The Influence of CO2 Curing on the Mechanical Performance and the Corresponding Chloride Ion Resistance of Alkali-Activated Compound Mineral Admixtures
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Specimen Preparation
2.3. Measurement
3. Results and Discussions
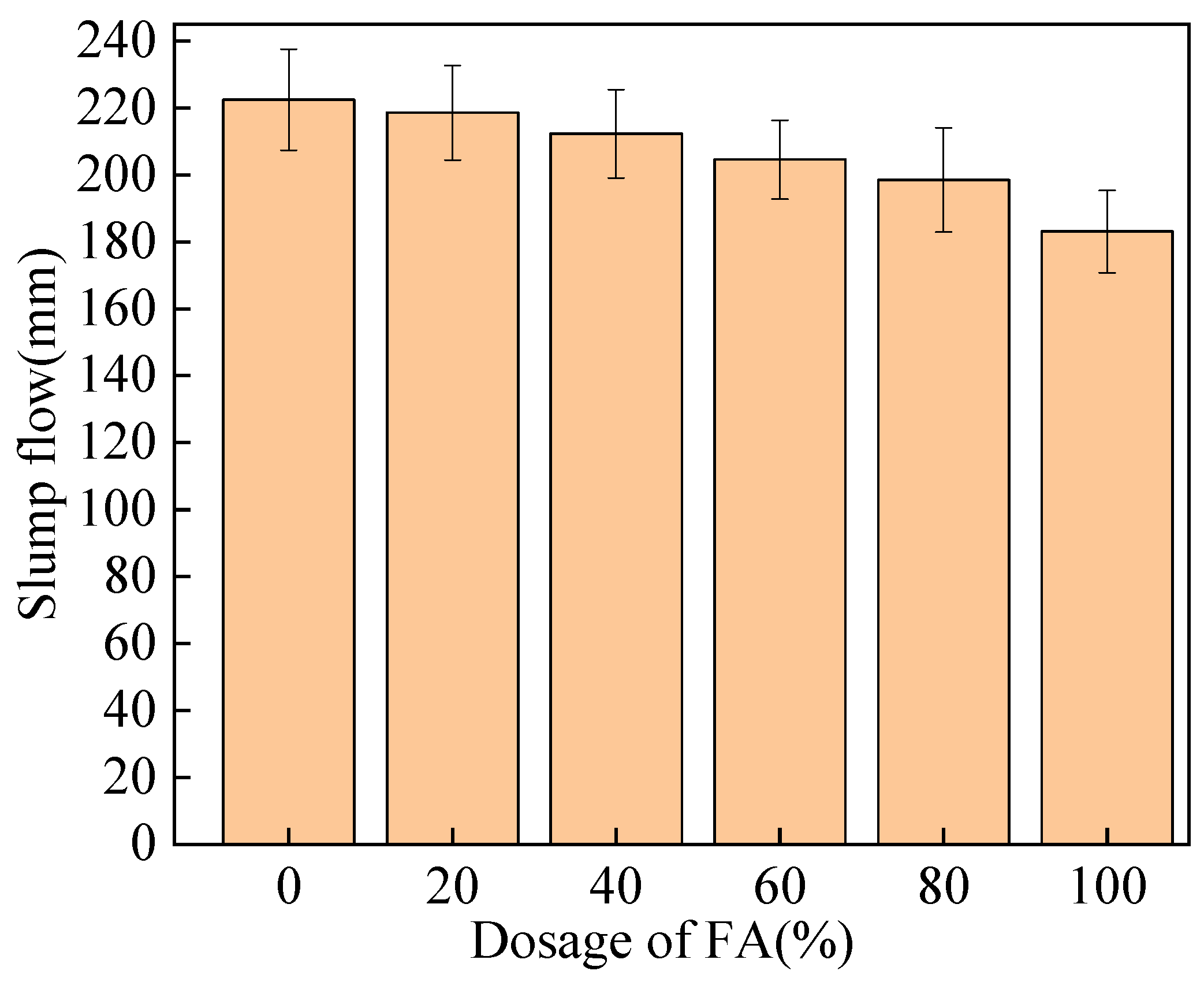
3.1. Influence of Steel Fibers on Working Performance
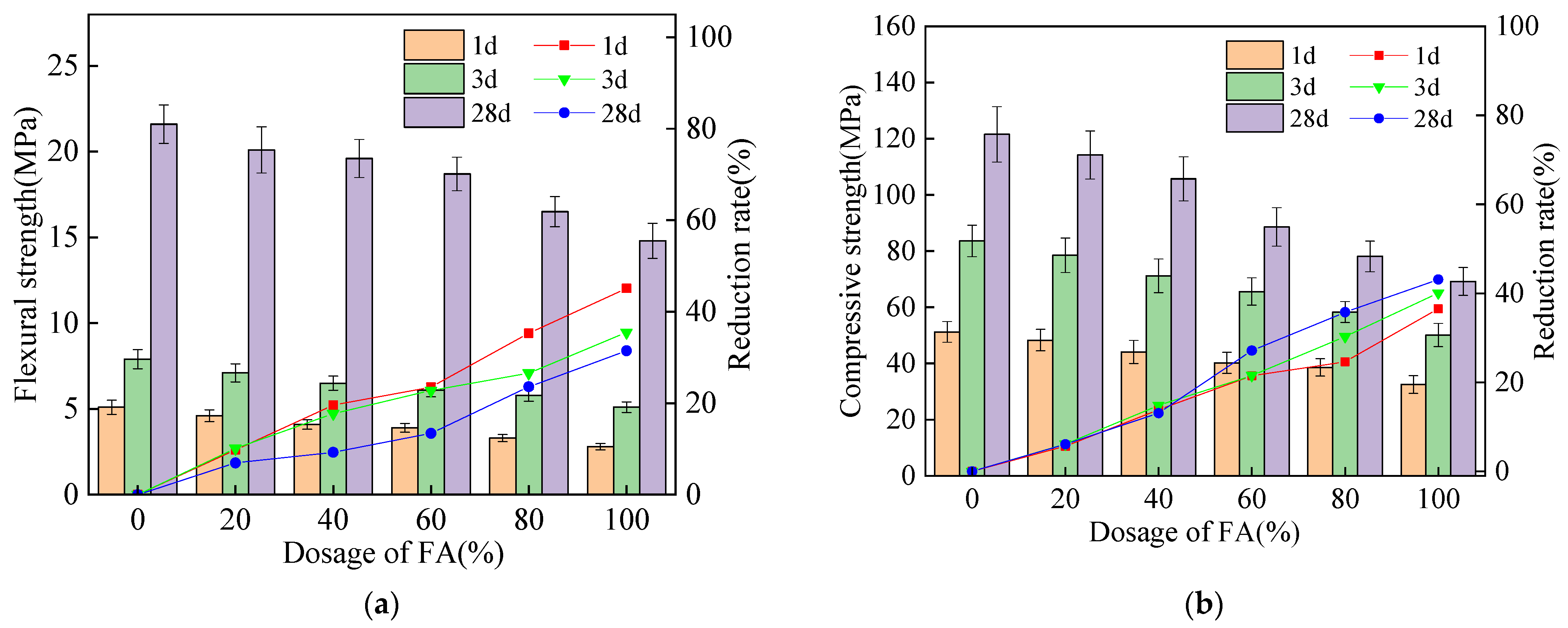
3.2. The Mechanical Strengths
4. Conclusions
- (1)
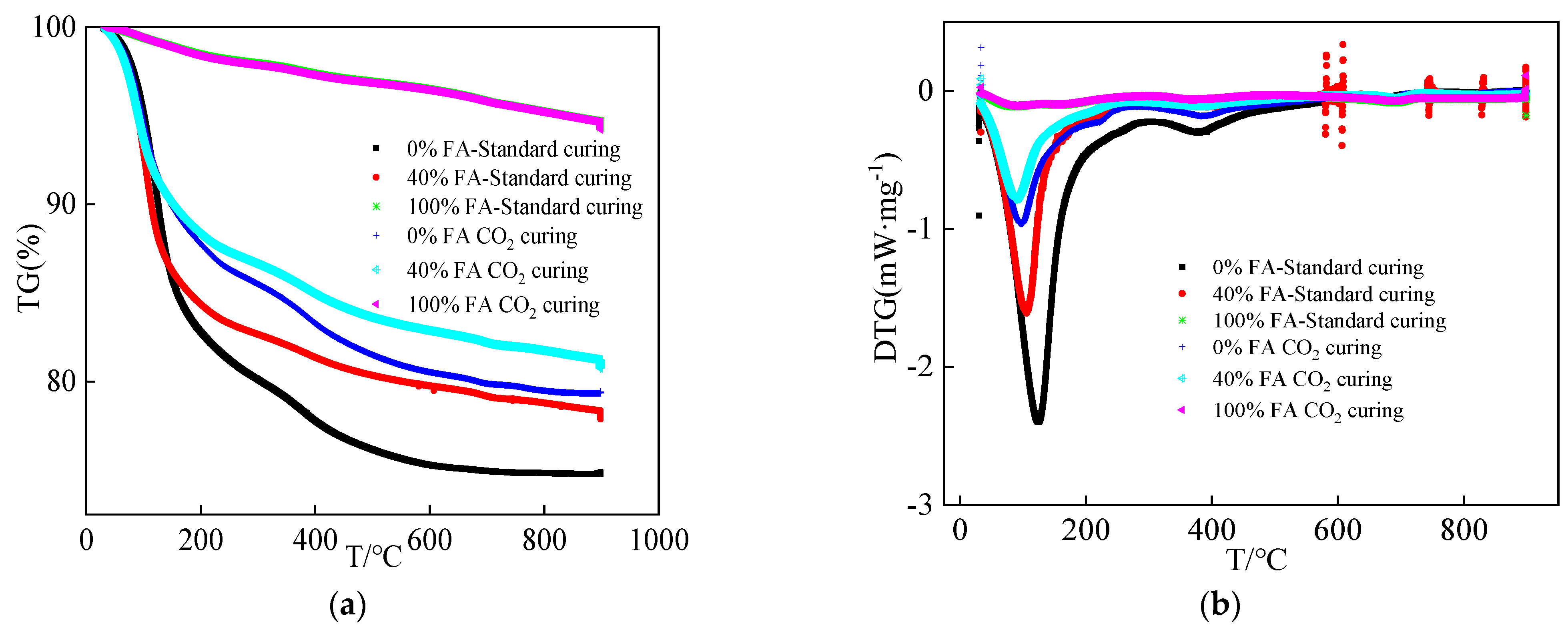
- The mechanical strengths of alkali-activated compound mineral admixtures are decreased by the addition of FA. Moreover, CO2 curing is effective in improving the mechanical strength. The addition of FA can decrease the flexural and compressive strengths of alkali-activated compound mineral admixtures by 32.6%–47.6% and 35.4%–42.3%, respectively. Meanwhile, CO2 curing can improve the flexural and compressive strengths of alkali-activated compound mineral admixtures by 16.7%–26.5% and 15.4%–23.1%, respectively. When, the BFS content is higher, the improving effect of CO2 curing is higher.
- (2)
- The addition of BFS and CO2 curing demonstrate an increasing effect on the drying shrinkage rate of alkali-activated compound mineral admixtures. Due to the addition of BFS, the drying shrinkage rate is decreased by 23.5%–32.1%, while, with CO2 curing, the drying shrinkage rate is increased by 36.5%–63.1%.
- (3)
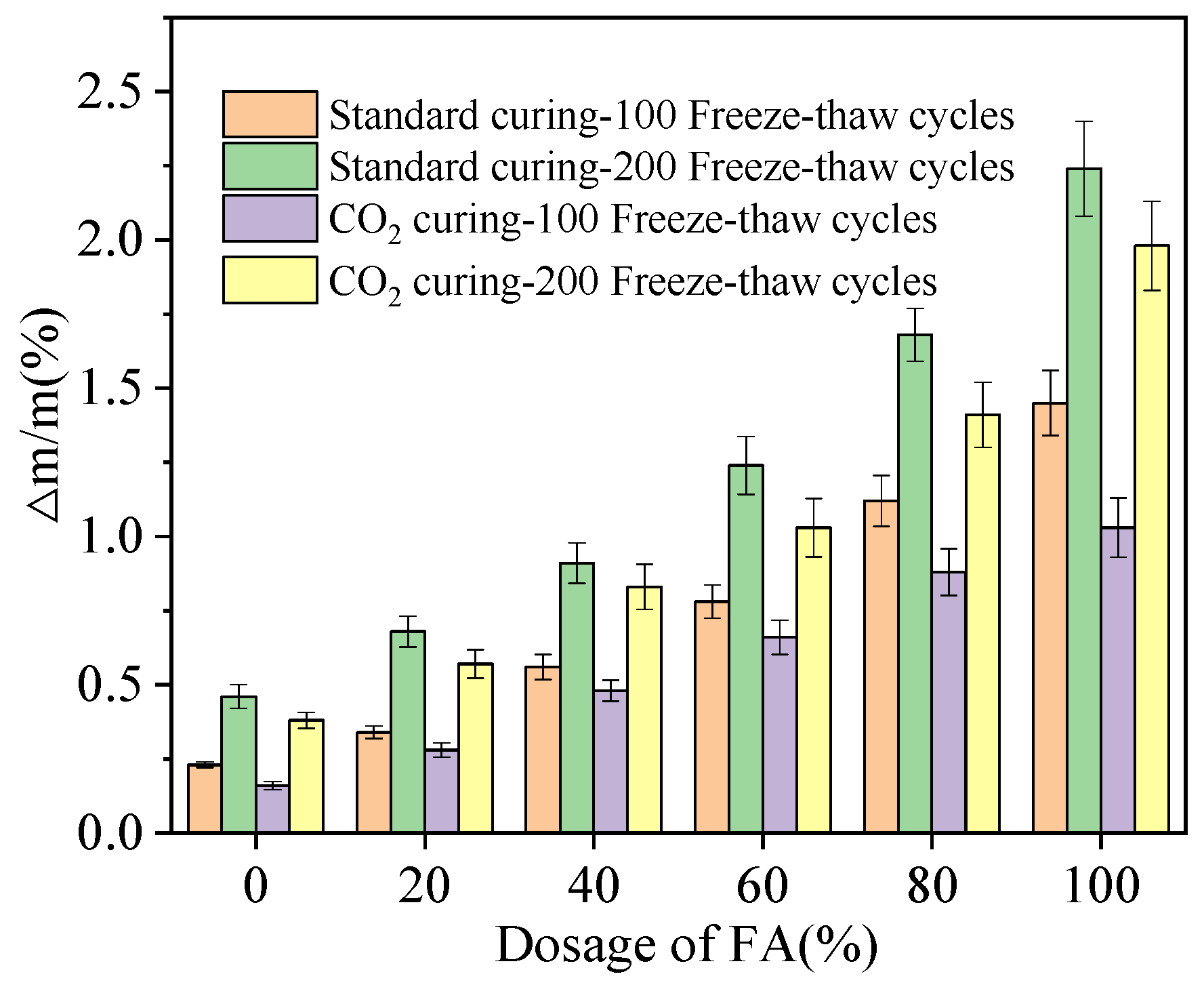
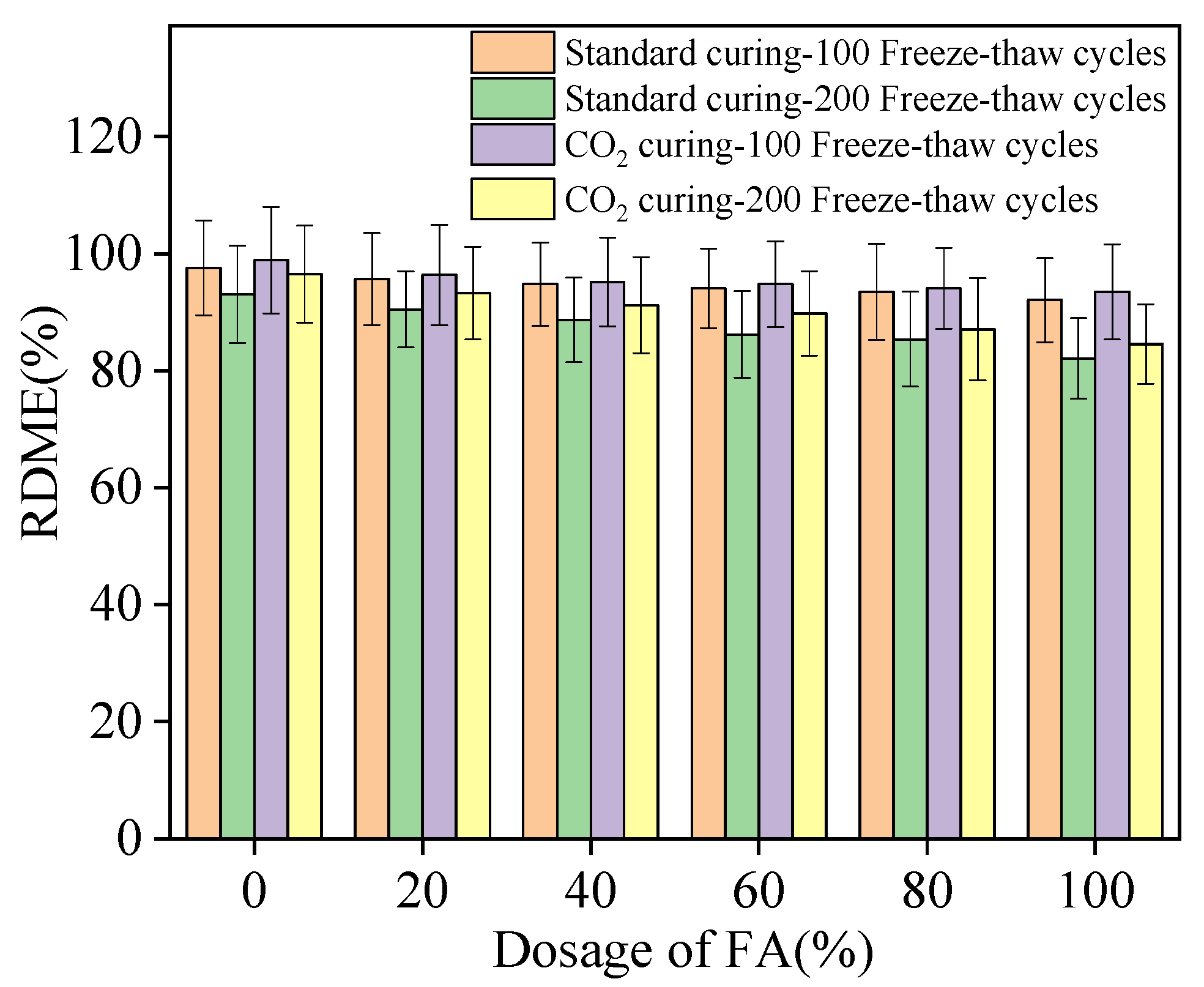
- The chloride ion impermeability and resistance of NaCl freeze–thaw cycles of alkali-activated compound mineral admixtures are improved by the increasing dosages of BFS and CO2 curing.
- (4)
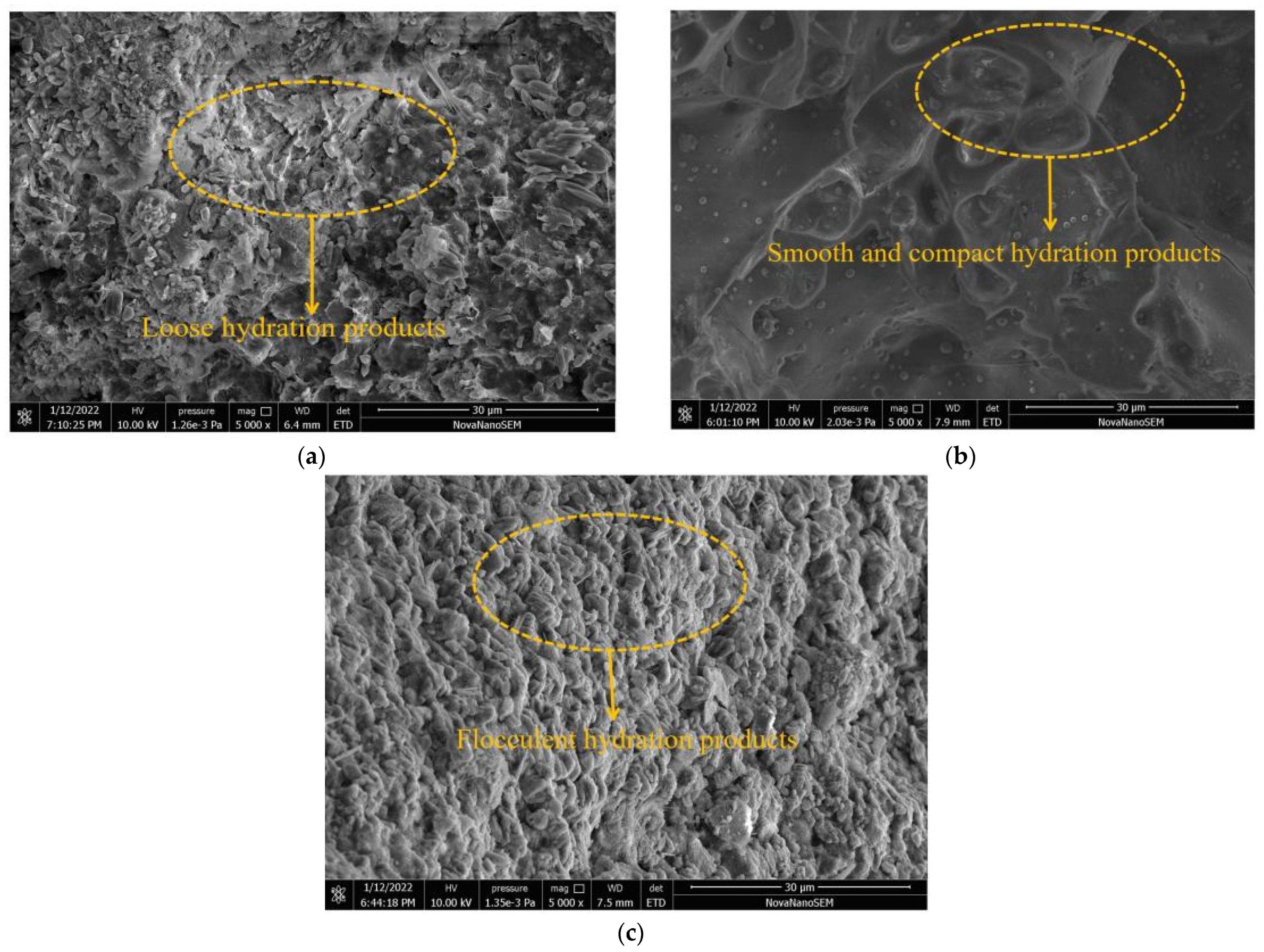
- CO2 curing is able to increase the production of CaCO3, while when the dosage of BFS is higher, the CaCO3 product is higher. The addition of BFS can improve the compactness of the hydration products and CO2 curing can increase the amount of rough hydration products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Li, X.; Tan, Y. Effect of aging on fatigue performance of cement emulsified asphalt repair material. Constr. Build. Mater. 2021, 292, 123417. [Google Scholar] [CrossRef]
- Shah, J. Laboratory characterization of controlled low-strength material and its application to construction of flexible pipe drainage system. Health Manpow. Manag. 2000, 19, 30–59. [Google Scholar]
- Haselbach, L.; Valavala, S.; Montes, F. Permeability predictions for sand clogged Portland cement pervious concrete pavement systems. J. Environ. Manag. 2005, 81, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, X.; Zhang, Q. Evaluating performance of concrete pavement joint repair using different materials to reduce reflective cracking in asphalt concrete overlay. Road Mater. Pavement Des. 2014, 15, 966–976. [Google Scholar] [CrossRef]
- Huang, G.; Wang, H.; Shi, F. Coupling Effect of Salt Freeze-Thaw Cycles and Carbonation on the Mechanical Performance of Quick Hardening Sulphoaluminate Cement-Based Reactive Powder Concrete with Basalt Fibers. Coatings 2021, 11, 1142. [Google Scholar] [CrossRef]
- Ju, Y.; Zhao, J.; Wang, D.; Song, Y. Experimental study on flexural behaviour of reinforced reactive powder concrete pole. Constr. Build. Mater. 2021, 312, 125399. [Google Scholar] [CrossRef]
- Liu, M.; Tan, H.; He, X. Effects of nano-SiO2 on early strength and microstructure of steam-cured high volume flfly ash cement system. Constr. Build. Mater. 2019, 194, 350–359. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, H.; Shen, W.; Xu, G.; Ma, B.; Ji, X. Nano-silica and silica fume modifified cement mortar used as Surface Protection Material to enhance the impermeability. Cem. Concr. Compos. 2018, 92, 7–17. [Google Scholar] [CrossRef]
- Liska, M.; Jin, F.; Yi, Y.; Al-Tabbaa, A. Mechanism of reactive magnesia-ground granulated blastfurnace slag (GGBS) soil stabilization. Can. Geotech. J. 2016, 53, 773–782. [Google Scholar]
- Celikten, S.; Sarldemir, M.; Deneme, I.O. Mechanical and microstructural properties of alkali-activated slag and slag+ fly ash mortars exposed to high temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Li, S.; Feng, Y.; Yang, J.; Cerny, R. Expansion mechanism and properties of magnesium oxide expansive hydraulic cement for engineering applications. Adv. Mater. Sci. Eng. 2021, 2021, 5542072. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Zhang, P. Hydration and hardening properties of reactive magnesia and Portland cement composite. Constr. Build. Mater. 2022, 327, 126779. [Google Scholar] [CrossRef]
- Ozata, S.; Akturk, B.; Yuzer, N. Utilization of waste Cappadocia earth as a natural pozzolan in alkali activation: A parametric study. Constr. Build. Mater. 2022, 329, 127192. [Google Scholar] [CrossRef]
- Marple, M.; Koroglu, B.; Morrison, K.; Crowhurst, J.; Balachandra, A.; Soroushian, P.; Mason, H.E. Accelerated carbonation and structural transformation of blast furnace slag by mechanochemical alkali-activation. Cem. Concr. Res. 2022, 156, 106760. [Google Scholar] [CrossRef]
- Matějková, P.; Matějka, V.; Sabovčík, T.; Gryžbon, L.; Vlček, J. Alkali Activation of Ground Granulated Blast Furnace Slag and Low Calcium Fly Ash Using “One-Part” Approach. J. Sustain. Metall. 2022, 8, 511–521. [Google Scholar] [CrossRef]
- Supriya, J.; Raut, A. Performance parameter analysis of magnesia based cement products-a review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1197, 012078. [Google Scholar] [CrossRef]
- Glukhovsky, V.D.; Rostovskaja, G.S.; Rumyna, G.V. High strength slag—Alkaline cements. In Proceedings of the 7th International Congress on the Chemistry of Cement, Paris, France, September 1980. [Google Scholar]
- Alomayri, T.; Adesina, A.; Das, S. Influence of amorphous raw rice husk ash as precursor and curing condition on the performance of alkali activated concrete. Case Stud. Constr. Materials 2021, 15, e00777. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Dai, J. Effects of delayed addition of polycarboxylate ether on one-part alkali-activated fly ash/slag pastes: Adsorption, reaction kinetics, and rheology. Constr. Build. Mater. 2022, 323, 126611. [Google Scholar] [CrossRef]
- Feng, S.; Zhu, J.; Wang, R.; Qu, Z.; Song, L.; Wang, H. The Influence of CaO and MgO on the Mechanical Properties of Alkali-Activated Blast Furnace Slag Powder. Materials 2022, 15, 6128. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2022, 56, 101861. [Google Scholar] [CrossRef]
- Adesina, A. Recent advances in the concrete industry to reduce its carbon dioxide emissions. Environ. Chall. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Witoon, T.; Lapkeatseree, V.; Numpilai, T.; Cheng, C.; Limtrakul, J. CO2 hydrogenation to light olefins over mixed Fe-Co-K-Al oxides catalysts prepared via precipitation and reduction methods. Chem. Eng. J. 2022, 428, 131389. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Nijpanich, S.; Chanlek, N.; Kidkhunthod, P.; Cheng, C.; Ng, K.; Vo, D.N.; Ittisanronnachai, S.; Wattanakit, C.; et al. Enhanced CO2 hydrogenation to higher alcohols over K-Co promoted In2O3 catalysts. Chem. Eng. J. 2022, 431, 133211. [Google Scholar] [CrossRef]
- AL-Ameeri, A.S.; ImranRafiq, M.; Tsioulou, O.; Rybdylova, O. Impact of climate change on the carbonation in concrete due to carbon dioxide ingress: Experimental investigation and modelling. J. Build. Eng. 2021, 44, 102594. [Google Scholar] [CrossRef]
- Kamal, N.L.M.; Itam, Z.; Sivaganese, Y.; Beddu, S. Carbon dioxide sequestration in concrete and its effects on concrete compressive strength. Mater. Today Proc. 2020, 31, A18–A21. [Google Scholar] [CrossRef]
- Zhu, J.; Qu, Z.; Liang, S.; Li, B.; Du, T.; Wang, H. The macro-scopic and microscopic properties of cement paste with carbon dioxide curing. Materials 2022, 15, 1578. [Google Scholar] [CrossRef]
- Smirnov, V.G.; Manakov, A.Y.; Dyrdin, V.V.; Ismagilov, Z.R.; Mikhailova, E.S.; Rodionova, T.V. The formation of carbon dioxide hydrate from water sorbed by coals. Fuel 2018, 228, 123–131. [Google Scholar] [CrossRef]
- Branston, J.; Das, S.; Kenno, S.; Taylor, C. Mechanical behaviour of basalt fifibre reinforced concrete. Constr. Build. Mater. 2016, 124, 878–886. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Tensile behavior contrast of basalt and glass fifibers after chemical treatment. Mater. Des. 2010, 31, 4244–4250. [Google Scholar] [CrossRef]
- Shi, X.; Fay, L.; Peterson, M.; Yang, Z. Freeze-thaw damage and chemical change of a portland cement concrete in the presence of diluted deicers. Mater. Struct. 2010, 43, 933–946. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Liu, J. Effects of salt freeze-thaw cycles and cyclic loading on the piezoresistive properties of carbon nanofibers mortar. Constr. Build. Mater. 2018, 177, 192–201. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Su, Y.; He, X.; Tan, H.; Yang, W.; Strnadel, B. Eco-friendly treatment of low-calcium coal fly ash for high pozzolanic reactivity: A step towards waste utilization in sustainable building material. J. Clean. Prod. 2019, 238, 117962. [Google Scholar] [CrossRef]
- Chao, L.; Duy, H.; Mitiku, D.; Vu, A. Improving the strength and engineering properties of alkali-activated slag–rice husk ash paste at the early ages with addition of various magnesium oxide content. Int. J. Struct. Civ. Eng. Res. 2019, 8, 210–214. [Google Scholar]
- Qin, L.; Gao, X. Properties of coal gangue-Portland cement mixture with carbonation. Fuel 2019, 245, 1–12. [Google Scholar] [CrossRef]
- Faridmehr, I.; Bedon, C.; Fahim, G.; Nikoo, M.; Baghban, M. Assessment of Mechanical Properties and Structural Morphology of Alkali-Activated Mortars with Industrial Waste Materials. Sustainability 2021, 13, 2062. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]



| Types | 0.3 μm | 1 μm | 4 μm | 8 μm | 64 μm | 360 μm |
|---|---|---|---|---|---|---|
| BFS | 0.03 | 3.5 | 19.6 | 35.0 | 97.9 | 100 |
| FA | 12.3 | 66.2 | 100 | 100 | 100 | 100 |
| Types | SiO2 | Al2O3 | FexOy | MgO | CaO | SO3 | K2O | Na2O | Ti2O | LI |
|---|---|---|---|---|---|---|---|---|---|---|
| BFS | 34.1 | 14.7 | 0.2 | 9.7 | 35.9 | 0.2 | 3.5 | - | - | - |
| FA | 55.00 | 20.00 | 6.00 | 10.20 | 4.50 | 0.11 | 1.26 | 2.13 | 0.06 | 0.74 |
| Number | Water | P·O | SAC | SF | GGBS | Quartz Sand | Water-Reducer | Li2SO4 | Calcium Formate | Tartaric Acid | Defoamer | Steel Fibers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 244.4 | 370.5 | 370.5 | 370.5 | 111.1 | 978 | 20.3 | 0.6 | 2.6 | 1.9 | 0.6 | 78.5 |
| 2 | 244.4 | 370.5 | 370.5 | 370.5 | 111.1 | 978 | 24.4 | 0.6 | 2.6 | 1.9 | 0.6 | 117.75 |
| 3 | 244.4 | 370.5 | 370.5 | 370.5 | 111.1 | 978 | 12.2 | 0.6 | 2.6 | 1.9 | 0.6 | 157 |
| 4 | 244.4 | 370.5 | 370.5 | 370.5 | 111.1 | 978 | 16.3 | 0.6 | 2.6 | 1.9 | 0.6 | 196.25 |
| 5 | 244.4 | 370.5 | 370.5 | 370.5 | 111.1 | 978 | 20.3 | 0.6 | 2.6 | 1.9 | 0.6 | 235.5 |
| Equation | FA Dosage (%) | a | b | c | R2 |
|---|---|---|---|---|---|
| 0 | −2.14 × 10−5 | 0.0056 | 0.45 | 0.99 | |
| 20 | −1.50 × 10−5 | 0.0036 | 0.33 | 0.93 | |
| 40 | −4.20 × 10−5 | 0.0025 | 0.10 | 0.99 | |
| 60 | 4.28 × 10−5 | 0.0014 | 0.06 | 0.99 | |
| 80 | 8.57 × 10−6 | 0.0004 | 0.04 | 0.98 | |
| 100 | −7.14 × 10−8 | 1.20 × 10−4 | 0.02 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Yang, J.; Wang, H.; Jin, X. The Influence of CO2 Curing on the Mechanical Performance and the Corresponding Chloride Ion Resistance of Alkali-Activated Compound Mineral Admixtures. Coatings 2022, 12, 1920. https://doi.org/10.3390/coatings12121920
Peng L, Yang J, Wang H, Jin X. The Influence of CO2 Curing on the Mechanical Performance and the Corresponding Chloride Ion Resistance of Alkali-Activated Compound Mineral Admixtures. Coatings. 2022; 12(12):1920. https://doi.org/10.3390/coatings12121920
Chicago/Turabian StylePeng, Ling, Junchao Yang, Hui Wang, and Xiaoqing Jin. 2022. "The Influence of CO2 Curing on the Mechanical Performance and the Corresponding Chloride Ion Resistance of Alkali-Activated Compound Mineral Admixtures" Coatings 12, no. 12: 1920. https://doi.org/10.3390/coatings12121920
APA StylePeng, L., Yang, J., Wang, H., & Jin, X. (2022). The Influence of CO2 Curing on the Mechanical Performance and the Corresponding Chloride Ion Resistance of Alkali-Activated Compound Mineral Admixtures. Coatings, 12(12), 1920. https://doi.org/10.3390/coatings12121920






