Research Progress on Antibacterial Coatings for Preventing Implant-Related Infection in Fractures: A Literature Review
Abstract
1. Introduction
2. Metallic Coating Materials
2.1. Ag NPs
2.2. Cu NPs
2.3. ZnO NPs
2.4. TiO2 NPs
3. Organic Coating Materials
3.1. Antibiotics as Coating Materials
| Antibiotics | Classification | Antimicrobial Mechanism | Target Bacterial Species | Example of the Application on the Coating | Ref. |
|---|---|---|---|---|---|
| Gentamicin | aminoglycoside | Blocking of ribosomal protein synthesis | E. Coli P. aeruginosa S. aureus | Gentamicin loaded chemically crosslinked layer-by-layer hydrogel coatings of poly(methacrylic acid) yielded high bacterial killing efficiencies for S. aureus and E. coli. | [86] |
| Vancomycin | glycopeptides | Blocking of cell wall synthesis | Most Gram-positive bacteria | Vancomycin-coated steel plates can inhibit Staphylococcus aureus colonization In a sheep infection model. | [95] |
| tobramycin | aminoglycoside | Blocking of ribosomal protein synthesis | E. Coli P. aeruginosa | Tobramycin-periapatite coating on the titanium implants can effectively prevent infection in rabbits. | [98] |
| Cephalothin | Cephalosporins | Blocking of cell wall synthesis | S.Aureus E. Coli Staphylococcus epidermidis | Bioactive mesoporous titanium dioxide coating loaded with Cephalothin exhibits a sufficient antibacterial effect against E. coli. | [99] |
| minocycline | Tetracycline | Blocking of ribosomal protein synthesis | S.Aureus Streptococcus pneumoniae E. Coli Enterococcus faecalis | Minocycline-loaded chitosan/alginate multilayer coating on titanium substrates Improved the sustainability of minocycline release and is able to kill both phytoplankton and adherent bacteria. | [100] |
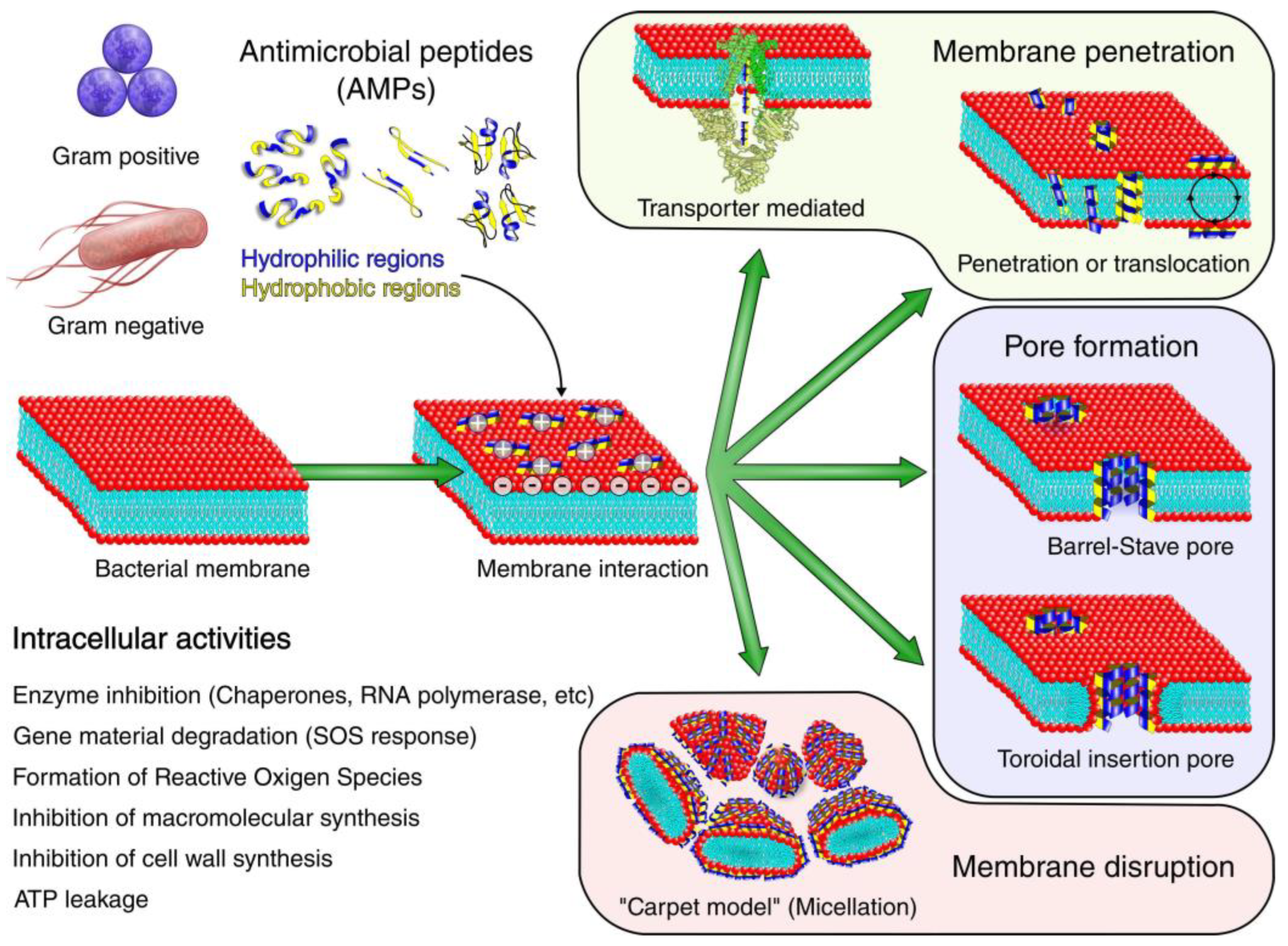
3.2. AMPs as Coating Materials
3.3. Novel Organic Coating Materials
4. Inorganic Coating Materials
4.1. Se NPs
4.2. Ca-P–Coated Carriers
4.3. Titanium-Iodine (Ti-I) Coatings
4.4. Diamond-Like Carbon (DLC) Coatings
5. Polymeric Coating Materials
5.1. Sol-Gel Membrane Carriers
5.2. Poly-D,L-Lactic Acid (PDLLA) Carriers
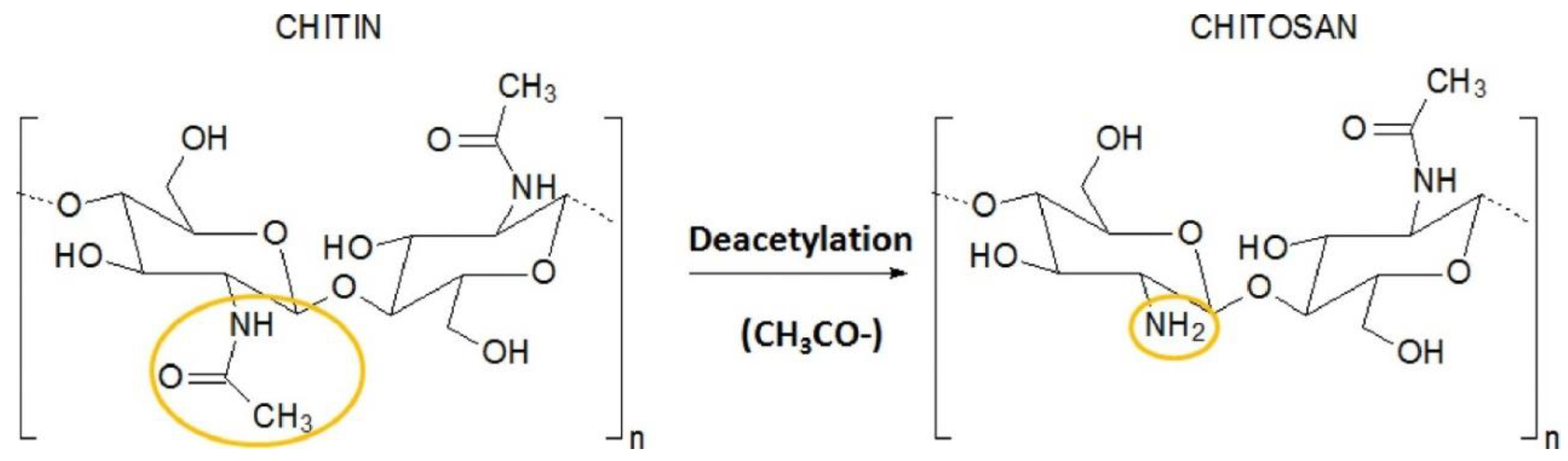
5.3. Chitosan Carriers
5.4. Phosphatidylcholine Carriers
5.5. Polylactic-Glycolic Acid Copolymer Carriers
5.6. Dendrimer Coatings
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, L.; Zhao, C.; Xiong, Y.; Pan, C.; Wang, A. Preparation, release profiles, and antibacterial properties of vancomycin-loaded poly(D,L-lactic) titanium alloy plates. Orthopedics 2009, 32, 324. [Google Scholar] [CrossRef]
- Metsemakers, W.-J.; Smeets, B.; Nijs, S.; Hoekstra, H. Infection after fracture fixation of the tibia: Analysis of healthcare utilization and related costs. Injury 2017, 48, 1204–1210. [Google Scholar] [CrossRef]
- Olesen, U.K.; Pedersen, N.J.; Eckardt, H.; Lykke-Meyer, L.; Bonde, C.T.; Singh, U.M.; McNally, M. The cost of infection in severe open tibial fractures treated with a free flap. Int. Orthop. 2017, 41, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Bezstarosti, H.; Van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; Obremskey, W.; McNally, M.A.; Metsemakers, W.J.; Verhofstad, M.H.J. Insights into treatment and outcome of fracture-related infection: A systematic literature review. Arch. Orthop. Trauma Surg. 2019, 139, 61–72. [Google Scholar] [CrossRef]
- Cierny, G.; Mader, J.T.; Penninck, J.J. A clinical staging system for adult osteomyelitis. Clin. Orthop. Relat. Res. 2003, 414, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Cierny, G.; Mader, J.T. Approach to adult osteomyelitis. Orthop. Rev. 1987, 16, 259–270. [Google Scholar]
- Stoodley, P.; Ehrlich, G.D.; Sedghizadeh, P.P.; Hall-Stoodley, L.; Baratz, M.E.; Altman, D.T.; Sotereanos, N.G.; Costerton, J.W.; Demeo, P. Orthopaedic biofilm infections. Curr. Orthop. Pract. 2011, 22, 558–563. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Buhlin, K.; Dufrêne, Y.F.; Gomelsky, M.; Moroni, A.; Ramstedt, M.; Rumbaugh, K.P.; Schulte, T.; Sun, L.; Åkerlund, B.; et al. Biofilm formation—What we can learn from recent developments. J. Intern. Med. 2018, 284, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Shi, Q.S.; Huang, X.M.; Xie, X.B. The Three Bacterial Lines of Defense against Antimicrobial Agents. Int. J. Mol. Sci. 2015, 16, 21711–21733. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Kumar, C.G.; Anand, S.K. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. JCMA 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Froyman, W.; Landolfo, C.; De Cock, B.; Wynants, L.; Sladkevicius, P.; Testa, A.C.; Van Holsbeke, C.; Domali, E.; Fruscio, R.; Epstein, E.; et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): A 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. 2019, 20, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal infections: Mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Anwar, H.; Dasgupta, M.K.; Costerton, J.W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob. Agents Chemother. 1990, 34, 2043–2046. [Google Scholar] [CrossRef]
- Khoury, A.E.; Lam, K.; Ellis, B.; Costerton, J.W. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1992, 38, M174–M178. [Google Scholar] [CrossRef]
- Nadell, C.D.; Drescher, K.; Wingreen, N.S.; Bassler, B.L. Extracellular matrix structure governs invasion resistance in bacterial biofilms. ISME J. 2015, 9, 1700–1709. [Google Scholar] [CrossRef]
- Bagge, N.; Hentzer, M.; Andersen, J.B.; Ciofu, O.; Givskov, M.; Høiby, N. Dynamics and spatial distribution of β-lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2004, 48, 1168–1174. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Germain, E.; Roghanian, M.; Gerdes, K.; Maisonneuve, E. Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases. Proc. Natl. Acad. Sci. USA 2015, 112, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mah, T.F. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Wilton, M.; Charron-Mazenod, L.; Moore, R.; Lewenza, S. Extracellular DNA Acidifies Biofilms and Induces Aminoglycoside Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Walters, M.C., 3rd; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef]
- Billings, N.; Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The extracellular matrix Component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Chiang, W.C.; Nilsson, M.; Jensen, P.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef]
- Secor, P.R.; Sweere, J.M.; Michaels, L.A.; Malkovskiy, A.V.; Lazzareschi, D.; Katznelson, E.; Rajadas, J.; Birnbaum, M.E.; Arrigoni, A.; Braun, K.R.; et al. Filamentous Bacteriophage Promote Biofilm Assembly and Function. Cell Host Microbe 2015, 18, 549–559. [Google Scholar] [CrossRef]
- Borriello, G.; Werner, E.; Roe, F.; Kim, A.M.; Ehrlich, G.D.; Stewart, P.S. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 2004, 48, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Joshi-Datar, A.; Lepine, F.; Bauerle, E.; Olakanmi, O.; Beer, K.; McKay, G.; Siehnel, R.; Schafhauser, J.; Wang, Y.; et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 2011, 334, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Rybtke, M.; Givskov, M.; Høiby, N.; Twetman, S.; Tolker-Nielsen, T. The dlt genes play a role in antimicrobial tolerance of Streptococcus mutans biofilms. Int. J. Antimicrob. Agents 2016, 48, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.; Pitts, B.; Pellock, B.; Walker, G.C.; Stewart, P.S.; O’Toole, G.A. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003, 426, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Gillis, R.J.; White, K.G.; Choi, K.H.; Wagner, V.E.; Schweizer, H.P.; Iglewski, B.H. Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2005, 49, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [PubMed]
- Driffield, K.; Miller, K.; Bostock, J.M.; O’Neill, A.J.; Chopra, I. Increased mutability of Pseudomonas aeruginosa in biofilms. J. Antimicrob. Chemother. 2008, 61, 1053–1056. [Google Scholar] [CrossRef]
- Dalton, T.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Watters, C.; Griswold, J.A.; Rumbaugh, K.P. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS ONE 2011, 6, e27317. [Google Scholar] [CrossRef]
- Rijnaarts, H.H.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Zehnder, A.J. Bacterial Adhesion under Static and Dynamic Conditions. Appl. Environ. Microbiol. 1993, 59, 3255–3265. [Google Scholar] [CrossRef]
- Tan, G.; Xu, J.; Chirume, W.M.; Zhang, J.; Zhang, H.; Hu, X. Antibacterial and Anti-Inflammatory Coating Materials for Orthopedic Implants: A Review. Coatings 2021, 11, 1401. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Sengupta, J.; Ghosh, S.; Datta, P.; Gomes, A.; Gomes, A. Physiologically important metal nanoparticles and their toxicity. J. Nanosci. Nanotechnol. 2014, 14, 990–1006. [Google Scholar] [CrossRef] [PubMed]
- Papp, A.; Horváth, T.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M.; Berkesi, D.S.; Kozma, G.; Kónya, Z.; Wilhelm, I.; Patai, R.; et al. Presence of Titanium and Toxic Effects Observed in Rat Lungs, Kidneys, and Central Nervous System in vivo and in Cultured Astrocytes in vitro on Exposure by Titanium Dioxide Nanorods. Int. J. Nanomed. 2020, 15, 9939–9960. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Behrendt, A.K.; Beythien, M.; Huber, J.; Zufraß, T.; Butschkau, A.; Mittlmeier, T.; Vollmar, B. New TiAg composite coating for bone prosthesis engineering shows promising microvascular compatibility in the murine dorsal skinfold chamber model. J. Mater. Sci. Mater. Med. 2015, 26, 5373. [Google Scholar] [CrossRef] [PubMed]
- Ichimaru, H.; Harada, A.; Yoshimoto, S.; Miyazawa, Y.; Mizoguchi, D.; Kyaw, K.; Ono, K.; Tsutsuki, H.; Sawa, T.; Niidome, T. Gold Coating of Silver Nanoplates for Enhanced Dispersion Stability and Efficient Antimicrobial Activity against Intracellular Bacteria. Langmuir ACS J. Surf. Colloids 2018, 34, 10413–10418. [Google Scholar] [CrossRef] [PubMed]
- Hurtuková, K.; Vašinová, T.; Kasálková, N.S.; Fajstavr, D.; Rimpelová, S.; Pavlíčková, V.S.; Švorčík, V.; Slepička, P. Antibacterial Properties of Silver Nanoclusters with Carbon Support on Flexible Polymer. Nanomaterials 2022, 12, 2658. [Google Scholar] [CrossRef]
- Nie, Y.; Kalapos, C.; Nie, X.; Murphy, M.; Hussein, R.; Zhang, J. Superhydrophilicity and antibacterial property of a Cu-dotted oxide coating surface. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 25. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wang, X.; Wang, Y.; Hang, R.; Huang, X.; Tang, B.; Chu, P.K. Effects of copper nanoparticles in porous TiO(2) coatings on bacterial resistance and cytocompatibility of osteoblasts and endothelial cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 110–120. [Google Scholar] [CrossRef]
- Maimaiti, B.; Zhang, N.; Yan, L.; Luo, J.; Xie, C.; Wang, Y.; Ma, C.; Ye, T. Stable ZnO-doped hydroxyapatite nanocoating for anti-infection and osteogenic on titanium. Colloids Surf. B Biointerfaces 2020, 186, 110731. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.; Allaker, R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. Part A 2015, 103, 981–989. [Google Scholar] [CrossRef]
- Ivanova, I.A.; Pavlova, E.L.; Stoyanova, D.S.; Angelov, O.I. Antibacterial effect of TiO(2):Cu:Ag thin coatings on Pseudomonas strain measured by microbiological and ATP assays. J. Basic Microbiol. 2019, 59, 1165–1172. [Google Scholar] [CrossRef]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Phillips, P.; Hastings, R. Reduction of bacterial contamination in a healthcare environment by silver antimicrobial technology. J. Infect. Prev. 2009, 10, 6–12. [Google Scholar] [CrossRef]
- Furkert, F.H.; Sörensen, J.H.; Arnoldi, J.; Robioneck, B.; Steckel, H. Antimicrobial efficacy of surface-coated external fixation pins. Curr. Microbiol. 2011, 62, 1743–1751. [Google Scholar] [CrossRef]
- Shao, W.; Zhao, Q. Effect of corrosion rate and surface energy of silver coatings on bacterial adhesion. Colloids Surf. B Biointerfaces 2010, 76, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Arens, D.; Zeiter, S.; Nehrbass, D.; Ranjan, N.; Paulin, T.; Alt, V. Antimicrobial silver-coating for locking plates shows uneventful osteotomy healing and good biocompatibility results of an experimental study in rabbits. Injury 2020, 51, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Ekrikaya, S.; Yilmaz, E.; Celik, C.; Demirbuga, S.; Ildiz, N.; Demirbas, A.; Ocsoy, I. Investigation of ellagic acid rich-berry extracts directed silver nanoparticles synthesis and their antimicrobial properties with potential mechanisms towards Enterococcus faecalis and Candida albicans. J. Biotechnol. 2021, 341, 155–162. [Google Scholar] [CrossRef]
- Celik, C.; Ildiz, N.; Ocsoy, I. Building block and rapid synthesis of catecholamines-inorganic nanoflowers with their peroxidase-mimicking and antimicrobial activities. Sci. Rep. 2020, 10, 2903. [Google Scholar] [CrossRef]
- Wahab, M.A.; Luming, L.; Matin, M.A.; Karim, M.R.; Aijaz, M.O.; Alharbi, H.F.; Abdala, A.; Haque, R. Silver Micro-Nanoparticle-Based Nanoarchitectures: Synthesis Routes, Biomedical Applications, and Mechanisms of Action. Polymers 2021, 13, 2870. [Google Scholar] [CrossRef]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver Nanoparticle-Based Nanocomposites for Combating Infectious Pathogens: Recent Advances and Future Prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chen, M.H.; Lee, B.H.; Hsieh, K.H.; Tu, Y.K.; Lin, J.J.; Chang, C.H. Evenly distributed thin-film Ag coating on stainless plate by tricomponent Ag/silicate/PU with antimicrobial and biocompatible properties. ACS Appl. Mater. Interfaces 2014, 6, 20324–20333. [Google Scholar] [CrossRef] [PubMed]
- Rosário, F.; Hoet, P.; Santos, C.; Oliveira, H. Death and cell cycle progression are differently conditioned by the AgNP size in osteoblast-like cells. Toxicology 2016, 368–369, 103–115. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir ACS J. Surf. Colloids 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Hoene, A.; Prinz, C.; Walschus, U.; Lucke, S.; Patrzyk, M.; Wilhelm, L.; Neumann, H.G.; Schlosser, M. In vivo evaluation of copper release and acute local tissue reactions after implantation of copper-coated titanium implants in rats. Biomed. Mater. 2013, 8, 035009. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Fellner, M.; Zhang, C.; Sui, D.; Hu, J. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 2017, 3, e1700344. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 2139021–2139023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef]
- Hui, A.; Yang, F.; Yan, R.; Kang, Y.; Wang, A. Palygorskite-Based Organic-Inorganic Hybrid Nanocomposite for Enhanced Antibacterial Activities. Nanomaterials 2021, 11, 3230. [Google Scholar] [CrossRef]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. Part A 2006, 78, 595–604. [Google Scholar] [CrossRef]
- Suh, K.S.; Lee, Y.S.; Seo, S.H.; Kim, Y.S.; Choi, E.M. Effect of zinc oxide nanoparticles on the function of MC3T3-E1 osteoblastic cells. Biol. Trace Elem. Res. 2013, 155, 287–294. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Ur Rehman, Z. Review of Recent Advances in Polylactic Acid/TiO(2) Composites. Material 2019, 12, 3659. [Google Scholar] [CrossRef]
- Younis, A.B.; Haddad, Y.; Kosaristanova, L.; Smerkova, K. Titanium dioxide nanoparticles: Recent progress in antimicrobial applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2022, e1860. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?--Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Barford, J.; Yeung, K.L.; Si, G. Non-UV based germicidal activity of metal-doped TiO2 coating on solid surfaces. J. Environ. Sci. (China) 2007, 19, 745–750. [Google Scholar] [CrossRef]
- Tyllianakis, M.; Dalas, E.; Christofidou, M.; Kallitsis, J.K.; Chrissanthopoulos, A.; Koutsoukos, P.G.; Bartzavali, C.; Gourdoupi, N.; Papadimitriou, K.; Oikonomou, E.K.; et al. Novel composites materials from functionalized polymers and silver coated titanium oxide capable for calcium phosphate induction, control of orthopedic biofilm infections: An “in vitro” study. J. Mater. Sci. Mater. Med. 2010, 21, 2201–2211. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Xu, Y.; Gu, Q.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed. 2014, 9, 3027–3036. [Google Scholar] [CrossRef]
- Albright, V.; Zhuk, I.; Wang, Y.; Selin, V.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C.; Sukhishvili, S.A. Self-defensive antibiotic-loaded layer-by-layer coatings: Imaging of localized bacterial acidification and pH-triggering of antibiotic release. Acta Biomater. 2017, 61, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Hatton, P.V.; Le Maitre, C.; Smith, T.J.; Akid, R. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated titanium. Bone Jt. J. 2021, 103-b, 522–529. [Google Scholar] [CrossRef]
- Yu, X.; Wu, H.; Li, J.; Xie, Z. Antibiotic cement-coated locking plate as a temporary internal fixator for femoral osteomyelitis defects. Int. Orthop. 2017, 41, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, S.; Fu, J.; Sun, D.; Wang, S.; Xie, Z.; Wang, Y. Investigating clinical characteristics and prognostic factors in patients with chronic osteomyelitis of humerus. Burn. Trauma 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wang, X.; Yu, S.; Wu, H.; Shen, J.; Huang, Q.; Xie, Z. An antibiotic cement-coated locking plate as a temporary fixation for treatment of infected bone defects: A new method of stabilization. J. Orthop. Surg. Res. 2020, 15, 44. [Google Scholar] [CrossRef]
- Wang, G.; Luo, W.; Zhou, Y.; Zhu, Z.; Zhao, Z.; Liu, S.; Li, J.; Feng, X.; Zheng, Y.; Liang, J.; et al. Custom-Made Antibiotic Cement-Coated Nail for the Treatment of Infected Bone Defect. BioMed Res. Int. 2021, 2021, 6693906. [Google Scholar] [CrossRef]
- Keller, D.M.; Pizzo, R.A.; Patel, J.N.; Viola, A.; Yoon, R.S.; Liporace, F.A. Use of antibiotic-cement coated locking plates in the setting of periprosthetic infection and infected nonunion. Injury 2022, 53, 2567–2572. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Edin, M.L.; Miclau, T.; Lester, G.E.; Lindsey, R.W.; Dahners, L.E. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin. Orthop. Relat. Res. 1996, 333, 245–251. [Google Scholar] [CrossRef]
- Stewart, S.; Barr, S.; Engiles, J.; Hickok, N.J.; Shapiro, I.M.; Richardson, D.W.; Parvizi, J.; Schaer, T.P. Vancomycin-modified implant surface inhibits biofilm formation and supports bone-healing in an infected osteotomy model in sheep: A proof-of-concept study. J. Bone Jt. Surg. Am. Vol. 2012, 94, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Prevention the formation of biofilm on orthopedic implants by melittin thin layer on chitosan/bioactive glass/vancomycin coatings. J. Mater. Sci. Mater. Med. 2021, 32, 75. [Google Scholar] [CrossRef]
- Freischmidt, H.; Armbruster, J.; Reiter, G.; Grützner, P.A.; Helbig, L.; Guehring, T. Individualized Techniques of Implant Coating with an Antibiotic-Loaded, Hydroxyapatite/Calcium Sulphate Bone Graft Substitute. Ther. Clin. Risk Manag. 2020, 16, 689–694. [Google Scholar] [CrossRef]
- Moojen, D.J.; Vogely, H.C.; Fleer, A.; Nikkels, P.G.; Higham, P.A.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J. Prophylaxis of infection and effects on osseointegration using a tobramycin-periapatite coating on titanium implants--an experimental study in the rabbit. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 710–716. [Google Scholar] [CrossRef]
- Xia, W.; Grandfield, K.; Hoess, A.; Ballo, A.; Cai, Y.; Engqvist, H. Mesoporous titanium dioxide coating for metallic implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 82–93. [Google Scholar] [CrossRef]
- Lv, H.; Chen, Z.; Yang, X.; Cen, L.; Zhang, X.; Gao, P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014, 42, 1464–1472. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.W.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Brogden, N.K.; Brogden, K.A. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int. J. Antimicrob. Agents 2011, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Omardien, S.; Brul, S.; Zaat, S.A. Antimicrobial Activity of Cationic Antimicrobial Peptides against Gram-Positives: Current Progress Made in Understanding the Mode of Action and the Response of Bacteria. Front. Cell Dev. Biol. 2016, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Loffredo, M.R.; Troiano, C.; Bobone, S.; Malanovic, N.; Eichmann, T.O.; Caprio, L.; Canale, V.C.; Park, Y.; Mangoni, M.L.; et al. Binding of an antimicrobial peptide to bacterial cells: Interaction with different species, strains and cellular components. Biochim. Et Biophys. Acta. Biomembr. 2020, 1862, 183291. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Wilson, D.N. Intracellular Antimicrobial Peptides Targeting the Protein Synthesis Machinery. Adv. Exp. Med. Biol. 2019, 1117, 73–89. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Noordin, S.; Masri, B.A.; Garbuz, D.S.; Duncan, C.P.; Hancock, R.E.; Wang, R. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1344–1352. [Google Scholar] [CrossRef]
- Tian, J.; Shen, S.; Zhou, C.; Dang, X.; Jiao, Y.; Li, L.; Ding, S.; Li, H. Investigation of the antimicrobial activity and biocompatibility of magnesium alloy coated with HA and antimicrobial peptide. J. Mater. Sci. Mater. Med. 2015, 26, 66. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Alves, D.; Pereira, M.O. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37--A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Marini, F.C.; Watson, K.; Zwezdaryk, K.J.; Dembinski, J.L.; LaMarca, H.L.; Tomchuck, S.L.; Zu Bentrup, K.H.; Danka, E.S.; Henkle, S.L.; et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3806–3811. [Google Scholar] [CrossRef]
- He, Y.; Mu, C.; Shen, X.; Yuan, Z.; Liu, J.; Chen, W.; Lin, C.; Tao, B.; Liu, B.; Cai, K. Peptide LL-37 coating on micro-structured titanium implants to facilitate bone formation in vivo via mesenchymal stem cell recruitment. Acta Biomater. 2018, 80, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Al-Baadani, M.A.; He, H.; Cai, L.; Wu, Z.; Yao, L.; Wu, X.; Wu, S.; Chen, M.; Zhang, H.; et al. Antibacterial and osteogenesis performances of LL37-loaded titania nanopores in vitro and in vivo. Int. J. Nanomed. 2019, 14, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Comparative mode of action of the antimicrobial peptide melimine and its derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 7063. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, X.; Liu, T.; Huang, Y.; Li, J. The effects of Peptide Mel4-coated titanium plates on infection rabbits after internal fixation of open fractures. Arch. Orthop. Trauma. Surg. 2022, 142, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gerits, E.; Kucharíková, S.; Van Dijck, P.; Erdtmann, M.; Krona, A.; Lövenklev, M.; Fröhlich, M.; Dovgan, B.; Impellizzeri, F.; Braem, A.; et al. Antibacterial activity of a new broad-spectrum antibiotic covalently bound to titanium surfaces. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Tan, L.; Liu, X.; Li, J.; Wu, S. A facile fabrication of novel stuff with antibacterial property and osteogenic promotion utilizing red phosphorus and near-infrared light. Bioact. Mater. 2019, 4, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Hooyberghs, G.; Robijns, S.; De Weerdt, A.; Kucharíková, S.; Tournu, H.; Braem, A.; Čeh, K.; Majdič, G.; Španič, T.; et al. An antibiofilm coating of 5-aryl-2-aminoimidazole covalently attached to a titanium surface. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1908–1919. [Google Scholar] [CrossRef]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochem. Mosc. 2022, 87, S168–S177. [Google Scholar] [CrossRef]
- Truong, L.B.; Medina-Cruz, D.; Mostafavi, E.; Rabiee, N. Selenium Nanomaterials to Combat Antimicrobial Resistance. Molecules 2021, 26, 3611. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, S.; Gadd, G.M.; McGrath, J.; Rooney, D.W.; Zhao, Q. Fungal-derived selenium nanoparticles and their potential applications in electroless silver coatings for preventing pin-tract infections. Regen. Biomater. 2022, 9, rbac013. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Liu, G.D.; Pan, C.J.; Chen, J.Y.; Zhou, Y.G.; Xiao, S.H.; Wang, Y.; Yu, H.J. Preparation, release profiles and antibacterial properties of vancomycin-loaded Ca-P coating titanium alloy plate. J. Mater. Sci. Mater. Med. 2011, 22, 989–995. [Google Scholar] [CrossRef]
- Pan, C.J.; Dong, Y.X.; Zhang, Y.Y.; Nie, Y.D.; Zhao, C.H.; Wang, Y.L. Enhancing the antibacterial activity of biomimetic HA coatings by incorporation of norvancomycin. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2011, 16, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, e00682-20. [Google Scholar] [CrossRef]
- Shirai, T.; Shimizu, T.; Ohtani, K.; Zen, Y.; Takaya, M.; Tsuchiya, H. Antibacterial iodine-supported titanium implants. Acta Biomater. 2011, 7, 1928–1933. [Google Scholar] [CrossRef]
- Inoue, D.; Kabata, T.; Ohtani, K.; Kajino, Y.; Shirai, T.; Tsuchiya, H. Inhibition of biofilm formation on iodine-supported titanium implants. Int. Orthop. 2017, 41, 1093–1099. [Google Scholar] [CrossRef]
- Ueoka, K.; Kabata, T.; Tokoro, M.; Kajino, Y.; Inoue, D.; Takagi, T.; Ohmori, T.; Yoshitani, J.; Ueno, T.; Yamamuro, Y.; et al. Antibacterial Activity in Iodine-coated Implants Under Conditions of Iodine Loss: Study in a Rat Model Plus In Vitro Analysis. Clin. Orthop. Relat. Res. 2021, 479, 1613–1623. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 72–84. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q.; Liu, Y.; Wang, S.; Abel, E.W. Reduction of bacterial adhesion on modified DLC coatings. Colloids Surf. B Biointerfaces 2008, 61, 182–187. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Santos, L.V.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Antibacterial activity of DLC and Ag–DLC films produced by PECVD technique. Diam. Relat. Mater. 2009, 18, 1010–1014. [Google Scholar] [CrossRef]
- Levon, J.; Myllymaa, K.; Kouri, V.P.; Rautemaa, R.; Kinnari, T.; Myllymaa, S.; Konttinen, Y.T.; Lappalainen, R. Patterned macroarray plates in comparison of bacterial adhesion inhibition of tantalum, titanium, and chromium compared with diamond-like carbon. J. Biomed. Mater. Res. Part A 2010, 92, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Rahmawan, Y.; Moon, M.W.; Kim, K.S.; Lee, K.R.; Suh, K.Y. Wrinkled, dual-scale structures of diamond-like carbon (DLC) for superhydrophobicity. Langmuir 2010, 26, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Helbig, R.; Nickerl, J.; Neinhuis, C.; Werner, C. Smart skin patterns protect springtails. PLoS ONE 2011, 6, e25105. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, C.; Xiong, Y.; Wang, A. Preparation, antibacterial properties and biocompatibility studies on vancomycin-poly(D,L)-lactic loaded plates. Int. Orthop. 2010, 34, 755–759. [Google Scholar] [CrossRef][Green Version]
- Fei, J.; Yu, H.J.; Pan, C.J.; Zhao, C.H.; Zhou, Y.G.; Wang, Y. Efficacy of a norvancomycin-loaded, PDLLA-coated plate in preventing early infection of rabbit tibia fracture. Orthopedics 2010, 33, 310. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Afroz, M.M.; Kashem, M.N.H.; Piash, K.; Islam, N. Saccharomyces Cerevisiae as an Untapped Source of Fungal Chitosan for Antimicrobial Action. Appl. Biochem. Biotechnol. 2021, 193, 3765–3786. [Google Scholar] [CrossRef]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef] [PubMed]
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Chesnutt, B.M.; Yuan, Y.; Yang, Y.; Appleford, M.; Oh, S.; McLaughlin, R.; Elder, S.H.; Ong, J.L. The integration of chitosan-coated titanium in bone: An in vivo study in rabbits. Implant. Dent. 2007, 16, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Pishbin, F.; Mouriño, V.; Flor, S.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition of gentamicin-loaded bioactive glass/chitosan composite coatings for orthopaedic implants. ACS Appl. Mater. Interfaces 2014, 6, 8796–8806. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Zhang, Y.; Diba, M.; Kolwijck, E.; Shao, J.; Jansen, J.A.; Yang, F.; Boccaccini, A.R.; Leeuwenburgh, S.C. Electrophoretic Deposition of Chitosan Coatings Modified with Gelatin Nanospheres To Tune the Release of Antibiotics. ACS Appl. Mater. Interfaces 2016, 8, 13785–13792. [Google Scholar] [CrossRef]
- Yu, W.-Z.; Zhang, Y.; Liu, X.; Xiang, Y.; Li, Z.; Wu, S. Synergistic antibacterial activity of multi components in lysozyme/chitosan/silver/hydroxyapatite hybrid coating. Mater. Des. 2018, 139, 351–362. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Xu, R.; Wang, W.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Electrochemically deposited chitosan/Ag complex coatings on biomedical NiTi alloy for antibacterial application. Surf. Coat. Technol. 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Han, B.; Tang, B.; Nimni, M.E. Combined effects of phosphatidylcholine and demineralized bone matrix on bone induction. Connect Tissue Res. 2003, 44, 160–166. [Google Scholar] [CrossRef]
- Jennings, J.A.; Carpenter, D.P.; Troxel, K.S.; Beenken, K.E.; Smeltzer, M.S.; Courtney, H.S.; Haggard, W.O. Novel Antibiotic-loaded Point-of-care Implant Coating Inhibits Biofilm. Clin. Orthop. Relat. Res. 2015, 473, 2270–2282. [Google Scholar] [CrossRef]
- Jennings, J.A.; Beenken, K.E.; Skinner, R.A.; Meeker, D.G.; Smeltzer, M.S.; Haggard, W.O.; Troxel, K.S. Antibiotic-loaded phosphatidylcholine inhibits staphylococcal bone infection. World J. Orthop. 2016, 7, 467–474. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; van den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Transl. Res. 2020, 10, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Schito, A.M. From Nanobiotechnology, Positively Charged Biomimetic Dendrimers as Novel Antibacterial Agents: A Review. Nanomaterials 2020, 10, 2022. [Google Scholar] [CrossRef]
- Khoo, X.; Hamilton, P.; O’Toole, G.A.; Snyder, B.D.; Kenan, D.J.; Grinstaff, M.W. Directed assembly of PEGylated-peptide coatings for infection-resistant titanium metal. J. Am. Chem. Soc. 2009, 131, 10992–10997. [Google Scholar] [CrossRef]
- Khoo, X.; O’Toole, G.A.; Nair, S.A.; Snyder, B.D.; Kenan, D.J.; Grinstaff, M.W. Staphylococcus aureus resistance on titanium coated with multivalent PEGylated-peptides. Biomaterials 2010, 31, 9285–9292. [Google Scholar] [CrossRef]
- Wang, L.; Erasquin, U.J.; Zhao, M.; Ren, L.; Zhang, M.Y.; Cheng, G.J.; Wang, Y.; Cai, C. Stability, antimicrobial activity, and cytotoxicity of poly(amidoamine) dendrimers on titanium substrates. ACS Appl. Mater. Interfaces 2011, 3, 2885–2894. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, L.; Liu, S.; Chen, J.; Ren, L.; Wang, Y. Antimicrobial Hyaluronic Acid/Poly(amidoamine) Dendrimer Multilayer on Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) Prepared by a Layer-by-Layer Self-Assembly Method. ACS Appl. Mater. Interfaces 2015, 7, 13876–13881. [Google Scholar] [CrossRef]
- Klaykruayat, B.; Siralertmukul, K.; Srikulkit, K. Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr. Polym. 2010, 80, 197–207. [Google Scholar] [CrossRef]
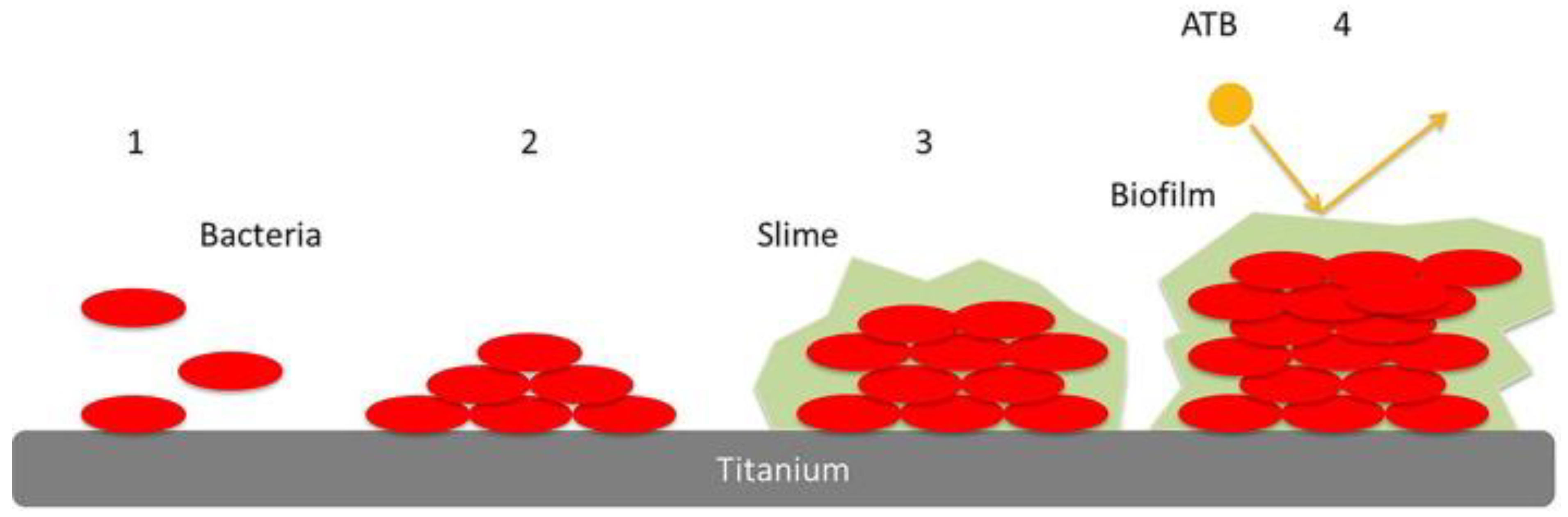
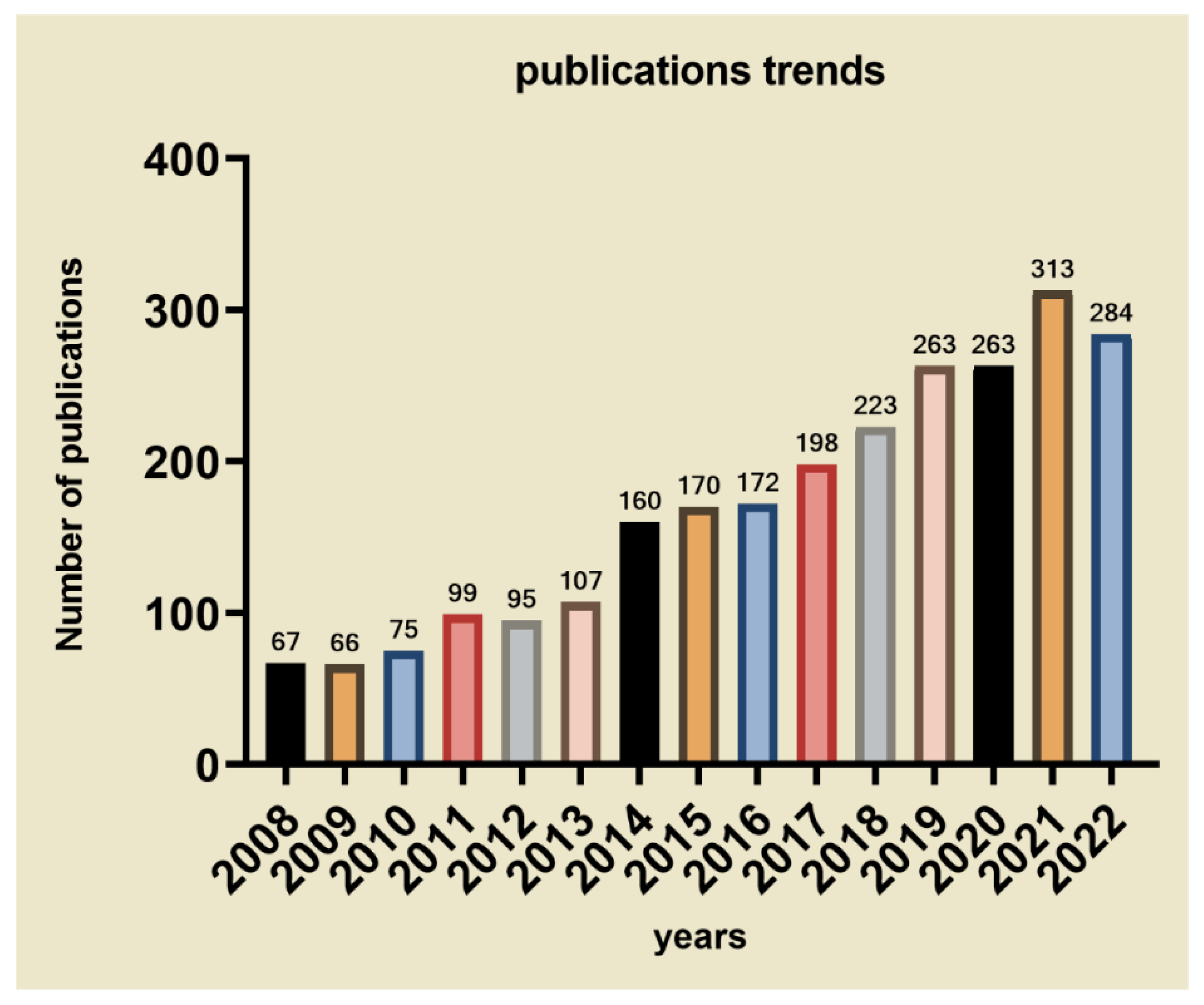
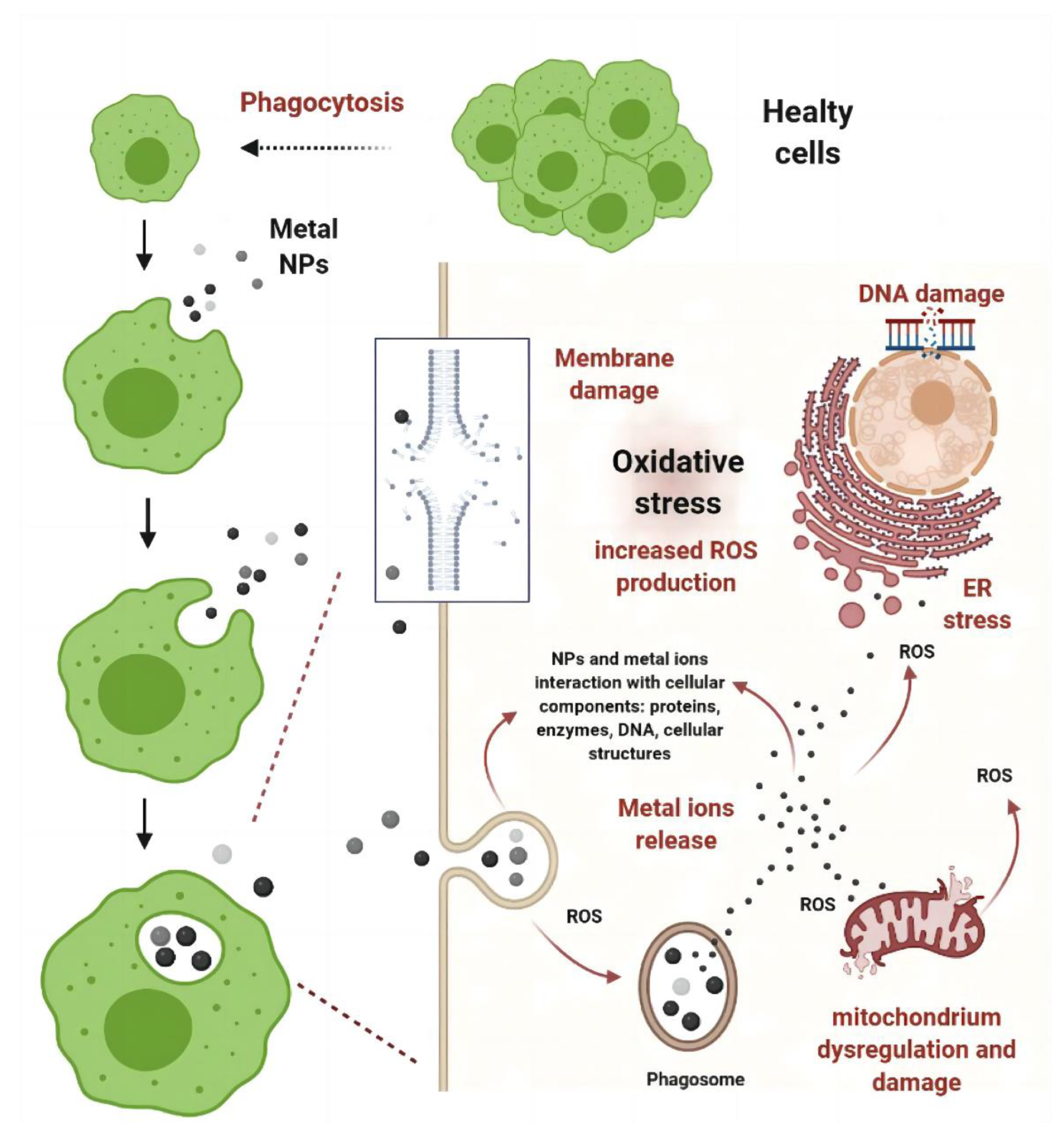
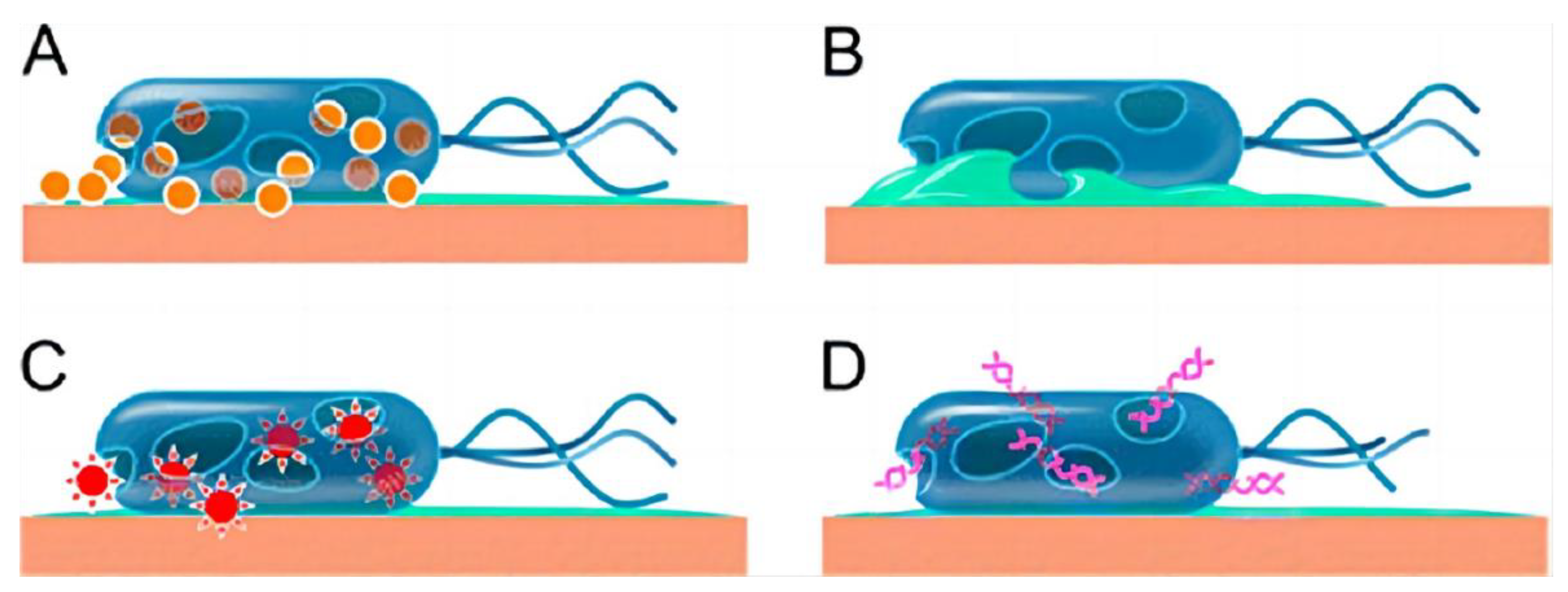
| Mechanisms | Examples | Ref. | |
|---|---|---|---|
| Biofilm matrix | Antimicrobial penetration | Biofilms of P. aeruginosa reduce the diffusion of tobramycin. | [25] |
| Polysaccharides | Psl(a exopolysaccharide produced by Pseudomonas aeruginosa) appears to play a role in resistance to colistin, polymyxin B, tobramycin and ciprofloxacin at early stages of biofilm development. | [26] | |
| Biofilms lacking Pel(a component of the biofilm glycocalyx in P. aeruginosa) were found to be more susceptible to tobramycin and gentamicin compared to wild-type biofilms. | [27] | ||
| antibiotic-degrading enzymes | K. pneumoniae biofilms produce β-lactamase that was found to effectively degrade ampicillin. | [28] | |
| Extracellular DNA | DNA added to P. aeruginosa biofilms from exogenous sources can become incorporated into the biofilm matrix, resulting in an increased level of resistance by 3-fold for tobramycin and by 2-fold for gentamicin. | [29] | |
| Bacteriophages | The presence of Pf phage allows P. aeruginosa to form liquid crystal biofilms with a higher tolerance to tobramycin. | [30] | |
| Nutritional limitation and stress responses | Physiological heterogeneity, hypoxia and reduced growth rate | The gradient of oxygen in P. aeruginosa biofilms can hypoxia the deep layers of the biofilm. Cell had decreased metabolic activity in the hypoxic zone, and this slow growth rate conferred tolerance to antibiotics. | [31] |
| Amino acid starvation and the stringent response | Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited P. aeruginosa. | [32] | |
| cell-wall-modifying enzymes | The dltABCD operon was a positive hit in a screen for biofilm-specific gentamicin tolerance genes in Streptococcus mutans, the increased negative charge of the ΔdltA strain promotes uptake of gentamicin. | [33] | |
| glycosyltransferases | The ndvB locus was identified as a P. aeruginosa biofilm-specific antibiotic resistance gene, ΔndvB biofilms were 16-fold more susceptible to tobramycin and 8-fold more susceptible to both gentamicin and ciprofloxacin than wild-type biofilms. | [34] | |
| efflux pumps | P. aeruginosa biofilm resistance to azithromycin was dependent on the presence of either the MexAB-OprM or the MexCD-OprJ pumps. | [35] | |
| genetic diversity | Horizontal gene transfer | In Staphylococcus aureus, the conjugal transfer frequency of a multidrug resistance plasmid was on the order of 10,000 times greater in biofilms than planktonic cultures. | [36] |
| Mutation frequency | The mutation frequency for selection of ciprofloxacin resistant mutants was approximately 2-log higher in P. aeruginosa biofilm cells than in planktonic cells. | [37] | |
| multispecies interactions | In an in vivo polymicrobial wound model, P. aeruginosa growing in a monospecies biofilm was 2-fold more susceptible to gentamicin treatment than P. aeruginosa in a polymicrobial biofilm with S. aureus, Enterococcus faecalis and Finegoldia magna. | [38] | |
| Metal Nanomaterials | Antimicrobial Mechanism | Characteristic | Example of the Application on the Coating | Ref. |
|---|---|---|---|---|
| AgNPs | Contact Killing ROS release and Oxidative stress | Efficient contact killing capability causes cell membrane perforation and leakage of intracellular compounds. | Silver-plated titanium plate implants showed better biocompatibility than pure titanium plates with metal implants, and successfully cured the rabbit infection. Triplate silver nanoparticle coatings with gold atoms have high antibacterial activity against bacteria. Silver nanoclusters enhanced the antimicrobial properties of the surface of flexible polymer. | [45,46,47] |
| CuNPs | Contact Killing ROS release and Oxidative stress | Compared to AgNPs, CuNPs have weaker antimicrobial properties and require higher concentrations to inhibit microorganisms. | Copper plate and copper dot oxide coatings had good antibacterial activity against E. coli and MRSA. Porous titanium dioxide coating of titanium doped with CuNPs deposited showed good antibacterial activity against S. aureus. | [48,49] |
| ZnO NPs | Contact Killing ROS release and Oxidative stress | Extensive antimicrobial spectrum good biocompatibility chemical stability low toxicity. | Zoxide and HA nanoparticle composite coatings reduced the number of Streptococcus, Anaerobes, and aerobic bacteria by 95%, 95%, and 90%, respectively. 100% ZnO NPs and 75% ZnONPs/25% HANPs composite-coated substrates have significant antimicrobial activity against Staphylococcus aureus. | [50,51] |
| TiO2 NPs | The generation of ROS and the degradation of biopolymers Change the surface features to a hydrophilic state | Photo-induced antimicrobial properties by ultraviolet (UV) light. Non-toxic to humans good chemical stability to biomaterials. | Nanocomposite TiO2:Cu:Ag coatings have a strong antimicrobial effect. | [52] |
| Apellation of Coatings | Classification of Dendrimers | Action Substrate | Target Bacterial Species | Characteristic | Ref. |
|---|---|---|---|---|---|
| Titanium-binding peptides/multivalent PEGylated-lysine dendrimer cores | Peptide-based Ds | Ti-coated slides | S. aureus | Antimicrobial properties of the coated improved with the number of TBP repeats | [156,157] |
| Poly(amidoamine) dendrimers | Cationic Ds | titanium substrates | P.aeruginosa, S. aureus | Higher bactericidal activity against Gram-negative bacteria than against Gram-positive bacteria durable bacteriophobic abilities good stability and biocompatibility | [158] |
| Hyaluronic Acid/Poly(amidoamine) Dendrimer Multilayer | Cationic Ds | Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) | E.coli | Durable anti-adhesive activity and bactericidal activity good biocompatibility | [159] |
| Cationic PAMAM-CTS-Ds | Cationic Ds | cotton fabric | S. aureus | Antibacterial activity comparable to natural CS films | [160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xiong, C.; Yu, Z.; Zhang, J.; Huang, Y.; Zhou, X. Research Progress on Antibacterial Coatings for Preventing Implant-Related Infection in Fractures: A Literature Review. Coatings 2022, 12, 1921. https://doi.org/10.3390/coatings12121921
Wang H, Xiong C, Yu Z, Zhang J, Huang Y, Zhou X. Research Progress on Antibacterial Coatings for Preventing Implant-Related Infection in Fractures: A Literature Review. Coatings. 2022; 12(12):1921. https://doi.org/10.3390/coatings12121921
Chicago/Turabian StyleWang, Hao, Chenwei Xiong, Zhentang Yu, Junjie Zhang, Yong Huang, and Xindie Zhou. 2022. "Research Progress on Antibacterial Coatings for Preventing Implant-Related Infection in Fractures: A Literature Review" Coatings 12, no. 12: 1921. https://doi.org/10.3390/coatings12121921
APA StyleWang, H., Xiong, C., Yu, Z., Zhang, J., Huang, Y., & Zhou, X. (2022). Research Progress on Antibacterial Coatings for Preventing Implant-Related Infection in Fractures: A Literature Review. Coatings, 12(12), 1921. https://doi.org/10.3390/coatings12121921








