Pulse Electrodeposited Super-Hydrophobic Ni-Co/WS2 Nanocomposite Coatings with Enhanced Corrosion-Resistance
Abstract
1. Introduction
2. Experimental
2.1. Materials and Specimen Preparation
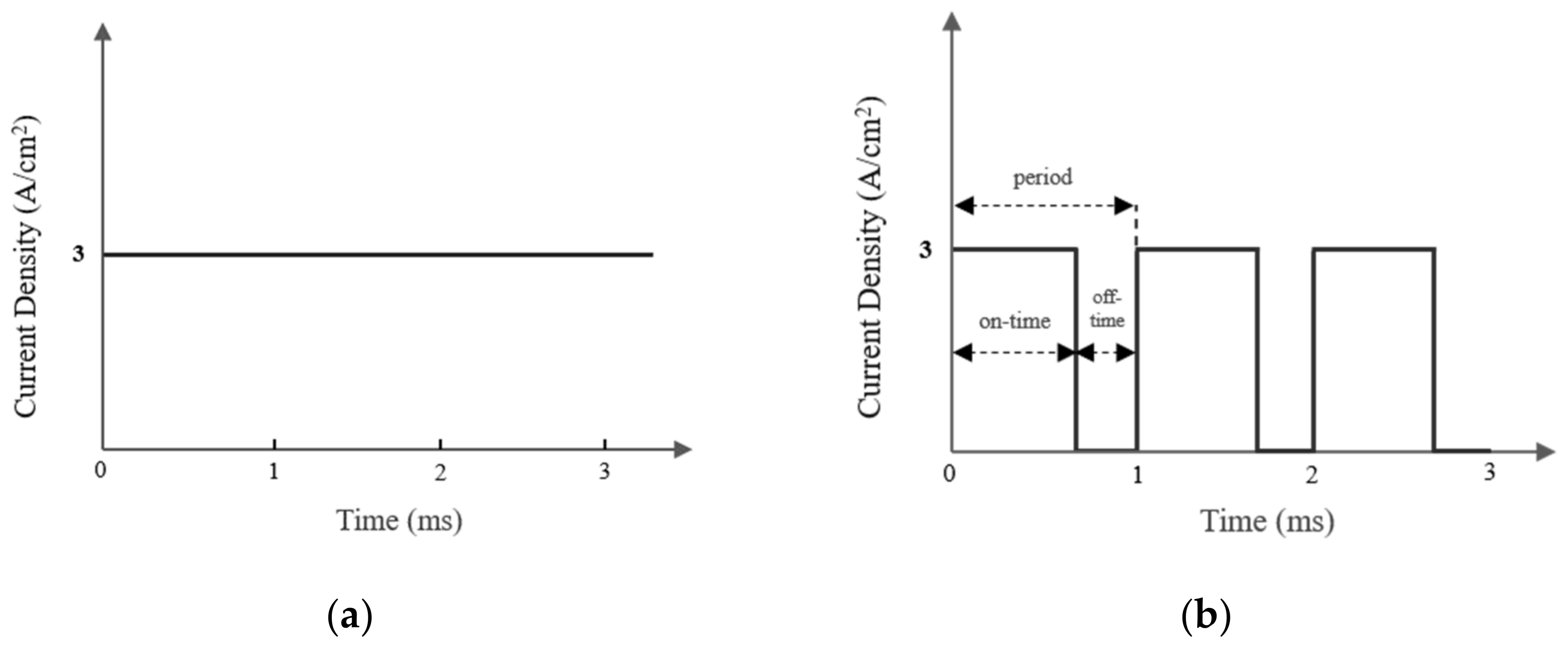
2.2. The Electrodeposition Process
2.3. Characterization
3. Results and Discussion
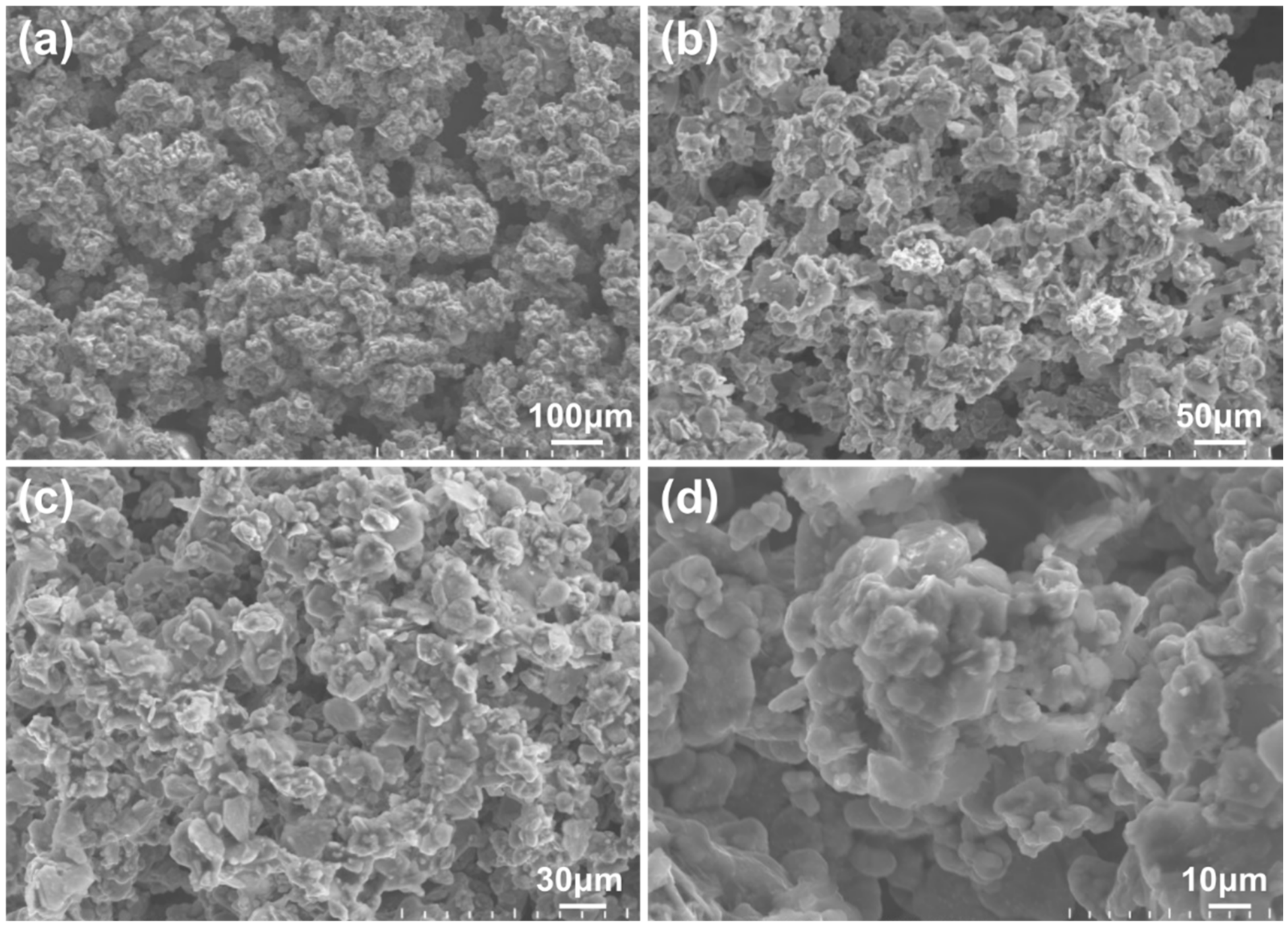
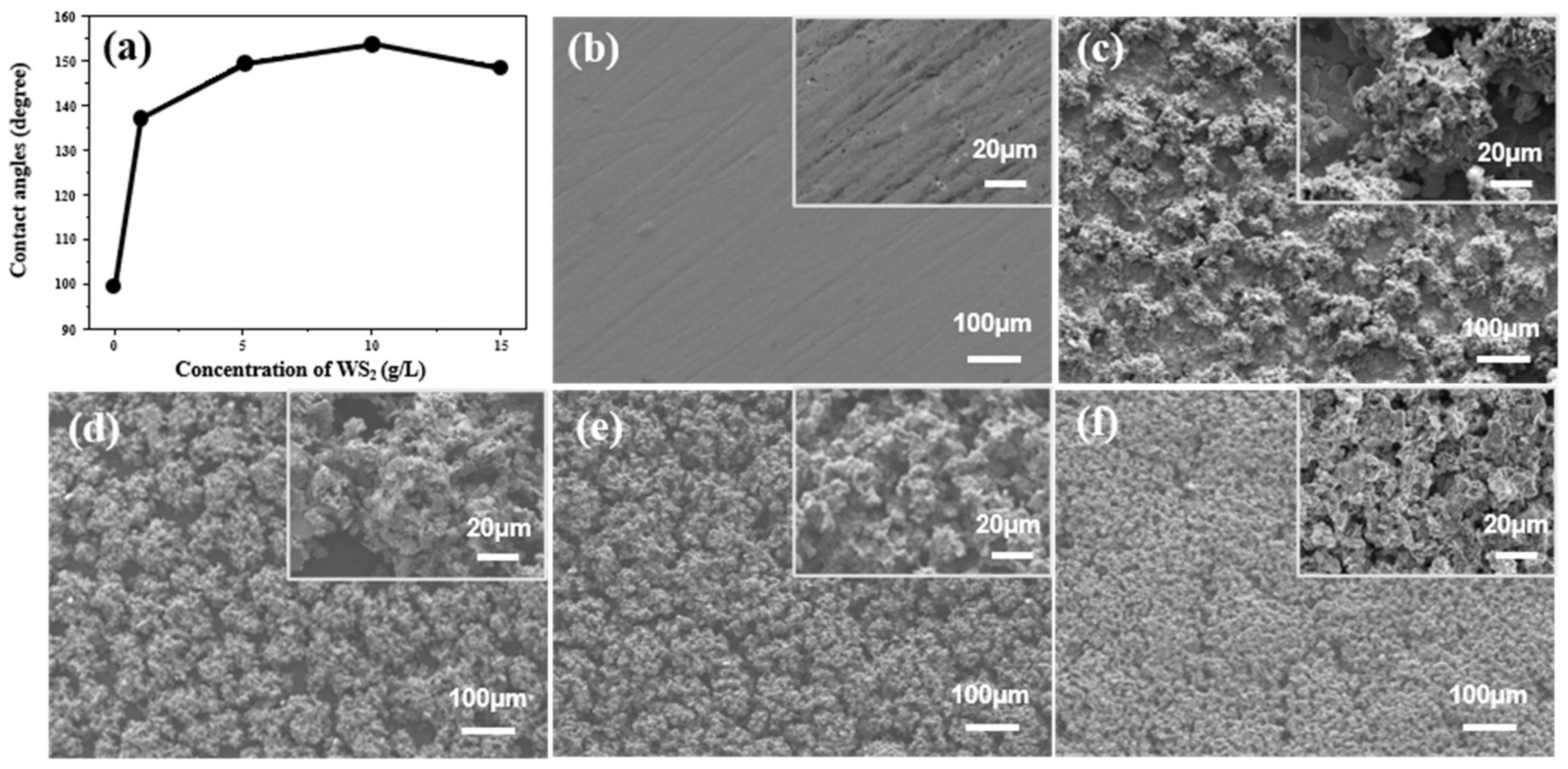
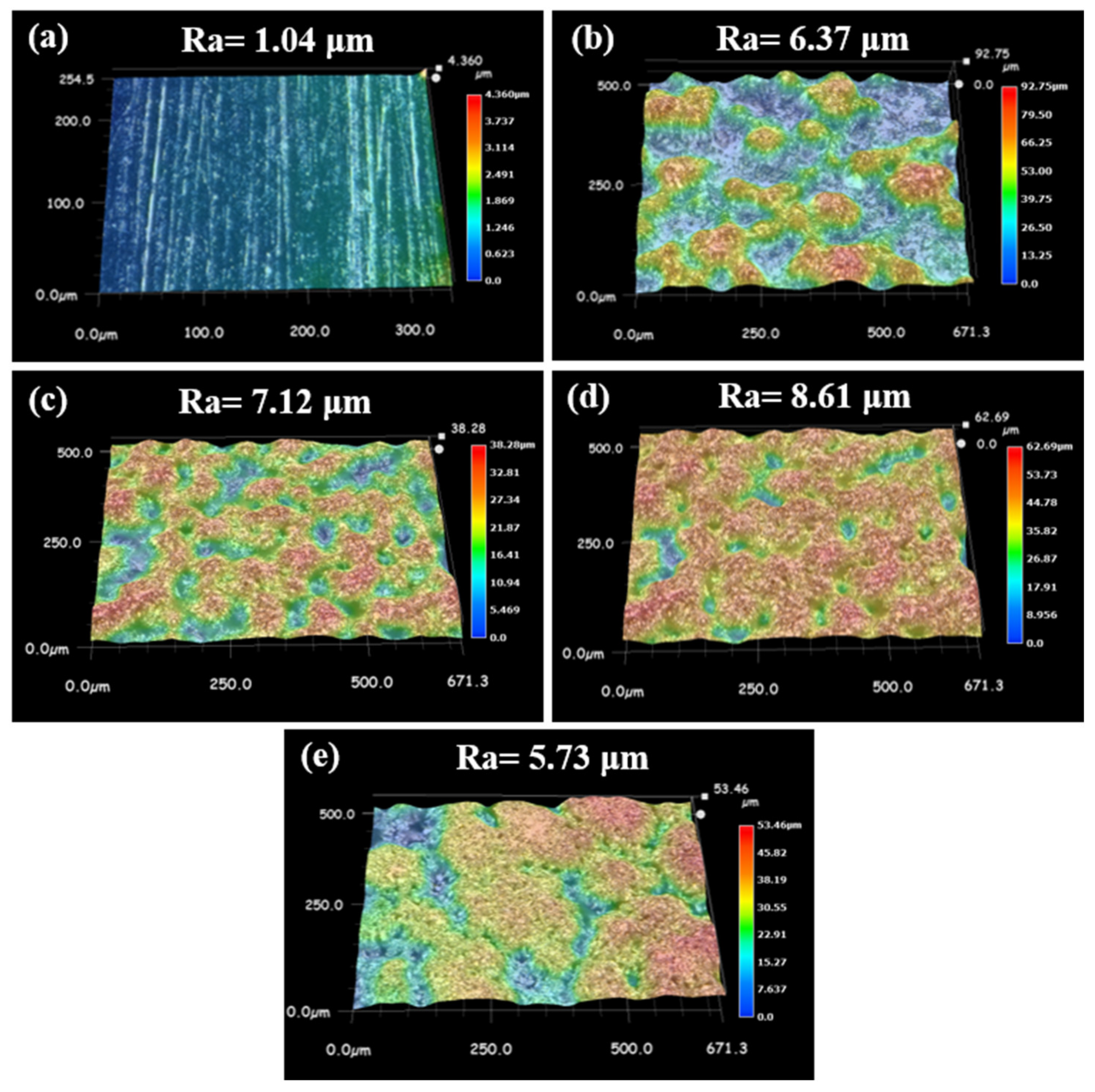
3.1. WS2-Induced Hydrophobic Microstructures on Ni-Co/WS2 Nanocomposite Coating Surface via Direct Current Electrodeposition
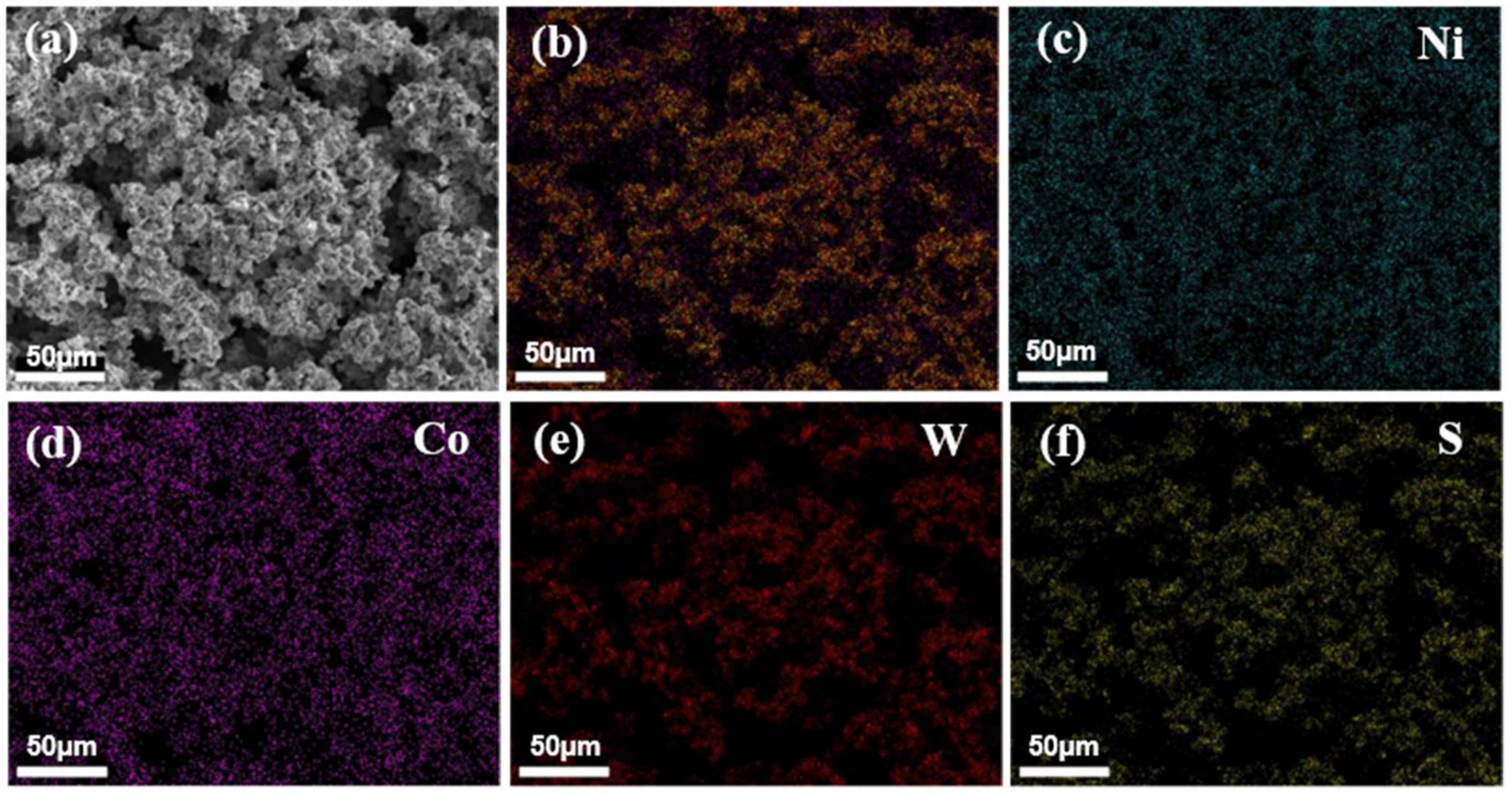
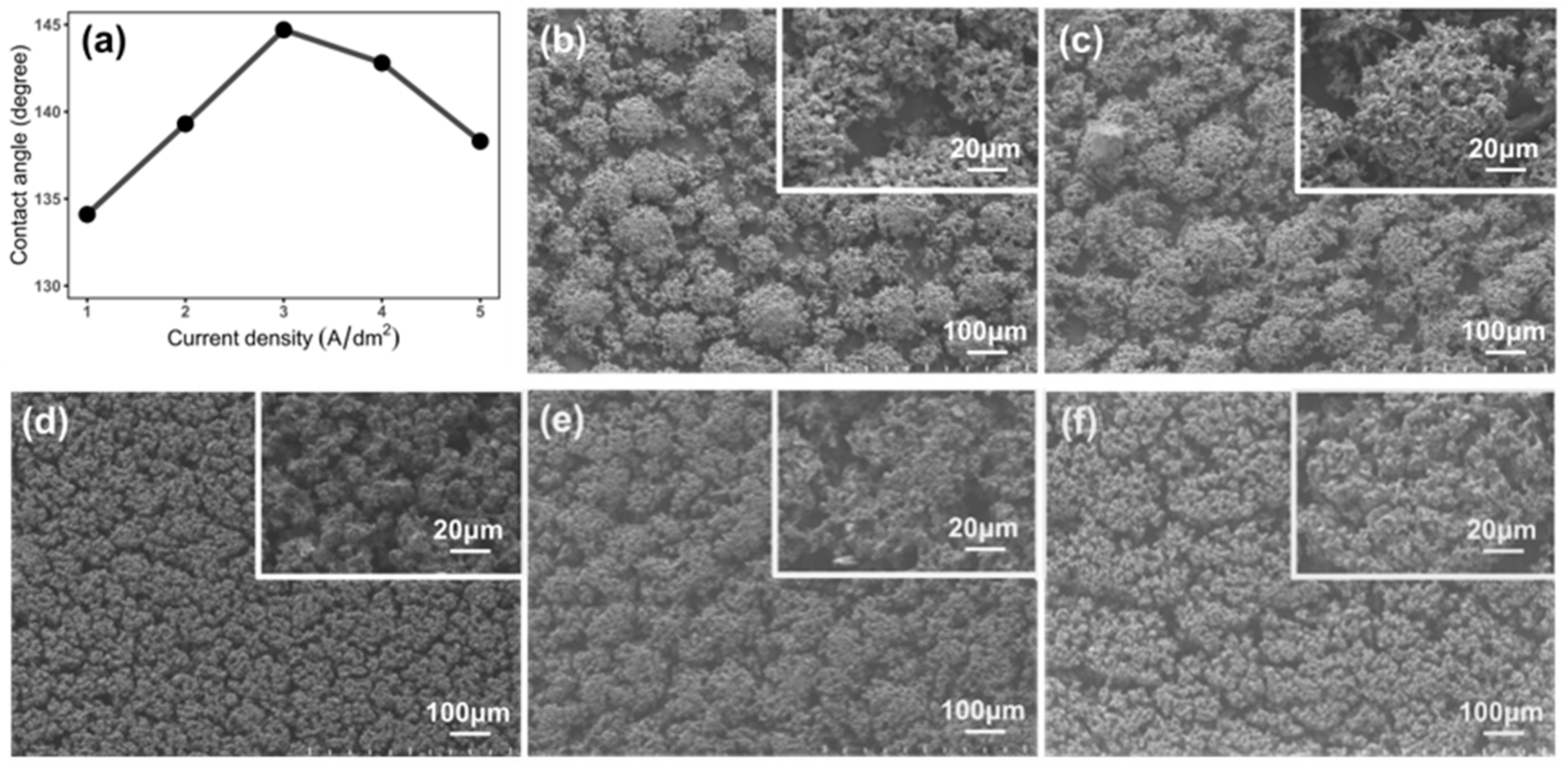
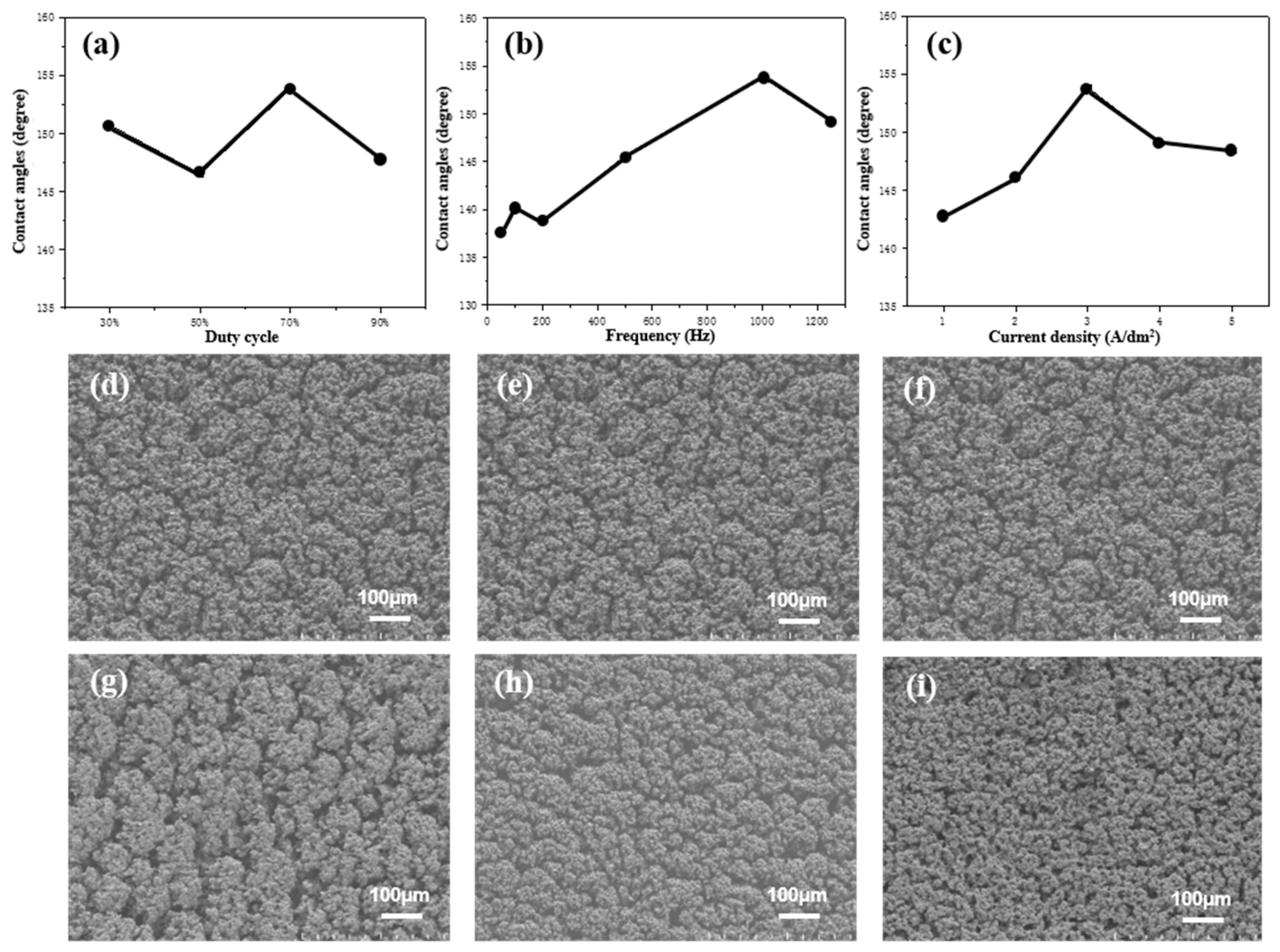
3.2. Improving the Surface Microstructures of Ni-Co/WS2 Nanocomposite Coating via Pulse Electrodeposition
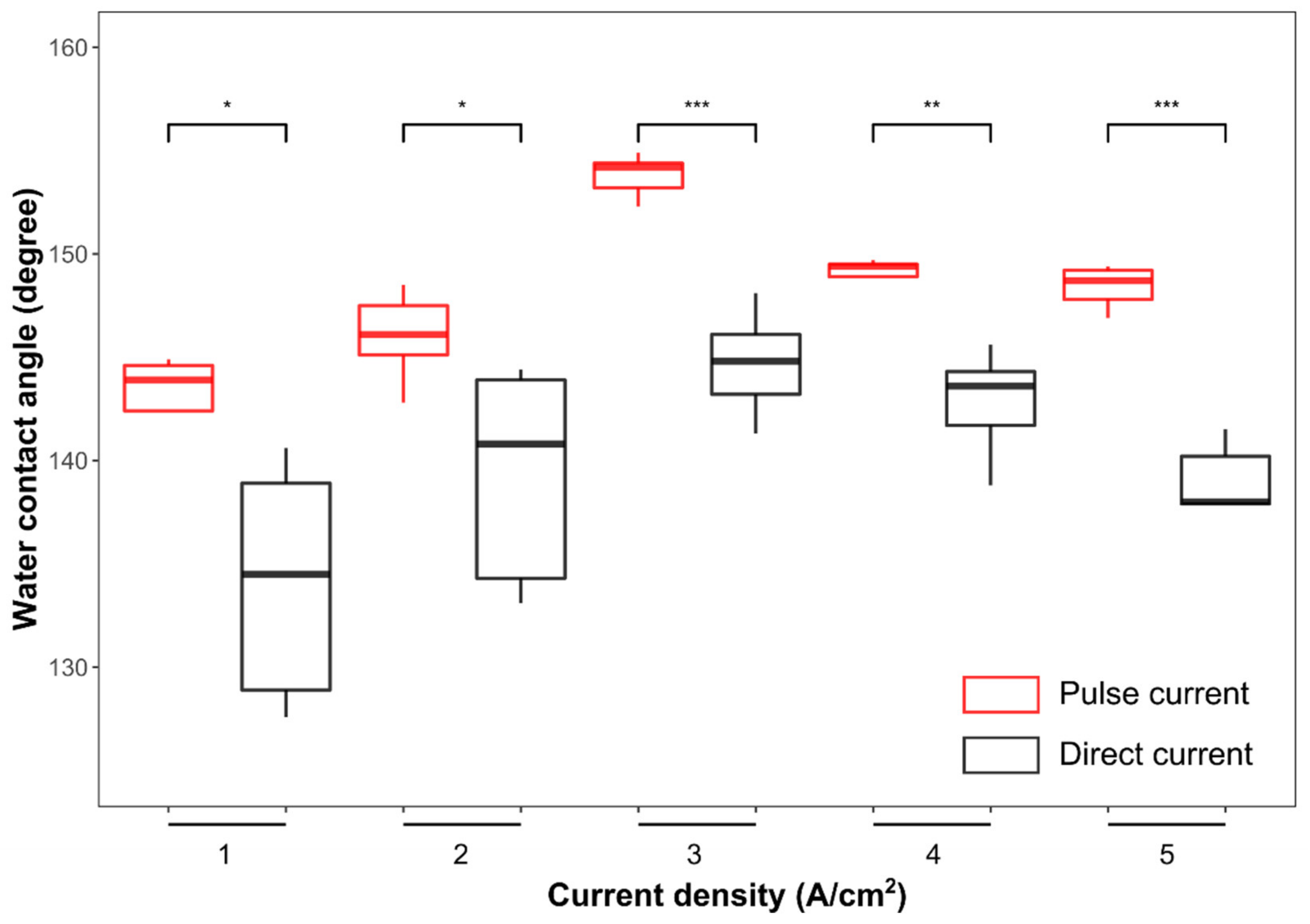
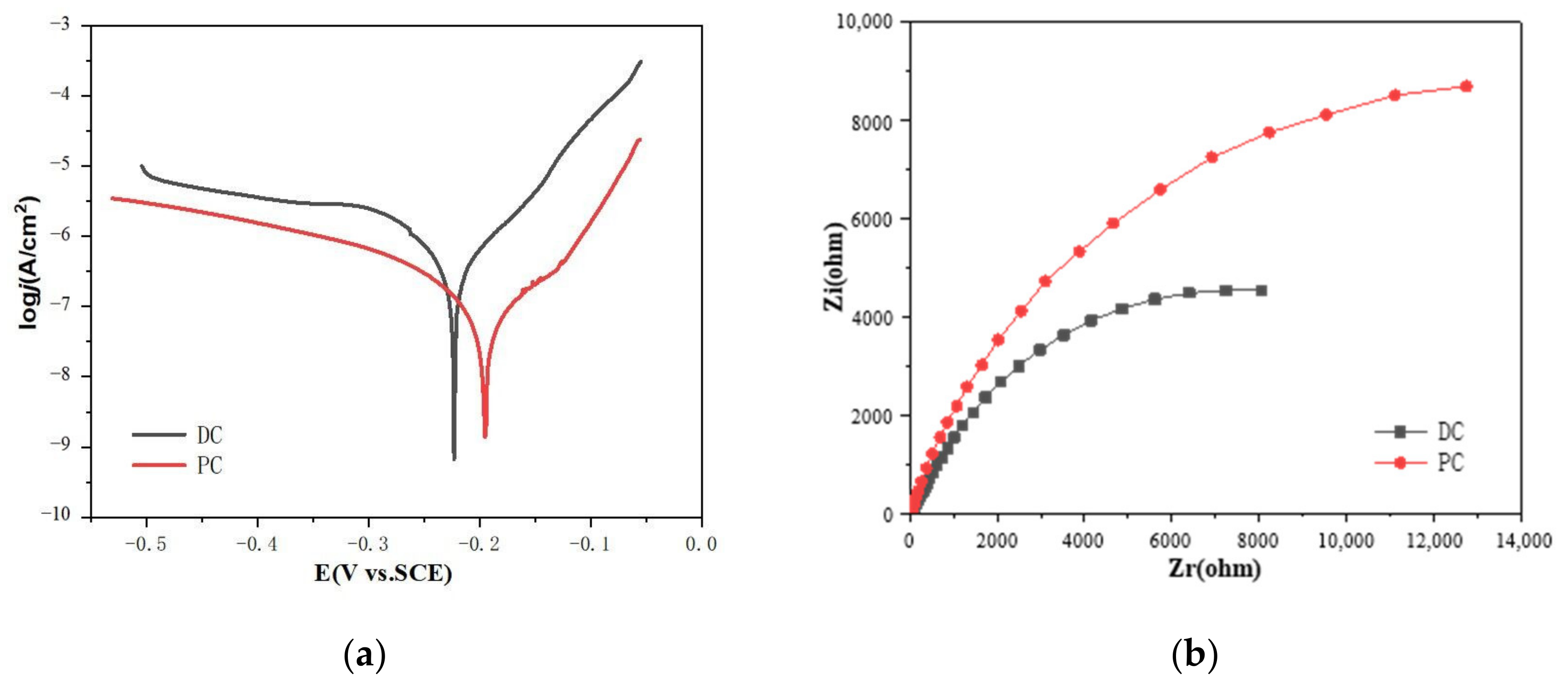
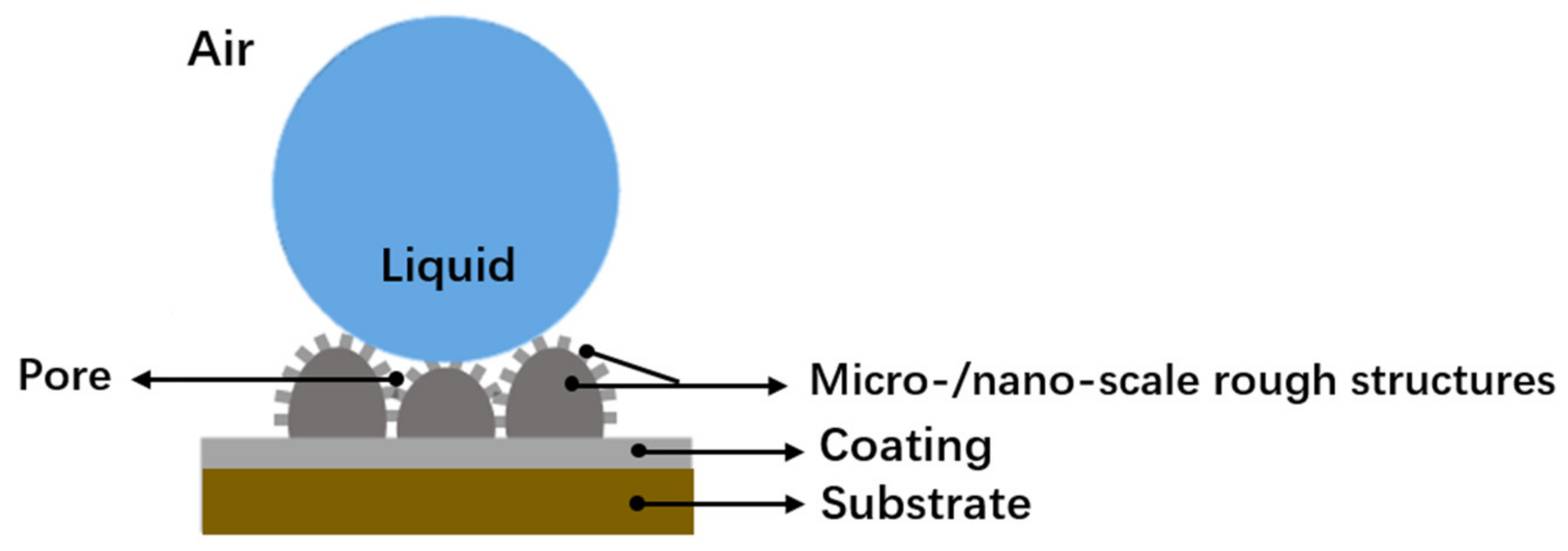
3.3. Mechanistic Understanding for the Hydrophobic Properties and Anti-Corrosion Behavior of the Ni-Co/WS2 Nanocomposite Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Zhang, D.; Qiu, R. Liquid/solid contact mode of super-hydrophobic film in aqueous solution and its effect on corrosion resistance. Corros. Sci. 2012, 54, 77–84. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Tian, Y. Fabrication of super-hydrophobic nickel film on copper substrate with improved corrosion inhibition by electrodeposition process. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 205–212. [Google Scholar] [CrossRef]
- Thiemig, D.; Bund, A. Characterization of electrodeposited Ni–TiO2 nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 2976–2984. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M. Alteration of corrosion and nanomechanical properties of pulse electrodeposited Ni/SiC nanocomposite coatings. J. Alloys Compd. 2014, 598, 236–242. [Google Scholar] [CrossRef]
- Morteza, A.; Safaei, H. Characterization of Ni-Cu matrix, Al2O3 reinforced nano-composite coatings prepared by electrodeposition. Appl. Surf. Sci. 2018, 456, 195–203. [Google Scholar]
- Safavi, M.S.; Fathi, M.; Mirzazadeh, S.; Ansarian, A.; Ahadzadeh, I. Perspectives in corrosion-performance of Ni–Cu coatings by adding Y2O3 nanoparticles. Surf. Eng. 2020, 37, 226–235. [Google Scholar] [CrossRef]
- Li, B.S.; Zhang, W.W.; Li, D.D. Synthesis and properties of a novel Ni–Co and Ni–Co/ZrO2 composite coating by DC electrodeposition. J. Alloys Compd. 2020, 821, 153258. [Google Scholar] [CrossRef]
- Popczyk, M.; Kubisztal, J.; Swinarew, A.S.; Waskiewicz, Z.; Stanula, A.; Knechtle, B. Corrosion Resistance of Heat-Treated Ni-W Alloy Coatings. Materials 2020, 13, 1172. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Peng, Z.J.; Liang, J.; Fu, S.H. Ni–Ti Nanocomposite Coatings Electro-Co-deposited from Deep Eutectic Solvent Containing Ti Nanoparticles. J. Electrochem. Soc. 2020, 167, 042502. [Google Scholar] [CrossRef]
- Bao, Q.L.; Zheng, W.X.; Chen, L.; Xu, Z.K.; Han, J.L.; Zhu, C.F. Optimization of plating process and corrosion behavior of nanocrystalline Ni-Mo coatings on pure aluminum. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128128. [Google Scholar] [CrossRef]
- Ma, C.; Wang, S.C.; Walsh, F.C. Electrodeposition of nanocrystalline nickel-cobalt binary alloy coatings: A review. Trans. Inst. Met. Finish. 2015, 93, 104–112. [Google Scholar] [CrossRef]
- Feng, L.B.; Li, H.; Song, Y.F.; Wang, Y.L. Formation process of a strong water-repellent alumina surface by the sol-gel method. Appl. Surf. Sci. 2010, 256, 3191–3196. [Google Scholar] [CrossRef]
- Crawford, R.J.; Ivanova, E.P. Natural Superhydrophobic Surfaces; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 7–25. [Google Scholar]
- Ünal, E.; Yaşar, A.; Karahan, I.H. A review of electrodeposited composite coatings with Ni-B alloy matrix. Mater. Res. Express 2019, 6, 092004. [Google Scholar] [CrossRef]
- Hossain, M.K.; Rubel, M.H.K.; Akbar, M.A.; Ahmed, M.H.; Haque, N.; Rahman, M.F.; Hossain, J.; Hossain, K.M. A review on recent applications and future prospects of rare earth oxides in corrosion and thermal barrier coatings, catalysts, tribological, and environmental sectors. Ceram. Int. 2022, 48, 32588–32612. [Google Scholar] [CrossRef]
- Rahman, M.T.; Hoque, M.A.; Rahman, G.T.; Gafur, M.A.; Khan, R.A.; Hossain, M.K. Evaluation of thermal, mechanical, electrical and optical properties of metal-oxide dispersed HDPE nanocomposites. Mater. Res. Express 2019, 6, 085092. [Google Scholar] [CrossRef]
- Hoque, M.A.; Ahmed, M.R.; Rahman, G.T.; Rahman, M.T.; Islam, M.A.; Khan, M.A.; Hossain, M.K. Fabrication and comparative study of magnetic Fe and α-Fe2O3 nanoparticles dispersed hybrid polymer (PVA + Chitosan) novel nanocomposite film. Results Phys. 2018, 10, 434–443. [Google Scholar] [CrossRef]
- Rahman, M.T.; Hoque, M.A.; Rahman, G.T.; Gafur, M.A.; Khan, R.A.; Hossain, M.K. Study on the mechanical, electrical and optical properties of metal-oxide nanoparticles dispersed unsaturated polyester resin nanocomposites. Results Phys. 2019, 13, 102264. [Google Scholar] [CrossRef]
- Biswas, M.C.; Chowdhury, A.; Hossain, M.M.; Hossain, M.K. Applications, drawbacks, and future scope of nanoparticle-based polymer composites. In Nanoparticle-Based Polymer Composites, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2022; pp. 243–275. [Google Scholar] [CrossRef]
- Khan, S.; Hossain, M.K. Classification and properties of nanoparticles. In Nanoparticle-Based Polymer Composites, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2022; pp. 15–54. [Google Scholar] [CrossRef]
- Rahman, M.T.; Hoque, M.A.; Rahman, G.T.; Azmi, M.M.; Gafur, M.A.; Khan, R.A.; Hossain, M.K. Fe2O3 nanoparticles dispersed unsaturated polyester resin based nanocomposites: Effect of gamma radiation on mechanical properties. Radiat. Eff. Defects Solids 2019, 174, 480–493. [Google Scholar] [CrossRef]
- Gao, P.Y.; Ma, Y.D.; Sun, W.W.; Yang, Y.; Zhang, C.; Cui, Y.H.; Wang, Y.W.; Dong, Y.C. Microstructure and properties of Al2O3-ZrO2-TiO2 composite coatings prepared by plasma spraying. Rare Met. 2020, 40, 1825–1834. [Google Scholar] [CrossRef]
- Gao, Q.S.; Yan, H.; Qin, Y.; Zhang, P.L.; Guo, J.L.; Chen, Z.F.; Yu, Z.S. Laser cladding Ti-Ni/TiN/TiW+TiS/WS2 self-lubricating wear resistant composite coating on Ti-6Al-4V alloy. Opt. Laser Technol. 2019, 113, 182–191. [Google Scholar] [CrossRef]
- Farayibi, P.K.; Folkes, J.; Clare, A.; Oyelola, O. Cladding of pre-blended Ti-6Al-4V and WC powder for wear resistant applications. Surf. Coat. Technol. 2011, 206, 372–377. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Zhang, D.; Cai, L.; Ren, P.; Hu, J.; Liao, D. Corrosion resistance of MoS2-modified titanium alloy micro-arc oxidation coating. Surf. Coat. Technol. 2022, 433, 128127. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W. Synthesis of Ni-Co-ZrO2 nanocomposites doped with ceria particles via electrodeposition as highly protective coating. J. Alloys Compd. 2020, 820, 153158. [Google Scholar] [CrossRef]
- Jin, P.; Sun, C.F.; Zhang, Z.H.; Zhou, C.Y.; Williams, T. Fabrication of the Ni-W-SiC thin film by pulse electrodeposition. Surf. Coat. Technol. 2020, 392, 125738. [Google Scholar] [CrossRef]
- Xu, Y.K.; Fan, M.Y.; Luo, Y.Q.; Chen, Y.N.; Hao, J.M.; Hou, X.H. Tribology and corrosion properties investigation of a pulse electrodeposition duplex hard-particle-reinforced Ni-Mo nanocomposite coating. Surf. Coat. Technol. 2020, 393, 125797. [Google Scholar] [CrossRef]
- Zhou, J.X.; Zhao, G.C.; Li, J.S.; Chen, J.; Zhang, S.Q.; Wang, J.; Walsh, F.C.; Wang, S.C.; Xue, Y.P. Electroplating of non-fluorinated superhydrophobic Ni/WC/WS2 composite coatings with high abrasive resistance. Appl. Surf. Sci. 2019, 487, 1329–1340. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Serebrennikova, I.; Birss, V.I. Structural and compositional properties of sol-gel formed Ni, Co and Ni-Co oxide films. J. Mater. Sci. 2001, 36, 4331–4343. [Google Scholar] [CrossRef]
- Sobolev, A.; Musin, A.; Whyman, G.; Borodianskiy, K.; Krichevski, O.; Kalashnikov, A.; Zinigrad, M. Stabilization of cubic phase in scandium-doped zirconia nanocrystals synthesized with sol-gel method. J. Am. Ceram. Soc. 2019, 102, 3236–3243. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Gofer, Y.; Borodianskiy, K.; Sobolev, A. Hydroxyapatite Coating on Ti-6Al-7Nb Alloy by Plasma Electrolytic Oxidation in Salt-Based Electrolyte. Materials 2022, 15, 7374. [Google Scholar] [CrossRef]
- Sahu, B.P.; Ray, M.; Mitra, R. Structure and properties of Ni1-xTixN thin films processed by reactive magnetron co-sputtering. Mater. Charact. 2020, 169, 110604. [Google Scholar] [CrossRef]
- He, J.L.; Yu, C.H.; Leyland, A.; Wilson, A.D.; Matthews, A. A comparative study of the cyclic thermal oxidation of PVD nickel aluminide coatings. Surf. Coat. Technol. 2002, 155, 67–79. [Google Scholar] [CrossRef]
- Dong, J.K.; Truong, Q.T.; Kim, J.I.; Suh, Y.; Moon, J.; Lee, S.-E.; Hong, B.H.; Woo, Y.S. Ultrahigh-strength multi-layer graphene-coated Ni film with interface-induced hardening. Carbon 2021, 178, 497–505. [Google Scholar]
- Borkar, T.; Harimkar, S.P. Effect of electrodeposition conditions and reinforcement content on microstructure and tribological properties of nickel composite coatings. Surf. Coat. Technol. 2011, 205, 4124–4134. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; Xu, Z.; Cai, F.; Zhang, Z.; Fu, P. Microstructure and corrosion behavior of Ti nanoparticles reinforced Ni–Ti composite coatings by electrodeposition. Mater. Des. 2015, 85, 39–46. [Google Scholar] [CrossRef]
- Roy, D.; Das, A.K.; Saini, R.; Singh, P.K.; Kumar, P.; Hussain, M.; Mandal, A.; Dixit, A.R. Pulse current co-deposition of Ni-WS2 nano-composite film for solid lubrication. Mater. Manuf. Process. 2017, 32, 365–372. [Google Scholar] [CrossRef]
- He, Y.; Sun, W.T.; Wang, S.C.; Reed, P.A.S.; Walsh, F.C. An electrodeposited Ni-P-WS2 coating with combined super-hydrophobicity and self-lubricating properties. Electrochim. Acta 2017, 245, 872–882. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K. Electroless Ni-W-P Coating and its nano-WS2 composite: Preparation and properties. Ind. Eng. Chem. Res. 2012, 51, 7932–7940. [Google Scholar] [CrossRef]
- Ghaziof, S.; Gao, W. The effect of pulse electroplating on Zn-Ni alloy and Zn-Ni-Al2O3 composite coatings. J. Alloys Compd. 2015, 622, 918–924. [Google Scholar] [CrossRef]
- Sheu, H.H.; Huang, P.C.; Tsai, L.C.; Hou, K.H. Effects of plating parameters on the Ni-P-Al2O3 composite coatings prepared by pulse and direct current plating. Surf. Coat. Technol. 2013, 235, 529–535. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Guo, Z.N.; Deng, Y.; Dong, H.S.; Li, X.Y.; Liu, J.W. Effect of pulse frequency on the one-step preparation of superhydrophobic surface by pulse electrodeposition. Appl. Surf. Sci. 2018, 458, 603–611. [Google Scholar] [CrossRef]
- Nemes, P.I.; Lekka, M.; Fedrizzi, L.; Muresan, L.M. Influence of the electrodeposition current regime on the corrosion resistance of Zn–CeO2 nanocomposite coatings. Surf. Coat. Technol. 2014, 252, 102–107. [Google Scholar] [CrossRef]
- Ganji, M.; Yousefnia, H.; Seyedraoufi, Z.S.; Shajari, Y. The corrosion behavior of Ni–Fe and Ni–Fe–TiC nanoparticles deposited using pulse electrodeposition on low-carbon steel. J. Aust. Ceram. Soc. 2022, 58, 1283–1295. [Google Scholar] [CrossRef]
- Xia, F.F.; Li, Q.; Ma, C.Y.; Zhao, D.; Ma, Z. Design and Properties of Ni-TiN/SiC Nano-coatings Prepared by Pulse Current Electrodeposition. Int. J. Electrochem. Sci. 2020, 15, 1813–1829. [Google Scholar] [CrossRef]
- Xu, S.; Hu, M.; Sun, J.; Weng, L.; Liu, W.; Gao, X. A simple strategy to tailor the microstructure and wear-resistance of sputtered WS2 films. Mater. Lett. 2018, 216, 179–181. [Google Scholar] [CrossRef]
- Sara, R.; Cheng, Z.; Sandip, H.; Boesl, B.; Agarwal, A. Effect of WS2 Addition on Tribological Behavior of Aluminum at Room and Elevated Temperatures. Tribol. Lett. 2017, 65, 76. [Google Scholar]
- Zhang, R.; Qiao, D.; Liu, X.; Guo, Z.; Cai, M.; Shi, L. A Facile and Effective Method to Improve the Dispersibility of WS2 Nanosheets in PAO8 for the Tribological Performances. Tribol. Int. 2018, 118, 60–70. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Song, R. Investigation of WS2-Embedded Al2O3 Coatings Prepared by Microarc Oxidation. J. Mater. Eng. Perform. 2020, 29, 1060–1067. [Google Scholar] [CrossRef]
- Pisoni, A.; Jacimovic, J.; Gaal, R.; Nafradi, B.; Berger, H.; Revay, Z.; Forro, L. Anisotropic transport properties of tungsten disulfide. Scr. Mater. 2016, 114, 48–50. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behavior of aluminum alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Guglielmi, N. Kinetics of the Deposition of Inert Particles from Electrolytic Baths. J. Electrochem. Soc. 1972, 119, 1009–1021. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Dolati, A. The kinetics of Ni–Co/SiC composite coatings electrodeposition. J. Alloys Compd. 2010, 504, 514–518. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Danilov, F.I. Kinetic model of composite coatings electrodeposition assuming irreversible adsorption of dispersed particles on a growing metal substrate. J. Electroanal. Chem. 2022, 918, 116463. [Google Scholar] [CrossRef]
- Prabhu, G.; Swaminatha, P.K.; Branko, N.P. Nanostructured Zn-Ni Alloys by Pulse Electrodeposition. In Proceedings of the AESF SUR/FIN Conference 2004 and Interfinish 2004 World Congress, Chicago, IL, USA, 28 June–1 July 2004. [Google Scholar]
- Harald, N.; Rold, H. Tailor-Made Nanomaterials Designed by Electrochemical Methods. Electrochim. Acta 2003, 49, 51–61. [Google Scholar]


| Composition | Concentration (g/L) | Function |
|---|---|---|
| WS2 | 0, 1, 5, 10, 15 | nanoparticles |
| NiSO4·6H2O | 200 | main salt ions |
| CoSO4·7H2O | 25 | main salt ions |
| NiCl2·7H2O | 40 | increase conductivity |
| H3BO3 | 40 | buffer pH |
| CTAB | 0.1 | dispersant of nanoparticles, reduce agglomeration |
| DC and PC Parameter | Value |
|---|---|
| pH | 3 ± 0.1 |
| Temperature (°C) | 40 |
| stirring speed | 300 r/min |
| Current density (A/dm2) | 1, 2, 3, 4, 5 |
| Electrodeposition time (min) | 20, 30, 40, 50, 60, 70 |
| PC Specific Parameter | Value |
| Duty ratio | 30%, 50%, 70%, 90% |
| Pulse frequency (Hz) | 50, 100, 200, 500, 1000, 1250 |
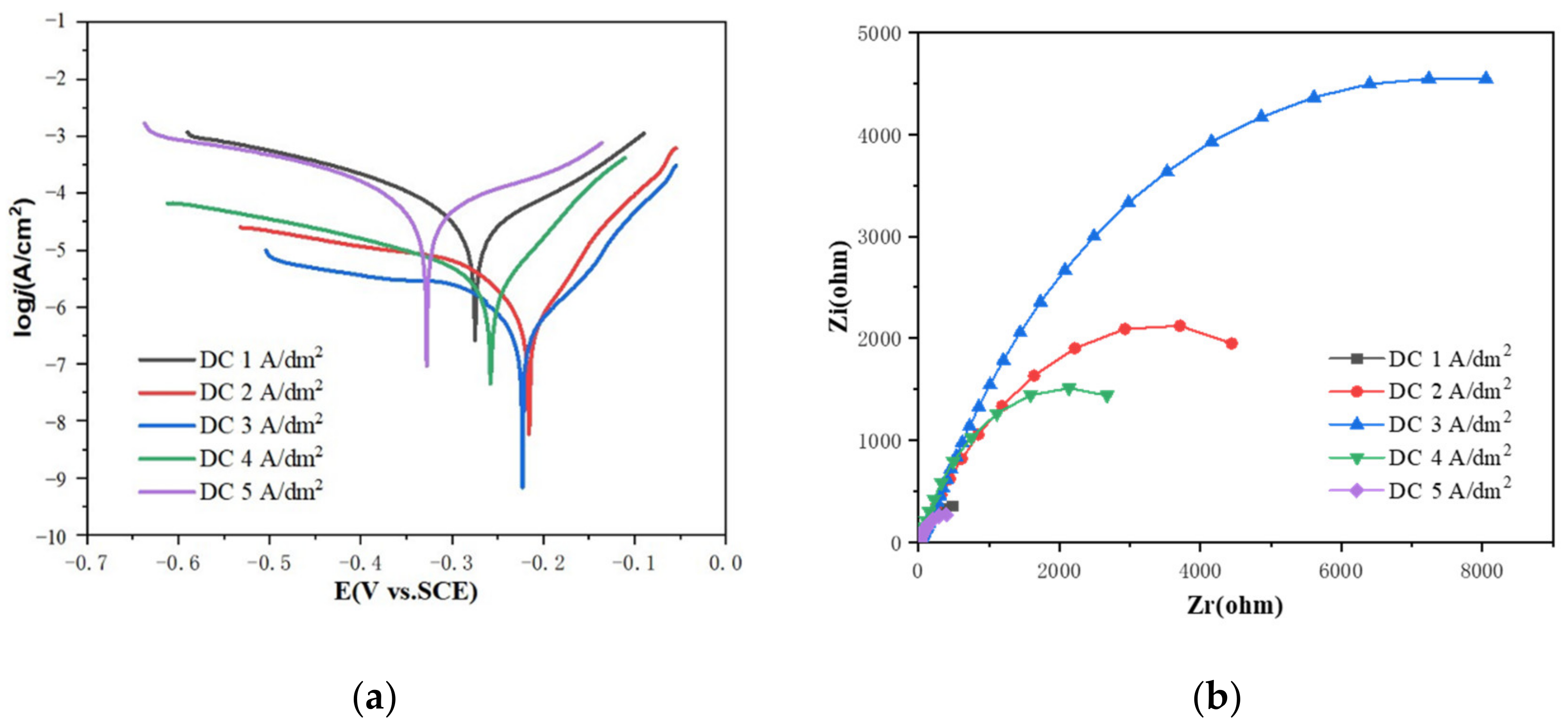
| Sample | Ecorr (V) | icorr (A/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|
| DC-j-1 | −0.275 | 5.29 × 10−5 | 0.618 |
| DC-j-2 | −0.216 | 1.06 × 10−6 | 1.24 × 10−2 |
| DC-j-3 | −0.222 | 6.17 × 10−7 | 7.22 × 10−3 |
| DC-j-4 | −0.258 | 3.76 × 10−6 | 4.40 × 10−2 |
| DC-j-5 | −0.328 | 1.49 × 10−4 | 1.74 |
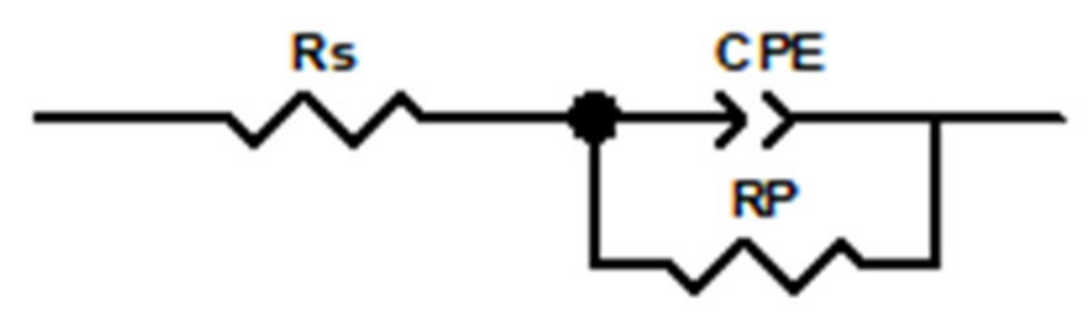
| Sample | Rs (Ω·cm2) | CPE Ω−1·sn·cm−2 | Rp (Ω·cm2) |
|---|---|---|---|
| DC-j-1 | 9.037 | 1.30 × 10−3 | 1810 |
| DC-j-2 | 8.798 | 1.53 × 10−4 | 8253 |
| DC-j-3 | 6.177 | 7.04 × 10−5 | 16,929 |
| DC-j-4 | 15.4 | 2.64 × 10−4 | 4939 |
| DC-j-5 | 7.347 | 2.16 × 10−3 | 1029 |
| Wt.% | Ni | Co | W | S | |
|---|---|---|---|---|---|
| Sample Number | |||||
| PC-j-3 | 42.65 | 15.14 | 31.27 | 10.94 | |
| DC-j-3 | 39.14 | 27.16 | 24.87 | 8.83 | |
| Sample | Ecorr (V) | icorr (A/cm2) | Corrosion Rate (mm/a) |
|---|---|---|---|
| DC-j-3 | −0.222 | 6.17 × 10−7 | 7.22 × 10−3 |
| PC-j-3 | −0.195 | 1.32 × 10−7 | 1.54 × 10−3 |
| Sample | Rs (Ω·cm2) | CPE Ω−1·sn·cm−2 | Rp (Ω·cm2) |
|---|---|---|---|
| DC-j-3 | 6.177 | 7.04 × 10−5 | 16,929 |
| PC-j-3 | 10.17 | 5.86 × 10−5 | 23,617 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Xue, Z.; Yan, S.; He, J.; Shao, Q.; Ge, W.; Lu, B. Pulse Electrodeposited Super-Hydrophobic Ni-Co/WS2 Nanocomposite Coatings with Enhanced Corrosion-Resistance. Coatings 2022, 12, 1897. https://doi.org/10.3390/coatings12121897
Wang M, Xue Z, Yan S, He J, Shao Q, Ge W, Lu B. Pulse Electrodeposited Super-Hydrophobic Ni-Co/WS2 Nanocomposite Coatings with Enhanced Corrosion-Resistance. Coatings. 2022; 12(12):1897. https://doi.org/10.3390/coatings12121897
Chicago/Turabian StyleWang, Meijiao, Zixiao Xue, Shaojiu Yan, Jin He, Qiuyue Shao, Wen Ge, and Baodi Lu. 2022. "Pulse Electrodeposited Super-Hydrophobic Ni-Co/WS2 Nanocomposite Coatings with Enhanced Corrosion-Resistance" Coatings 12, no. 12: 1897. https://doi.org/10.3390/coatings12121897
APA StyleWang, M., Xue, Z., Yan, S., He, J., Shao, Q., Ge, W., & Lu, B. (2022). Pulse Electrodeposited Super-Hydrophobic Ni-Co/WS2 Nanocomposite Coatings with Enhanced Corrosion-Resistance. Coatings, 12(12), 1897. https://doi.org/10.3390/coatings12121897




