Effect of Doping Content of MgO on Solar Absorptivity to IR Emissivity Ratio of Al2O3 Coatings
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Ceramic Preparation Method
2.2. Coating Preparation Method
2.3. Measurement of the Properties
3. Result and Discussion
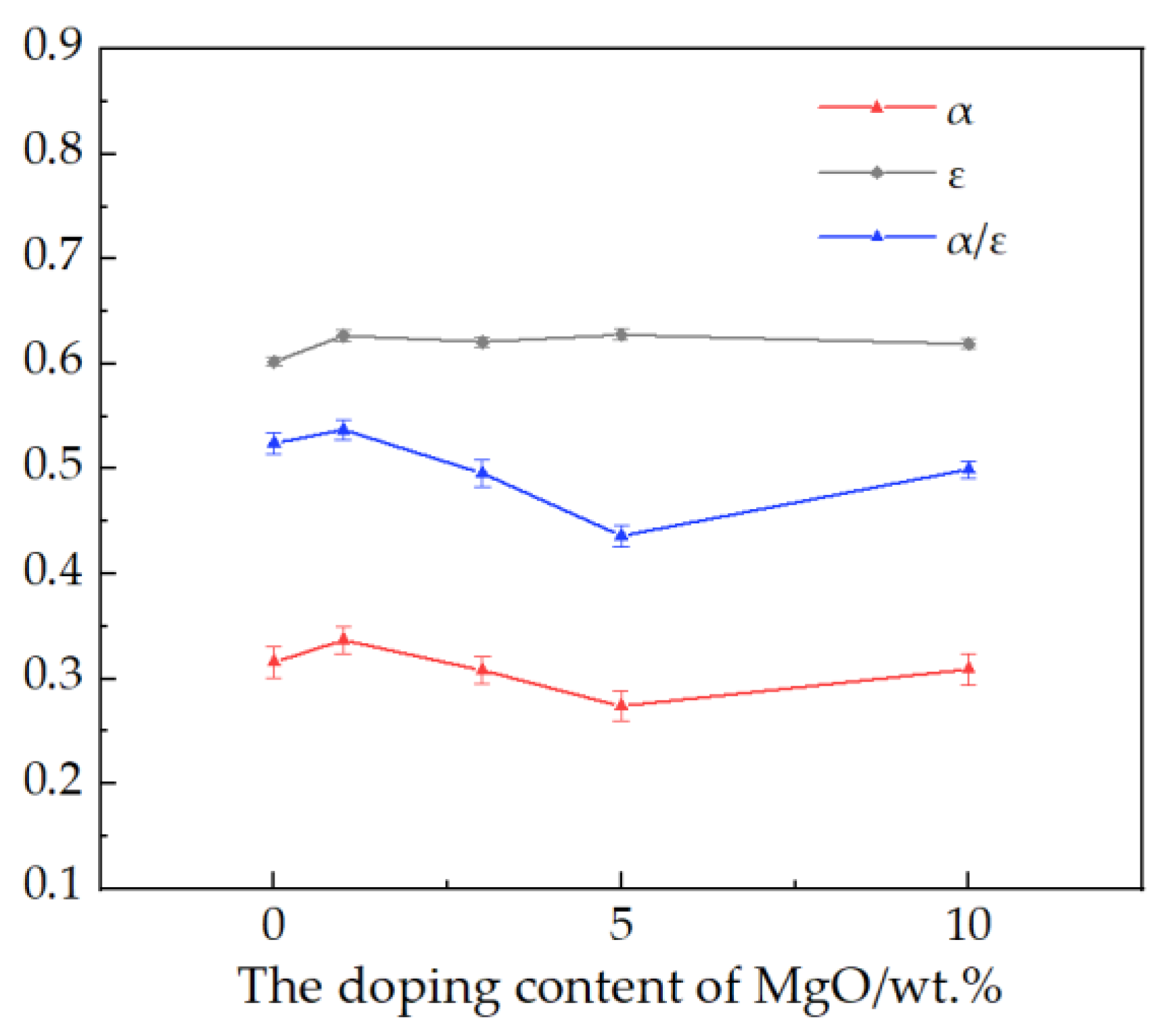
3.1. Effect of Doping Content of MgO on α/ε of Ceramic Pellet
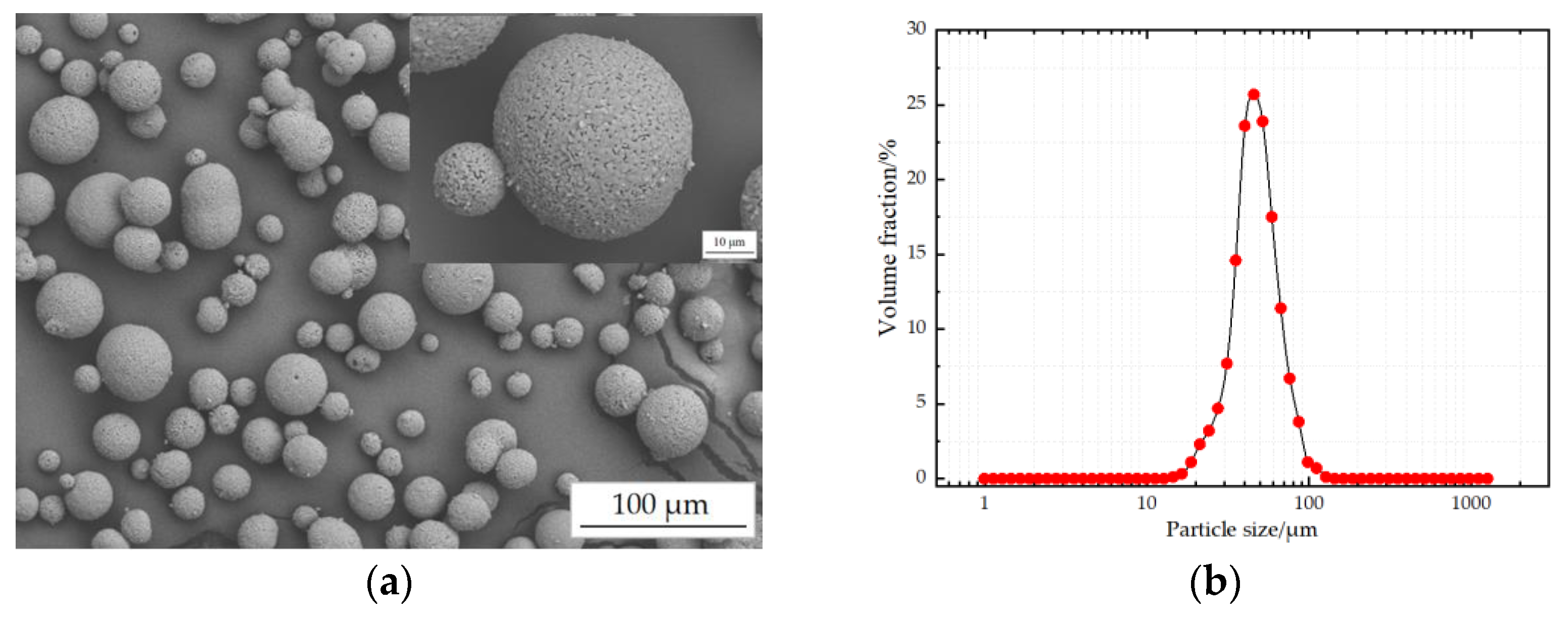
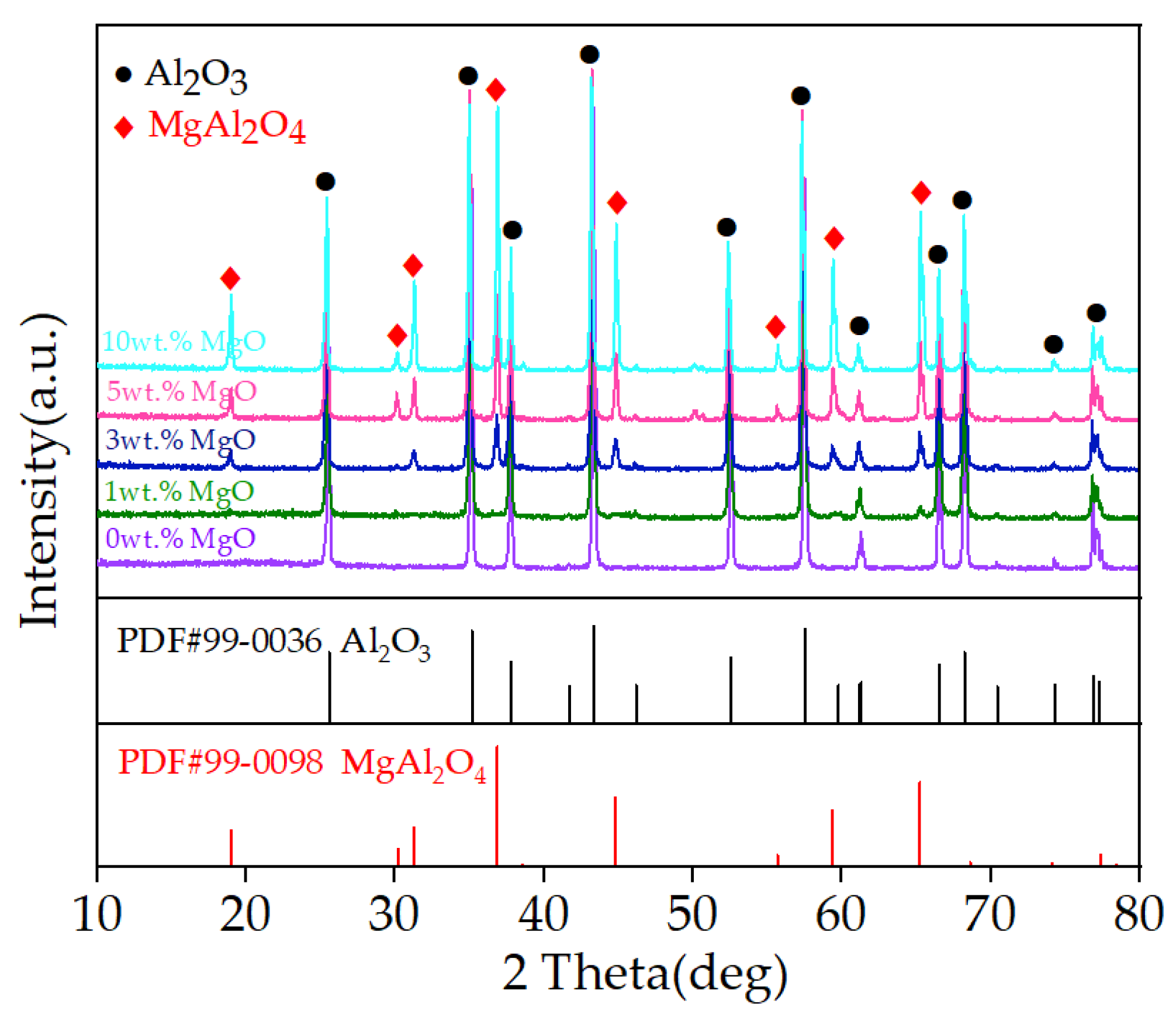
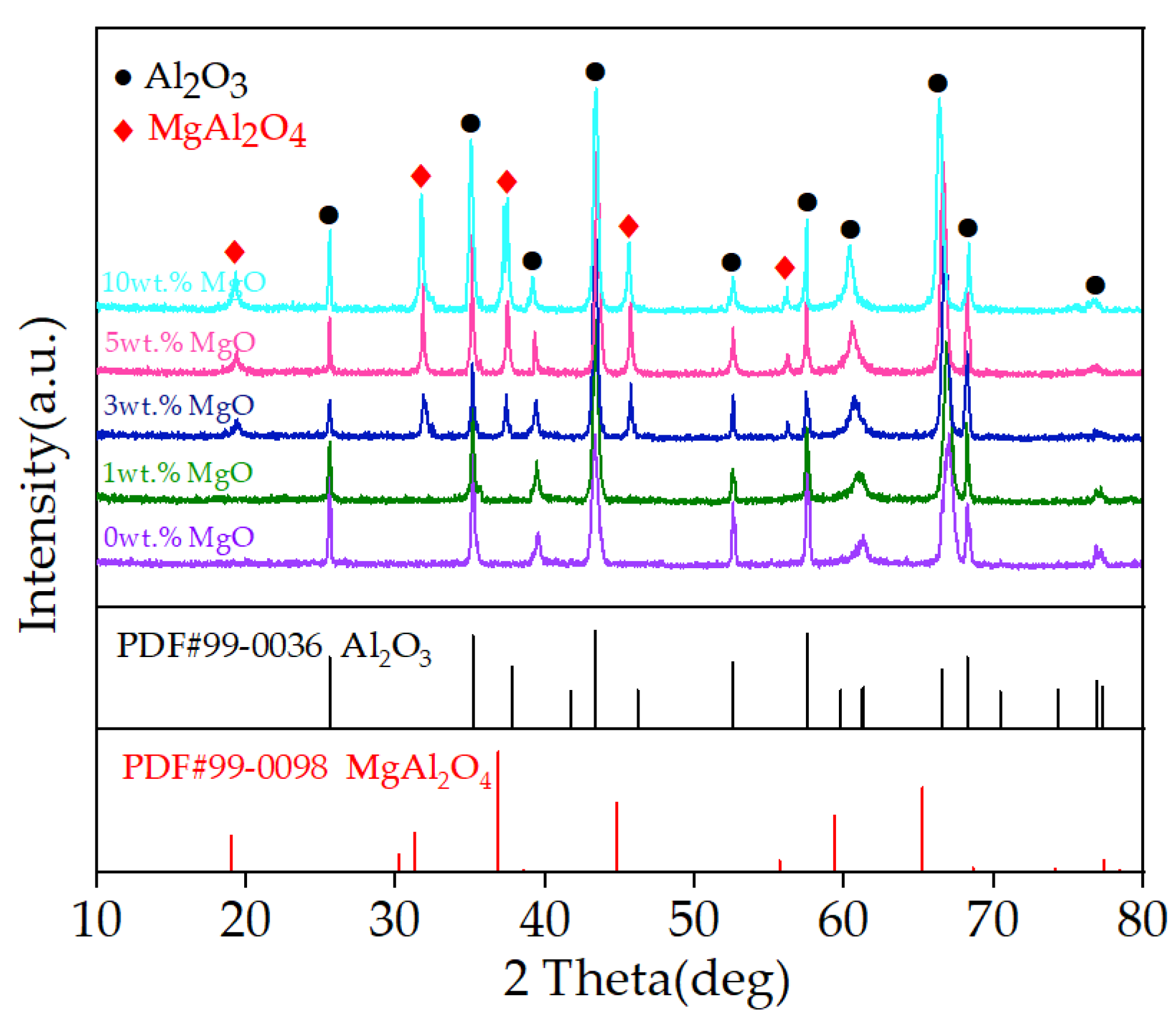
3.2. Properties of MgO-Doped Al2O3 Spraying Powders
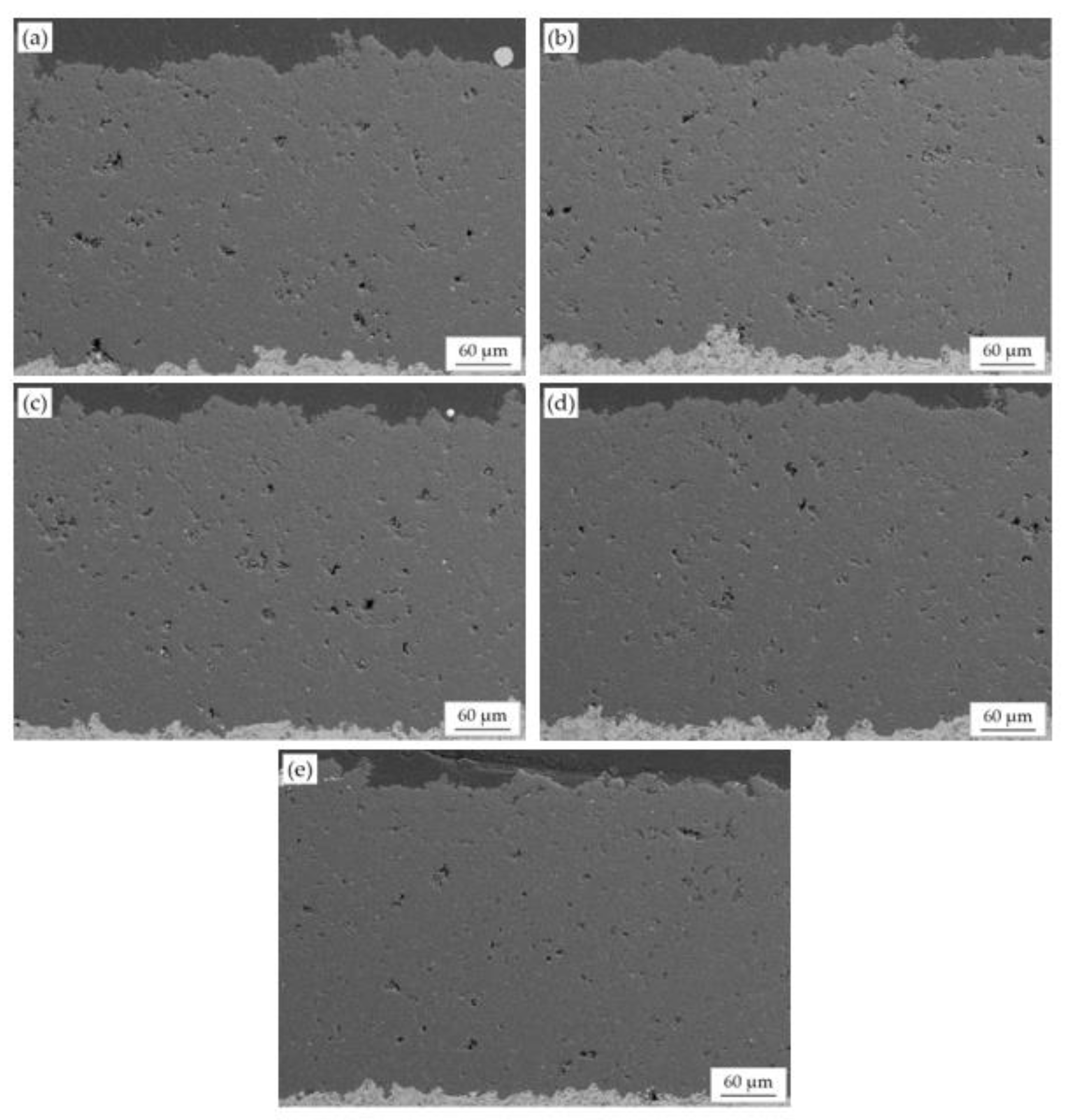
3.3. Properties of MgO-Doped Al2O3 Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, N.J.; Velli, M.C.; Bale, S.D.; Decker, R.; Driesman, A.; Howard, R.A.; Kasper, J.C.; Kinnison, J.; Kusterer, M.; Lario, D.; et al. The Solar Probe Plus Mission: Humanity’s First Visit to Our Star. Space Sci. Rev. 2016, 204, 7–48. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.P. Solar Probe Plus: Mission design challenges and trades. Acta Astronaut. 2010, 67, 1063–1072. [Google Scholar] [CrossRef]
- King, D.E.; Drewry, D.G.; Sample, J.L.; Clemons, D.E.; Caruso, K.S.; Potocki, K.A.; Eng, D.A.; Mehoke, D.S.; Mattix, M.P.; Thomas, M.E.; et al. Alumina Optical Surface Heat Shield for Use in Near-Solar Environment. Int. J. Appl. Ceram. Technol. 2009, 6, 355–361. [Google Scholar] [CrossRef]
- Brodu, E.; Balat-Pichelin, M.; Sans, J.L.; Freeman, M.D.; Kasper, J.C. Efficiency and behavior of textured high emissivity metallic coatings at high temperature. Mater. Des. 2015, 83, 85–94. [Google Scholar] [CrossRef]
- Wu, Y.B.; Ma, X.F.; Zhang, H.Z.; Zhou, Y. A New High Emissivity Coating on Ni-Based Superalloy Substrate. Rare Metal Mat. Eng. 2016, 45, 588–592. [Google Scholar] [CrossRef]
- Li, L.F.; Yu, K.; Zhang, K.H.; Liu, Y.F. Study of Ti-6Al-4V alloy spectral emissivity characteristics during thermal oxidation process. Int. J. Heat Mass Transf. 2016, 101, 699–706. [Google Scholar] [CrossRef]
- Liu, Y.F.; Xie, J.L.; Luo, M.; Peng, B.; Xu, C.; Deng, L.J. The synthesis and optical properties of Al/MnO2 composite pigments by ball-milling for low infrared emissivity and low lightness. Prog. Org. Coat. 2017, 108, 30–35. [Google Scholar] [CrossRef]
- Li, J.F.; Luo, Z.P. Fabrication and performances of preceramic polymer-based high-temperature High emissivity coating. J. Eur. Ceram. Soc. 2020, 40, 5217–5225. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Cheng, X.D.; Ye, W.P.; Min, J. Synthesis of NiCr2O4 spinel coatings with high emissivity by plasma spraying. Int. J. Miner. Metall. Mater. 2012, 19, 266–270. [Google Scholar] [CrossRef]
- Yang, K.; Rong, J.; Liu, C.G.; Zhao, H.Y.; Tao, S.Y.; Ding, C.X. Study on erosion-wear behavior and mechanism of plasma-sprayed alumina-based coatings by a novel slurry injection method. Tribol. Int. 2016, 93, 29–35. [Google Scholar] [CrossRef]
- Szczygiel, B.; Kolodziej, M. Composite Ni/Al2O3 coatings and their corrosion resistance. Electrochim. Acta 2005, 50, 4188–4195. [Google Scholar] [CrossRef]
- Shao, F.; Zhuang, Y.; Ni, J.X.; Sheng, J.; Zhao, H.Y.; Tao, S.Y.; Yang, K. Comparison of the microstructural characteristics and electrical properties of plasma sprayed Al2O3 and Al2O3-Ca2SiO4 coatings immersed in deionized water. Surf. Coat. Technol. 2021, 422, 127530. [Google Scholar] [CrossRef]
- Rangaraj, S.; Kokini, M. Interface thermal fracture in functionally graded zirconia-mullite-bond coat alloy thermal barrier coatings. Acta Mater. 2003, 51, 251–267. [Google Scholar] [CrossRef]
- Popov, A.I.; Lushchik, A.; Shablonin, E.; Vasil’chenko, E.; Kotomin, E.A.; Moskina, A.M.; Kuzovkov, V.N. Comparison of the F-type center thermal annealing in heavy-ion and neutron irradiated Al2O3 single crystals. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 433, 93–97. [Google Scholar] [CrossRef]
- Ananchenko, D.V.; Nikiforov, S.V.; Kuzovkov, V.N.; Popov, A.I.; Ramazanova, G.R.; Batalov, R.I.; Bayazitov, R.M.; Novikov, H.A. Radiation-induced defects in sapphire single crystals irradiated by a pulsed ion beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 466, 1–7. [Google Scholar] [CrossRef]
- Song, G.P.; He, S.F.; He, F.; Yao, Y.T.; Li, J.J.; Li, M.W.; He, X.D. Effect of doping graphene oxide on the structure and properties of SiO2 based high emissivity coatings. J. Appl. Polym. Sci. 2020, 137, 9. [Google Scholar] [CrossRef]
- Wang, S.M.; Kuang, F.H. Sol-gel Preparation and Infrared Radiation Property of Boron-substituted Cordierite Glass-ceramics. J. Mater. Sci. Technol. 2010, 26, 445–448. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Liu, S.; Zhang, X.K.; Xiang, Y. Effects of Cr3+ Concentration on the Crystallinity and Optical Properties of Cr-Doped Al2O3 Powders by Solid-State Reaction Method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022037. [Google Scholar] [CrossRef]
- Kim, B.N.; Hiraga, K.; Morita, K.; Yoshida, H.; Kagawa, Y. Light scattering in MgO-doped alumina fabricated by spark plasma sintering. Acta Mater. 2010, 58, 4527–4535. [Google Scholar] [CrossRef]
- Kim, B.N.; Hiraga, K.; Morita, K.; Yoshida, H. Microstructure and Optical Properties of MgO-Doped Transparent Alumina Manufactured by Spark Plasma Sintering. In Preprints of Annual Meeting of The Ceramic Society of Japan; The Ceramic Society of Japan: Gifu, Japan, 2010; Volume 654, pp. 2041–2044. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Ringer, S.; Sorrell, C.C. Defect Chemistry and Electrical Properties of La(1-x)Sr(x)CoO(3-delta) IV. Electrical Properties. Ionics 2001, 7, 388–393. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, F.; Liao, Q.L.; Liu, H.; Li, X.B. Synthesis and characterization of translucent MgO-doped Al2O3 hollow spheres in millimeter-scale. J. Alloys Compd. 2014, 608, 185–190. [Google Scholar] [CrossRef]
- Tian, J.; Zhu, W.R.; Li, H.D. Infrared radiant emissivities of ceramics with spinel structure. Int. J. Infrared Millim. Waves 1993, 14, 1855–1863. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, Y.M.; Weng, W.X.; Liu, B.L.; Li, Q. Influence of pores on mechanical properties of plasma sprayed coatings: Case study of YSZ thermal barrier coatings. Ceram. Int. 2018, 44, 21564–21577. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.Z.; Yang, T.; Fang, H.J.; Ye, D.D.; Li, D.P. Study on Influencing Factors of Solar Absorptance to IR Emittance Ratio of Al2O3 Coating by Atmospheric Plasma Spraying. Hot Work. Technol. 2021, 16, 43. [Google Scholar] [CrossRef]



| Components | Sample 1 (wt.%) | Sample 2 (wt.%) | Sample 3 (wt.%) | Sample 4 (wt.%) | Sample 5 (wt.%) |
|---|---|---|---|---|---|
| Al2O3 | 100 | 99 | 97 | 95 | 90 |
| MgO | 0 | 1 | 3 | 5 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Wang, W.; Ye, D.; Yang, T.; Li, D.; Yang, M.; Yuan, B.; Wang, Y. Effect of Doping Content of MgO on Solar Absorptivity to IR Emissivity Ratio of Al2O3 Coatings. Coatings 2022, 12, 1891. https://doi.org/10.3390/coatings12121891
Zhu H, Wang W, Ye D, Yang T, Li D, Yang M, Yuan B, Wang Y. Effect of Doping Content of MgO on Solar Absorptivity to IR Emissivity Ratio of Al2O3 Coatings. Coatings. 2022; 12(12):1891. https://doi.org/10.3390/coatings12121891
Chicago/Turabian StyleZhu, Han, Weize Wang, Dongdong Ye, Ting Yang, Dongpeng Li, Min Yang, Baohan Yuan, and Yihao Wang. 2022. "Effect of Doping Content of MgO on Solar Absorptivity to IR Emissivity Ratio of Al2O3 Coatings" Coatings 12, no. 12: 1891. https://doi.org/10.3390/coatings12121891






