Sedum Plumbizincicola Derived Functional Carbon for Activation of Peroxymonosulfate to Eliminate Bisphenol A: Performance and Reaction Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterization of FC
2.2.2. Adsorption
2.2.3. Catalytic Degradation of BPA
2.2.4. Analytical Methods
3. Results and Discussion
3.1. Characterizations
3.2. Adsorption
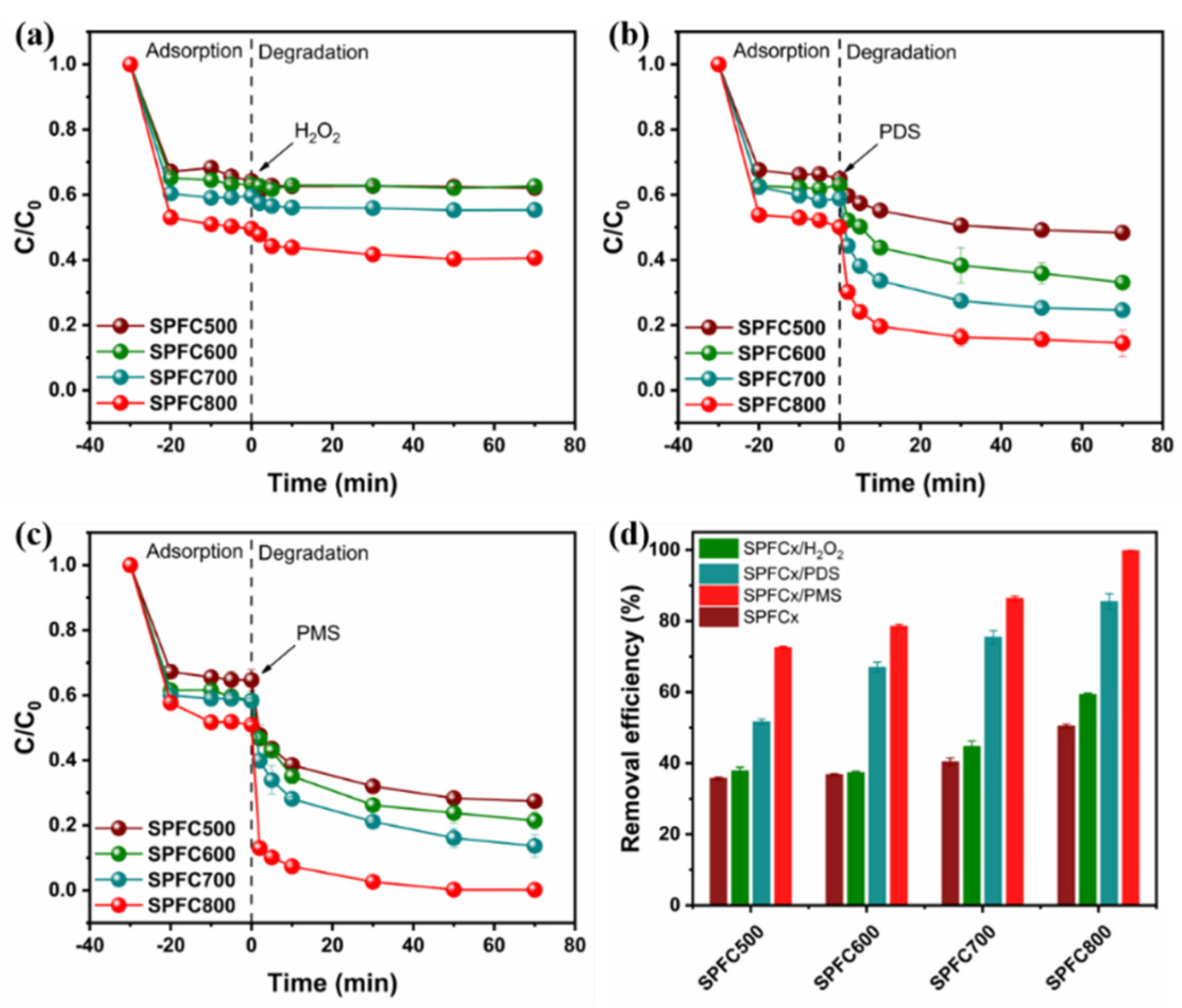
3.3. Catalytic Degradation Performance
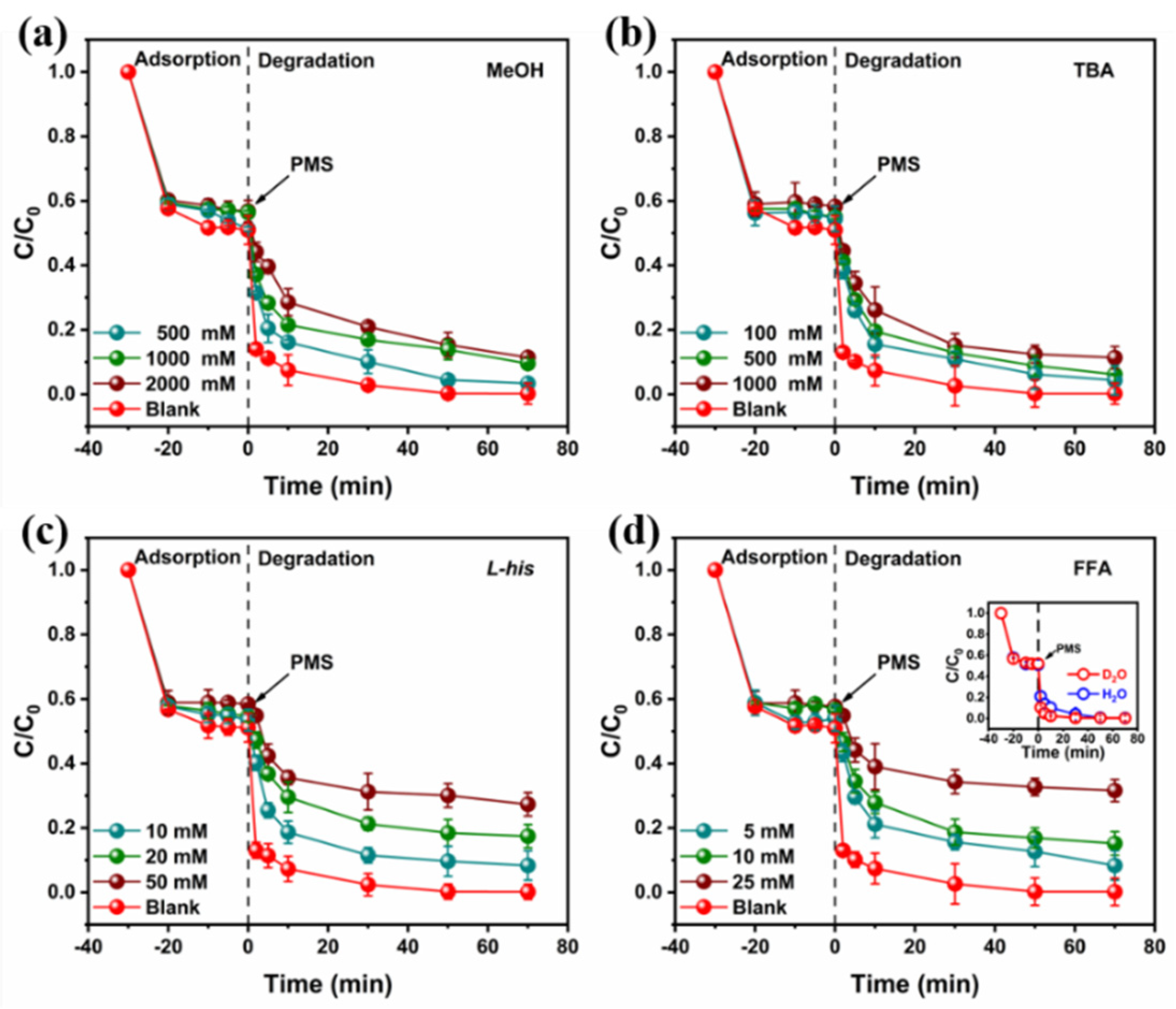
3.4. Catalytic Mechanism
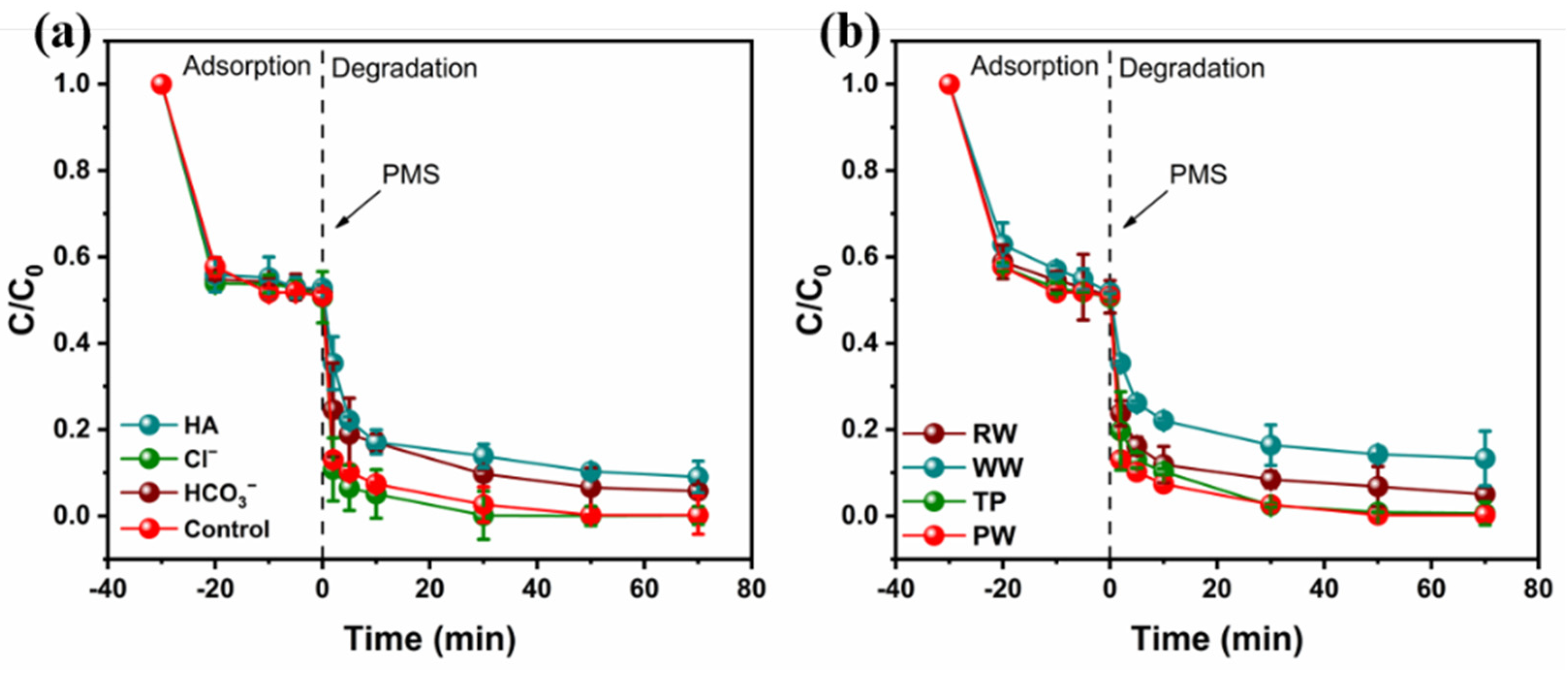
3.5. Degradation in Real Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef]
- Yin, Z.; Yu, J.; Han, X.; Wang, H.; Yang, Q.; Pan, H.; Lou, Y.; Zhuge, Y. A novel phytoremediation technology for polluted cadmium soil: Salix integra treated with spermidine and activated carbon. Chemosphere 2022, 306, 135582. [Google Scholar] [CrossRef]
- Sarma, H.; Islam, N.F.; Prasad, R.; Prasad, M.N.V.; Ma, L.Q.; Rinklebe, J. Enhancing phytoremediation of hazardous metal(loid)s using genome engineering CRISPR-Cas9 technology. J. Hazard. Mater. 2021, 414, 125493. [Google Scholar] [CrossRef]
- Fadhile Almansoory, A.; Abu Hasan, H.; Idris, M.; Sheikh Abdullah, S.R.; Anuar, N. Potential application of a biosurfactant in phytoremediation technology for treatment of gasoline-contaminated soil. Ecol. Eng. 2015, 84, 113–120. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.; Yao, Z.; Lin, Q.; Yan, B.; Cui, X.; He, Z.; Yang, X.; Wang, C.H.; Chen, G. Phytoremediation of Cd-contaminated farmland soil via various Sedum alfredii-oilseed rape cropping systems: Efficiency comparison and cost-benefit analysis. J. Hazard. Mater. 2021, 419, 126489. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, J.; Wang, X.; Pan, M.; Lin, Q.; Khan, K.Y.; Yan, B.; Li, T.; He, Z.; Yang, X.; et al. A review on the thermal treatment of heavy metal hyperaccumulator: Fates of heavy metals and generation of products. J. Hazard. Mater. 2021, 405, 123832. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Luo, J.; Pan, M.; Du, Y.; Liang, Y.; Li, T. Integrated glycolysis and pyrolysis process for multiple utilization and cadmium collection of hyperaccumulator Sedum alfredii. J. Hazard. Mater. 2022, 422, 126859. [Google Scholar] [CrossRef]
- Huo, X.; Zhou, P.; Zhang, J.; Liu, Y.; Cheng, X.; Liu, Y.; Li, W.; Zhang, Y. N, S-Doped porous carbons for persulfate activation to remove tetracycline: Nonradical mechanism. J. Hazard. Mater. 2020, 391, 122055. [Google Scholar] [CrossRef]
- Pei, X.; Peng, X.; Jia, X.; Wong, P.K. N-doped biochar from sewage sludge for catalytic peroxydisulfate activation toward sulfadiazine: Efficiency, mechanism, and stability. J. Hazard. Mater. 2021, 419, 126446. [Google Scholar] [CrossRef]
- Xiao, P.; Yi, X.; Wu, M.; Wang, X.; Zhu, S.; Gao, B.; Liu, Y.; Zhou, H. Catalytic performance and periodate activation mechanism of anaerobic sewage sludge-derived biochar. J. Hazard. Mater. 2021, 424, 127692. [Google Scholar] [CrossRef]
- Yu, J.; Feng, H.; Tang, L.; Pang, Y.; Zeng, G.; Lu, Y.; Dong, H.; Wang, J.; Liu, Y.; Feng, C.; et al. Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Prog. Mater. Sci. 2020, 111, 100654. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.; Yang, L. Engineered biochar reclaiming phosphate from aqueous solutions: Mechanisms and potential application as a slow-release fertilizer. Environ. Sci. Technol. 2013, 47, 8700–8708. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Luo, Z.; Du, S.; Yang, J.; Zhi, D.; Zhou, Y. Magnetic MgFe2O4/biochar derived from pomelo peel as a persulfate activator for levofloxacin degradation: Effects and mechanistic consideration. Bioresour. Technol. 2022, 346, 126547. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Yang, X.; Jia, X.; Cai, M.; Bao, Y. Preparation of Si-Mn/biochar composite and discussions about characterizations, advances in application and adsorption mechanisms. Chemosphere 2021, 281, 130946. [Google Scholar] [CrossRef]
- Long, Y.; Dai, J.; Zhao, S.; Huang, S.; Zhang, Z. Metal-organic framework-derived magnetic carbon for efficient decontamination of organic pollutants via periodate activation: Surface atomic structure and mechanistic considerations. J. Hazard. Mater. 2021, 424, 126786. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, X.; Xiang, W.; Zhang, J.; Cao, C.; Wang, H.; Hu, X.; Gao, B. Ball milling biochar with ammonia hydroxide or hydrogen peroxide enhances its adsorption of phenyl volatile organic compounds (VOCs). J. Hazard. Mater. 2021, 403, 123540. [Google Scholar] [CrossRef]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass derived N-doped biochar as efficient catalyst supports for CO2 methanation. J. CO2 Util. 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Feng, D.; Lü, J.; Guo, S.; Li, J. Biochar enhanced the degradation of organic pollutants through a Fenton process using trace aqueous iron. J. Environ. Chem. Eng. 2021, 9, 104677. [Google Scholar] [CrossRef]
- Anfar, Z.; Ait El Fakir, A.; Ait Ahsaine, H.; Zbair, M.; Farsad, S.; Morlet-Savary, F.; Jada, A.; El Alem, N. Nitrogen doped graphitic porous carbon from almond shells as an efficient persulfate activator for organic compound degradation. New J. Chem. 2020, 44, 9391–9401. [Google Scholar] [CrossRef]
- Dou, J.; Cheng, J.; Lu, Z.; Tian, Z.; Xu, J.; He, Y. Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process. Appl. Catal. B Environ. 2022, 301, 120832. [Google Scholar] [CrossRef]
- He, L.; Yang, C.; Ding, J.; Lu, M.-Y.; Chen, C.-X.; Wang, G.-Y.; Jiang, J.-Q.; Ding, L.; Liu, G.-S.; Ren, N.-Q.; et al. Fe, N-doped carbonaceous catalyst activating periodate for micropollutant removal: Significant role of electron transfer. Appl. Catal. B Environ. 2022, 303, 120880. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Si, Q.; Luo, H.; Zhao, Q.; Ren, N. Sludge-derived biochar as efficient persulfate activators: Sulfurization-induced electronic structure modulation and disparate nonradical mechanisms. Appl. Catal. B Environ. 2020, 279, 119361. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Z.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H.a. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for nitrobenzene hydrogenation. Appl. Catal. B Environ. 2020, 260, 118105. [Google Scholar] [CrossRef]
- Feng, Z.; Zhou, B.; Yuan, R.; Li, H.; He, P.; Wang, F.; Chen, Z.; Chen, H. Biochar derived from different crop straws as persulfate activator for the degradation of sulfadiazine: Influence of biomass types and systemic cause analysis. Chem. Eng. J. 2022, 440, 135669. [Google Scholar] [CrossRef]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.P.; Liu, Y.L.; Tian, S.Q.; Yang, J.J.; Wang, L.; Ma, J. Straw biochar enhanced removal of heavy metal by ferrate. J. Hazard. Mater. 2021, 416, 126128. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L. Preparation of straw biochar and application of constructed wetland in China: A review. J. Clean. Prod. 2020, 273, 123131. [Google Scholar] [CrossRef]
- Duan, R.; Ma, S.; Xu, S.; Wang, B.; He, M.; Li, G.; Fu, H.; Zhao, P. Soybean straw biochar activating peroxydisulfate to simultaneously eliminate tetracycline and tetracycline resistance bacteria: Insights on the mechanism. Water Res 2022, 218, 118489. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Geng, W.; Alvarez, P.J.J.; Long, M. 2D N-Doped Porous Carbon Derived from Polydopamine-Coated Graphitic Carbon Nitride for Efficient Nonradical Activation of Peroxymonosulfate. Environ. Sci. Technol. 2020, 54, 8473–8481. [Google Scholar] [CrossRef]
- Minakata, D.; Kamath, D.; Maetzold, S. Mechanistic Insight into the Reactivity of Chlorine-Derived Radicals in the Aqueous-Phase UV-Chlorine Advanced Oxidation Process: Quantum Mechanical Calculations. Environ. Sci. Technol. 2017, 51, 6918–6926. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhou, P.; Zhou, H.; Liu, W.; Zhang, H.; Zhou, C.; Lai, L.; Ao, Z.; Su, S.; Lai, B. Insights into the Electron-Transfer Mechanism of Permanganate Activation by Graphite for Enhanced Oxidation of Sulfamethoxazole. Environ. Sci. Technol. 2021, 55, 9189–9198. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.L.; Kim, K.H.; Kavitha, B.; Kumar, V.; Raza, N.; Kalagara, S. Photocatalytic degradation of bisphenol A in aqueous media: A review. J. Environ. Manag. 2018, 213, 189–205. [Google Scholar] [CrossRef]
- Moradi, F.G.M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Lu, C.S.; Tsai, H.Y.; Shaya, J.; Golovko, V.B.; Wang, S.Y.; Liu, W.J.; Chen, C.C. Degradation of sulfamethoxazole in water by AgNbO3 photocatalyst mediated by persulfate. RSC Adv. 2022, 12, 29709–29718. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, L.; Zhu, J.; Sun, Q.; Qu, W.; Yu, Y.; Zhao, Z.; Yu, Y.; Shao, G. Environmental contaminant BPA causes intestinal damage by disrupting cellular repair and injury homeostasis in vivo and in vitro. Biomed. Pharm. 2021, 137, 111270. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, Q.; Zhang, C.; Wang, R.; Deng, R.; Luo, H.; Li, T.; Li, J.; Chen, S.; Liu, C. Mn doped magnetic biochar as persulfate activator for the degradation of tetracycline. Chem. Eng. J. 2020, 391, 123532. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 396, 125119. [Google Scholar] [CrossRef]
- Qu, S.; Yuan, Y.; Yang, X.; Xu, H.; Mohamed, A.K.; Zhang, J.; Zhao, C.; Liu, L.; Wang, B.; Wang, X.; et al. Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation. Chem. Eng. J. 2022, 441, 135864. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Chen, D.; Ren, W.; Lin, H.; Zhang, H. Wood-based biochar as an excellent activator of peroxydisulfate for Acid Orange 7 decolorization. Chemosphere 2019, 231, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gao, X.; Pan, B. Nanoconfinement-Mediated Water Treatment: From Fundamental to Application. Environ. Sci. Technol. 2020, 54, 8509–8526. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Liu, E.; Ding, R.; Liu, K.; Liu, R.; Wang, L.; Yang, Z.; Jiang, H. Bean dregs-based activated carbon/copper ion supercapacitors. Electrochim. Acta 2016, 194, 394–404. [Google Scholar] [CrossRef]
- Chen, Y.-d.; Duan, X.; Zhang, C.; Wang, S.; Ren, N.-Q.; Ho, S.-H. Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem. Eng. J. 2020, 384, 123244. [Google Scholar] [CrossRef]




| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| SPFC500 | 36.09 | 0.0277 | 3.997 |
| SPFC600 | 59.88 | 0.0592 | 3.764 |
| SPFC700 | 78.29 | 0.0631 | 3.799 |
| SPFC800 | 121.57 | 0.0847 | 3.651 |
| Biochar | Pseudo First-Order | Pseudo Second-Order | ||||
|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | |
| SPFC500 | 17.771 (0.408) | 0.862 | 0.951 | 18.308 (0.302) | 0.0847 | 0.982 |
| SPFC600 | 18.084 (0.411) | 0.876 | 0.951 | 18.619 (0.307) | 0.0855 | 0.982 |
| SPFC700 | 20.399 (0.509) | 0.674 | 0.949 | 21.158 (0.323) | 0.0521 | 0.985 |
| SPFC800 | 25.184 (0.624) | 0.724 | 0.944 | 26.081 (0.418) | 0.0461 | 0.983 |
| Water Sample | pH | CODCr (mg L−1) | Cl− (mg L−1) | NH4+-N (mg L−1) | PO43− (mg L−1) |
|---|---|---|---|---|---|
| PW | 7.2 ± 0.03 | <10 | 0.47 | 0.09 | 0.02 |
| TW | 7.13 ± 0.03 | <10 | 12.6 | 1.37 | 0.138 |
| RW | 7.08 ± 0.03 | <10 | 8.52 | 2.14 | 0.577 |
| WW | 7.44 ± 0.03 | 572 ± 20 | 1388 | 4.11 | 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, Z.; Kang, R.; Niu, Y.; Su, W.; Wang, X.; Tian, D.; Xu, Y. Sedum Plumbizincicola Derived Functional Carbon for Activation of Peroxymonosulfate to Eliminate Bisphenol A: Performance and Reaction Mechanisms. Coatings 2022, 12, 1892. https://doi.org/10.3390/coatings12121892
Liu C, Chen Z, Kang R, Niu Y, Su W, Wang X, Tian D, Xu Y. Sedum Plumbizincicola Derived Functional Carbon for Activation of Peroxymonosulfate to Eliminate Bisphenol A: Performance and Reaction Mechanisms. Coatings. 2022; 12(12):1892. https://doi.org/10.3390/coatings12121892
Chicago/Turabian StyleLiu, Chao, Zhenxiang Chen, Ruiqin Kang, Yongsheng Niu, Wenhui Su, Xiaolong Wang, Dayong Tian, and Ying Xu. 2022. "Sedum Plumbizincicola Derived Functional Carbon for Activation of Peroxymonosulfate to Eliminate Bisphenol A: Performance and Reaction Mechanisms" Coatings 12, no. 12: 1892. https://doi.org/10.3390/coatings12121892







