90 | 0.40221 | 23.57 |
| Kinetic Model | Rate Constant (×10−2) | Half-Life Time (t1/2) | R2 (COD) |
|---|---|---|---|
| Zero-order | k0 = 2.406 | 68.25 min | 0.99431 |
| Pseudo-first-order | k1 = 1.196 | 57.94 min | 0.95846 |
| Pseudo-second-order | k2 = 1.086 | 28.04 min | 0.80346 |
| Photocatalyst | [RhB] (ppm) | Catalyst Dose (g/L) | Time (min) | Degradation (%) | Light Source | k1 (×10−2 min−1) | Ref. |
|---|---|---|---|---|---|---|---|
| CdO-ZnO nanocomposites | 20 | 0.5 | 120 | 87.0 | UV | 1.196 | This work |
| ZnO-Cu0.5O heterostructure | 10 | 0.05 | 120 | 73.5 | UV | 21.70 | [67] |
| CuO nano-whiskers | 1 | - | 260 | 84.0 | Visible | 0.71 | [68] |
| In-doped ZnO nanoparticles | 20 | 0.5 | 120 | 76.0 | UV | - | [69] |
| ZnO nanoparticles | 10 μM | 0.2 | 200 | 98.0 | Solar | 1.7 | [70] |
| Au-ZnO nanoparticles | 10 | 0.3 | 180 | 95.0 | UV | 2.47 | [71] |
| Ce-doped spinel CuFe2O4 | ~4.25 | 2.0 | 120 | 88.0 | Visible | - | [72] |
| TiO2/ZrO2 composites | 10 | 0.5 | 270 | 90.5 | UV | - | [73] |
| Cauliflower shaped ZnO | 10 | 0.5 | 120 | 75.0 | Solar | - | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, A.; Kumar, R.; Chauhan, M.S.; Kumar, R.; Ibrahim, A.A.; Alhamami, M.A.M.; Algadi, H.; Akhtar, M.S. Effective Fluorescence Detection of Hydrazine and the Photocatalytic Degradation of Rhodamine B Dye Using CdO-ZnO Nanocomposites. Coatings 2022, 12, 1959. https://doi.org/10.3390/coatings12121959
Umar A, Kumar R, Chauhan MS, Kumar R, Ibrahim AA, Alhamami MAM, Algadi H, Akhtar MS. Effective Fluorescence Detection of Hydrazine and the Photocatalytic Degradation of Rhodamine B Dye Using CdO-ZnO Nanocomposites. Coatings. 2022; 12(12):1959. https://doi.org/10.3390/coatings12121959
Chicago/Turabian StyleUmar, Ahmad, Ramesh Kumar, Mohinder Singh Chauhan, Rajesh Kumar, Ahmed A. Ibrahim, Mohsen A. M. Alhamami, Hassan Algadi, and Mohammad Shaheer Akhtar. 2022. "Effective Fluorescence Detection of Hydrazine and the Photocatalytic Degradation of Rhodamine B Dye Using CdO-ZnO Nanocomposites" Coatings 12, no. 12: 1959. https://doi.org/10.3390/coatings12121959
APA StyleUmar, A., Kumar, R., Chauhan, M. S., Kumar, R., Ibrahim, A. A., Alhamami, M. A. M., Algadi, H., & Akhtar, M. S. (2022). Effective Fluorescence Detection of Hydrazine and the Photocatalytic Degradation of Rhodamine B Dye Using CdO-ZnO Nanocomposites. Coatings, 12(12), 1959. https://doi.org/10.3390/coatings12121959









