Synthesis and Characterization of Urethane Acrylate Resin Based on 1,3-Propanediol for Coating Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
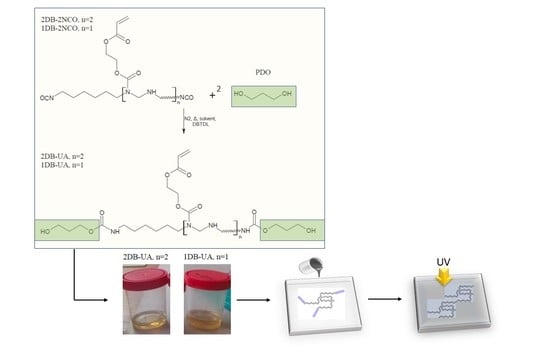
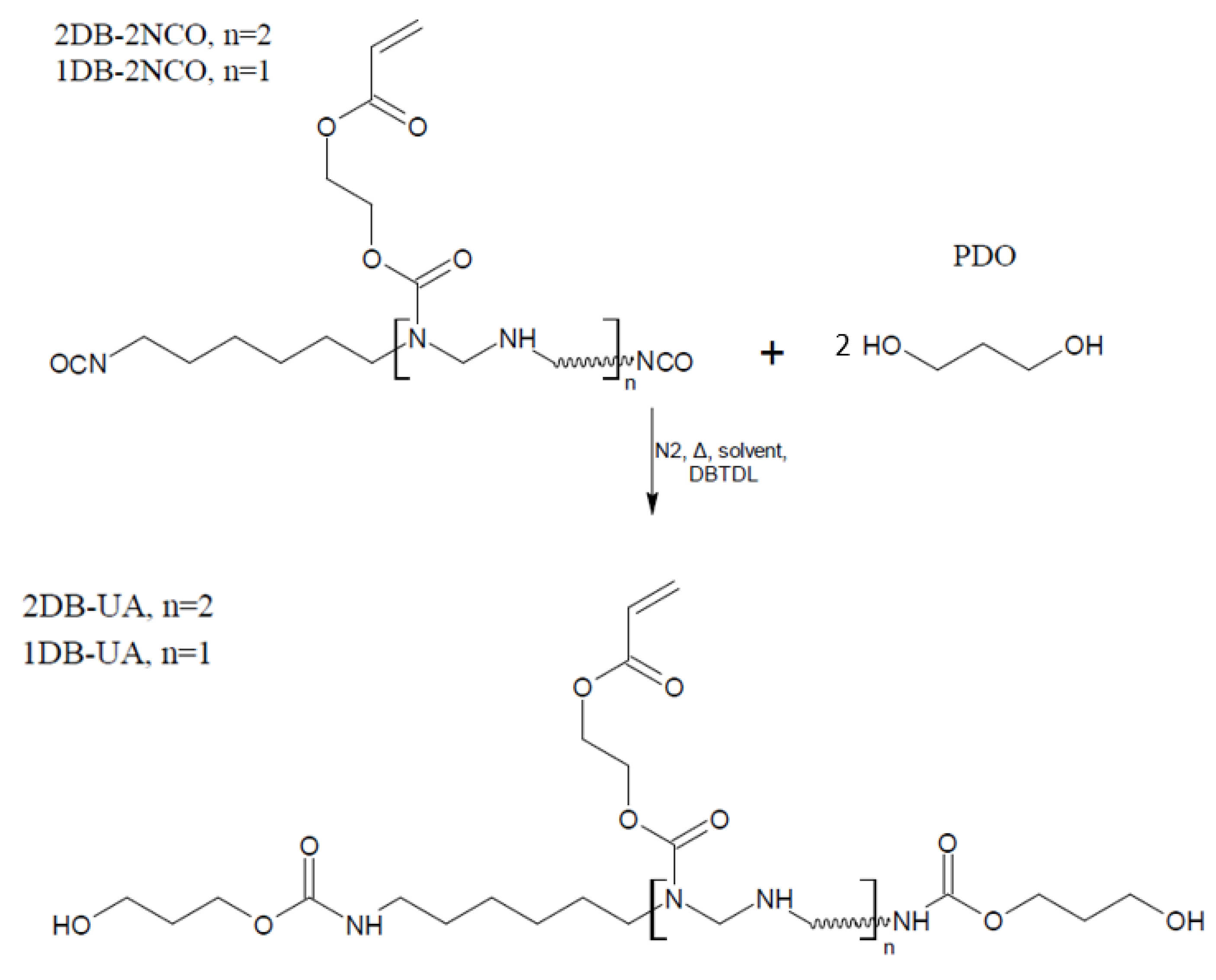
2.2. Synthesis of Sustainable Urethane Acrylate Resins
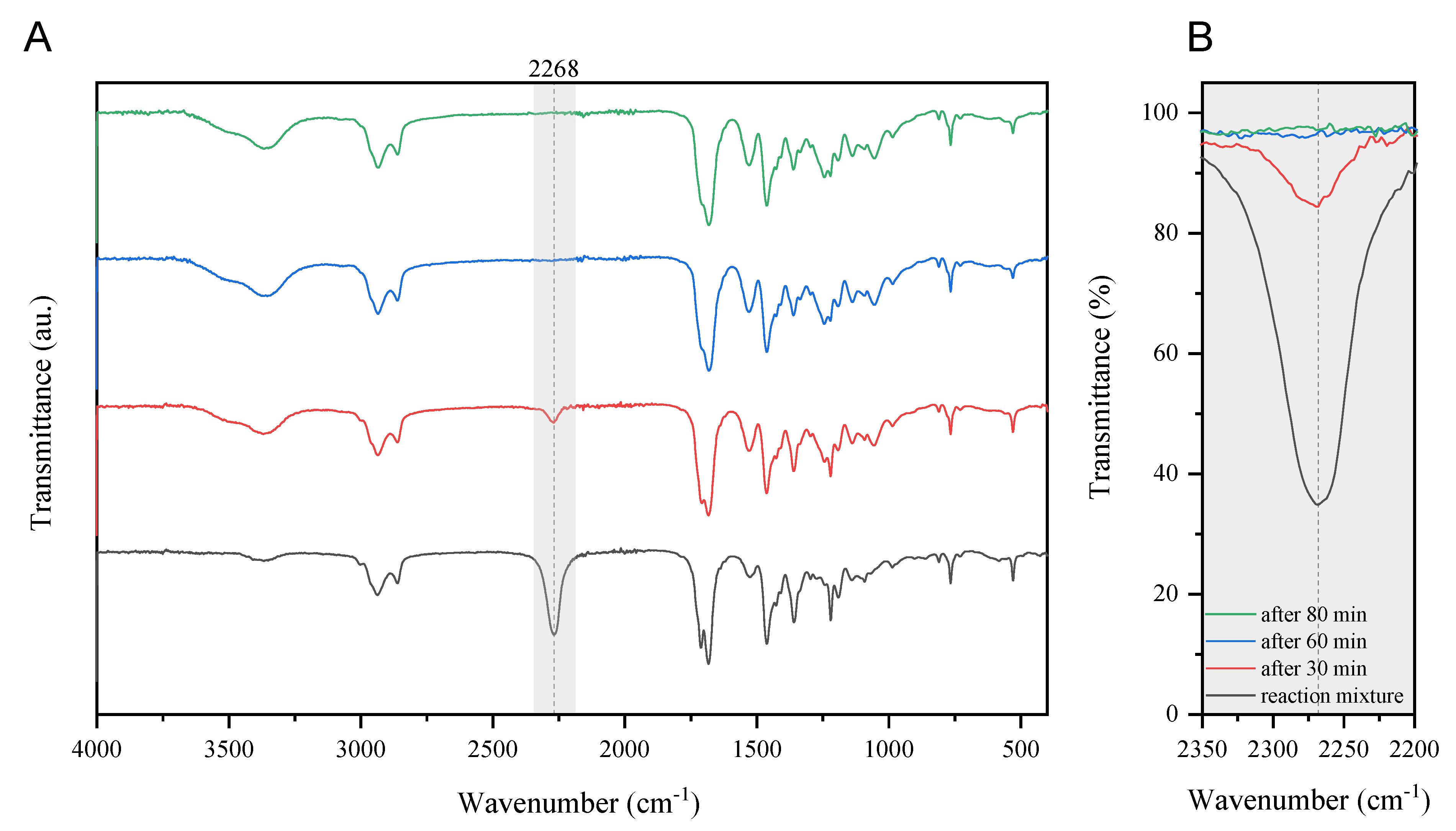
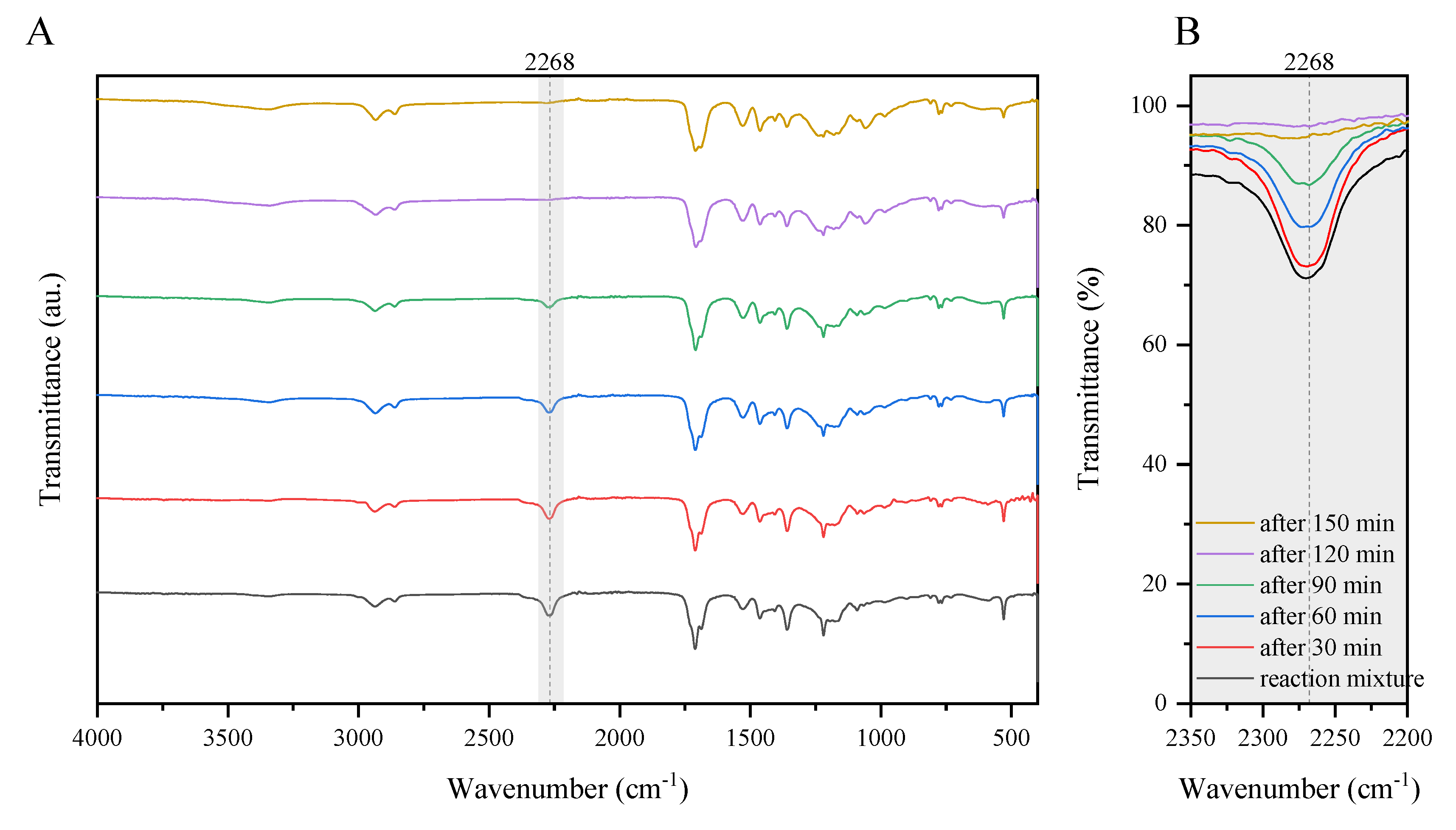
2.3. The FTIR Spectroscopy
2.4. Viscosity
2.5. Preparation of Coating Compositions and Curing Conditions
2.6. Characterization of the Cured Coatings
3. Results and Discussion
3.1. Approach to the Synthesis of Sustainable Urethane Acrylate Resins
3.2. Examination of the Curing Process and Properties of Cured Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Sharma, B.; Sauraj, S.; Kumar, B.; Pandey, A.; Dutt, D.; Negi, Y.S.; Maji, P.K.; Kulshreshtha, A. Synthesis of Waterborne Acrylic Copolymer Resin as a Binding Agent for the Development of Water-based Inks in the Printing Application. Polym. Eng. Sci. 2021, 61, 1569–1580. [Google Scholar] [CrossRef]
- Kumar, B.; Negi, Y.S. Synthesis and Thermal Properties of Novel Poly(Potassium 1-Hydroxy Acrylate-Co-Potassium Acrylate) Based Copolymer. Mater. Lett. 2018, 232, 196–201. [Google Scholar] [CrossRef]
- Kumar, B.; Deeba, F.; Priyadarshi, R.; Bano, S.; Kumar, A.; Negi, Y.S. Development of Novel Cross-Linked Carboxymethyl Cellulose/Poly(Potassium 1-Hydroxy Acrylate): Synthesis, Characterization and Properties. Polym. Bull. 2020, 77, 4555–4570. [Google Scholar] [CrossRef]
- Niesbach, A.; Adams, T.A.; Lutze, P. Semicontinuous Distillation of Impurities for the Production of Butyl Acrylate from Bio-Butanol and Bio-Acrylic Acid. Chem. Eng. Process. Process Intensif. 2013, 74, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Nagengast, J. Development and Optimization of a One-Step Liquid Phase Process for the Sustainable Production of Bio Acrylic Acid from Lactic Acid and Its Oligomers, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). 2021. Available online: https://d-nb.info/1237499100/34 (accessed on 1 October 2022).
- Lubis, M.A.R.; Handika, S.O.; Sari, R.K.; Iswanto, A.H.; Antov, P.; Kristak, L.; Lee, S.H.; Pizzi, A. Modification of Ramie Fiber via Impregnation with Low Viscosity Bio-Polyurethane Resins Derived from Lignin. Polymers 2022, 14, 2165. [Google Scholar] [CrossRef]
- Handika, S.O.; Lubis, M.A.R.; Sari, R.K.; Laksana, R.P.B.; Antov, P.; Savov, V.; Gajtanska, M.; Iswanto, A.H. Enhancing Thermal and Mechanical Properties of Ramie Fiber via Impregnation by Lignin-Based Polyurethane Resin. Materials 2021, 14, 6850. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Fatriasari, W.; Sari, R.K.; Antov, P.; Gajtanska, M.; Papadopoulos, A.N.; Pizzi, A. Bio-Based Polyurethane Resins Derived from Tannin: Source, Synthesis, Characterisation, and Application. Forests 2021, 12, 1516. [Google Scholar] [CrossRef]
- Fouilloux, H.; Thomas, C.M. Production and Polymerization of Biobased Acrylates and Analogs. Macromol. Rapid Commun. 2021, 42, 2000530. [Google Scholar] [CrossRef]
- Hernández, N.; Williams, R.C.; Cochran, E.W. The Battle for the “Green” Polymer. Different Approaches for Biopolymer Synthesis: Bioadvantaged vs. Bioreplacement. Org. Biomol. Chem. 2014, 12, 2834–2849. [Google Scholar] [CrossRef]
- Samyn, P.; Bosmans, J.; Cosemans, P. Comparative Study on Mechanical Performance of Photocurable Acrylate Coatings with Bio-Based versus Fossil-Based Components. Mater. Today Commun. 2022, 32, 104002. [Google Scholar] [CrossRef]
- Stouten, J.; Vanpoucke, D.E.P.; Van Assche, G.; Bernaerts, K.V. UV-Curable Biobased Polyacrylates Based on a Multifunctional Monomer Derived from Furfural. Macromolecules 2020, 53, 1388–1404. [Google Scholar] [CrossRef] [Green Version]
- Hermens, J.G.H.; Freese, T.; van den Berg, K.J.; van Gemert, R.; Feringa, B.L. A Coating from Nature. Sci. Adv. 2020, 6, eabe0026. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, J.; Lu, J.; Huang, J.; Zhang, F.; Hu, Y.; Liu, C.; An, R.; Miao, H.; Chen, Y.; et al. Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-Like Resins for UV-Curable Coatings. Polymers 2020, 12, 2165. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Tang, L.; Zhang, Z.; Gu, L.; Xu, Y.; Eger, C. Wear-Resistant and Transparent Acrylate-Based Coating with Highly Filled Nanosilica Particles. Tribol. Int. 2010, 43, 83–91. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.F.; Fernando, S.; Jagodzinski, K. Soy-Based, High Biorenewable Content UV Curable Coatings. Prog. Org. Coat. 2011, 71, 98–109. [Google Scholar] [CrossRef]
- Hu, Y.; Shang, Q.; Tang, J.; Wang, C.; Zhang, F.; Jia, P.; Feng, G.; Wu, Q.; Liu, C.; Hu, L.; et al. Use of Cardanol-Based Acrylate as Reactive Diluent in UV-Curable Castor Oil-Based Polyurethane Acrylate Resins. Ind. Crops Prod. 2018, 117, 295–302. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, S.; Zhou, X.; Yang, Z.; Yuan, T. A Novel Multi-Functional Bio-Based Reactive Diluent Derived from Cardanol for High Bio-Content UV-Curable Coatings Application. Prog. Org. Coat. 2020, 148, 105880. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Zhu, J.; Liu, X.; Wang, Z.; Yan, J. UV-Curable Coatings from Multiarmed Cardanol-Based Acrylate Oligomers. ACS Sustain. Chem. Eng. 2015, 3, 1313–1320. [Google Scholar] [CrossRef]
- Sung, J.; Sun, X.S. Cardanol Modified Fatty Acids from Camelina Oils for Flexible Bio-Based Acrylates Coatings. Prog. Org. Coat. 2018, 123, 242–253. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Sun, X.S. Epoxidized and Acrylated Epoxidized Camelina Oils for Ultraviolet-Curable Wood Coatings. J. Am. Oil Chem. Soc. 2018, 95, 1307–1318. [Google Scholar] [CrossRef]
- Tathe, D.S.; Jagtap, R.N. Biobased Reactive Diluent for UV-Curable Urethane Acrylate Oligomers for Wood Coating. J. Coat. Technol. Res. 2015, 12, 187–196. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on Transesterification of Non-Edible Sources for Biodiesel Production with a Focus on Economic Aspects, Fuel Properties and by-Product Applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Haas, T.; Jaeger, B.; Weber, R.; Mitchell, S.F.; King, C.F. New Diol Processes: 1,3-Propanediol and 1,4-Butanediol. Appl. Catal. A Gen. 2005, 280, 83–88. [Google Scholar] [CrossRef]
- Casali, S.; Gungormusler, M.; Bertin, L.; Fava, F.; Azbar, N. Development of a Biofilm Technology for the Production of 1,3-Propanediol (1,3-PDO) from Crude Glycerol. Biochem. Eng. J. 2012, 64, 84–90. [Google Scholar] [CrossRef]
- Ghozali, M.; Triwulandari, E.; Haryono, A. Preparation and Characterization of Polyurethane-Modified Epoxy with Various Types of Polyol: Preparation and Characterization of Polyurethane-Modified. Macromol. Symp. 2015, 353, 154–160. [Google Scholar] [CrossRef]
- Acevedo, M.; De La Campa, J.G.; Abajo, J.D. Synthesis and Characterization of Imide End-Capped Oligoesters of Terephthalic Acid and 2-Methyl-2-Propyl-1,3-Propanediol. J. Appl. Polym. Sci. 1990, 41, 163–176. [Google Scholar] [CrossRef]
- Tuan Ismail, T.N.M.; Ibrahim, N.A.; Sendijarevic, V.; Sendijarevic, I.; Schiffman, C.M.; Hoong, S.S.; Mohd Noor, M.A.; Poo Palam, K.D.; Yeong, S.K.; Idris, Z.; et al. Thermal and Mechanical Properties of Thermoplastic Urethanes Made from Crystalline and Amorphous Azelate Polyols. J. Appl. Polym. Sci. 2019, 136, 47890. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Nowak, M.; Mozelewska, K.; Czech, Z. Photocurable Coatings Based on Bio-Renewable Oligomers and Monomers. Materials 2021, 14, 7731. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Synthesis and Photopolymerization of Novel UV-Curable Macro-Photoinitiators. Prog. Org. Coat. 2020, 141, 105546. [Google Scholar] [CrossRef]
- Esen, D.S.; Karasu, F.; Arsu, N. The Investigation of Photoinitiated Polymerization of Multifunctional Acrylates with TX-BT by Photo-DSC and RT-FTIR. Prog. Org. Coat. 2011, 70, 102–107. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Li, Z.; Shen, Y.; Wang, P.; Wang, D.; Wang, X.; Hu, X. Preparation and Properties of UV-Curable Waterborne Silicon-Containing Polyurethane Acrylate Emulsion. Prog. Org. Coat. 2021, 160, 106503. [Google Scholar] [CrossRef]

| Raw Material Symbol | Chemical Name | Functionality | Mmol (g/mol) | Viscosity (20 °C, mPa·s) | ||
|---|---|---|---|---|---|---|
| Double Bonds | NCO Groups | OH Groups | ||||
| 2DB-2NCO | isocyanate-bearing urethane diacrylate | 2 | 2 | - | 700 | 10,000 |
| 1DB-2NCO | isocyanate-bearing urethane acrylate | 1 | 2 | - | 1200 | 20,000 |
| PDO | 1,3-propanediol | - | 2 | 76 | 55 | |
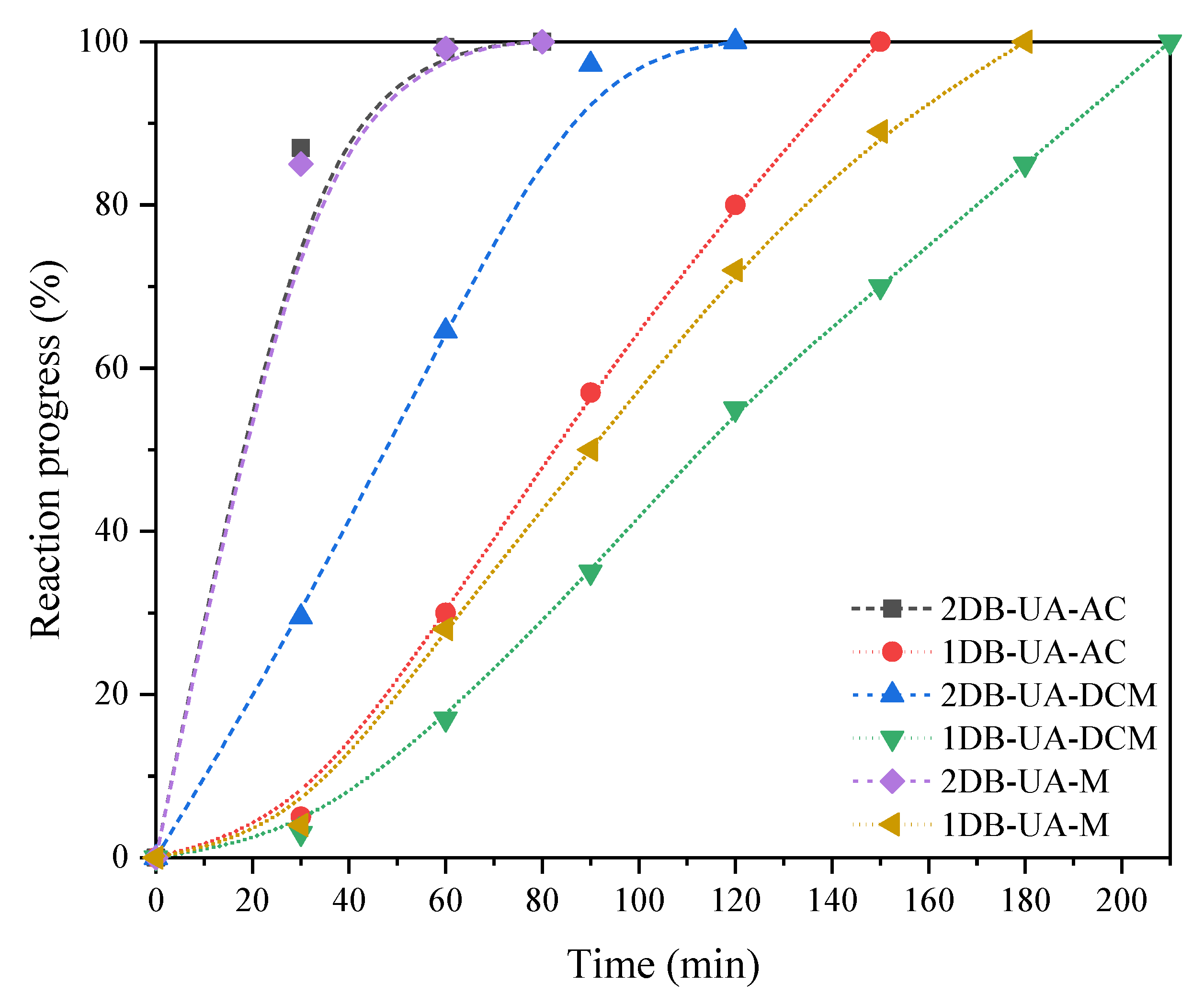
| Sample | nDB-NCO | nPDO | Solvent | R901 (%) | t1002 (min) |
|---|---|---|---|---|---|
| 2DB-UA-A | 0.04 | 0.08 | A | 100 | 80 |
| 1DB-UA-A | 57 | 150 | |||
| 2DB-UA-B | 0.088 | A | 100 | 80 | |
| 1DB-UA-B | 61 | 150 | |||
| 2DB-UA-AC | 0.04 | 0.08 | A | 100 | 80 |
| 1DB-UA-AC | 57 | 150 | |||
| 2DB-UA-DCM | DCM | 97 | 120 | ||
| 1DB-UA-DCM | 35 | 210 | |||
| 2DB-UA-M | none | 100 | 80 | ||
| 1DB-UA-M | 50 | 180 |
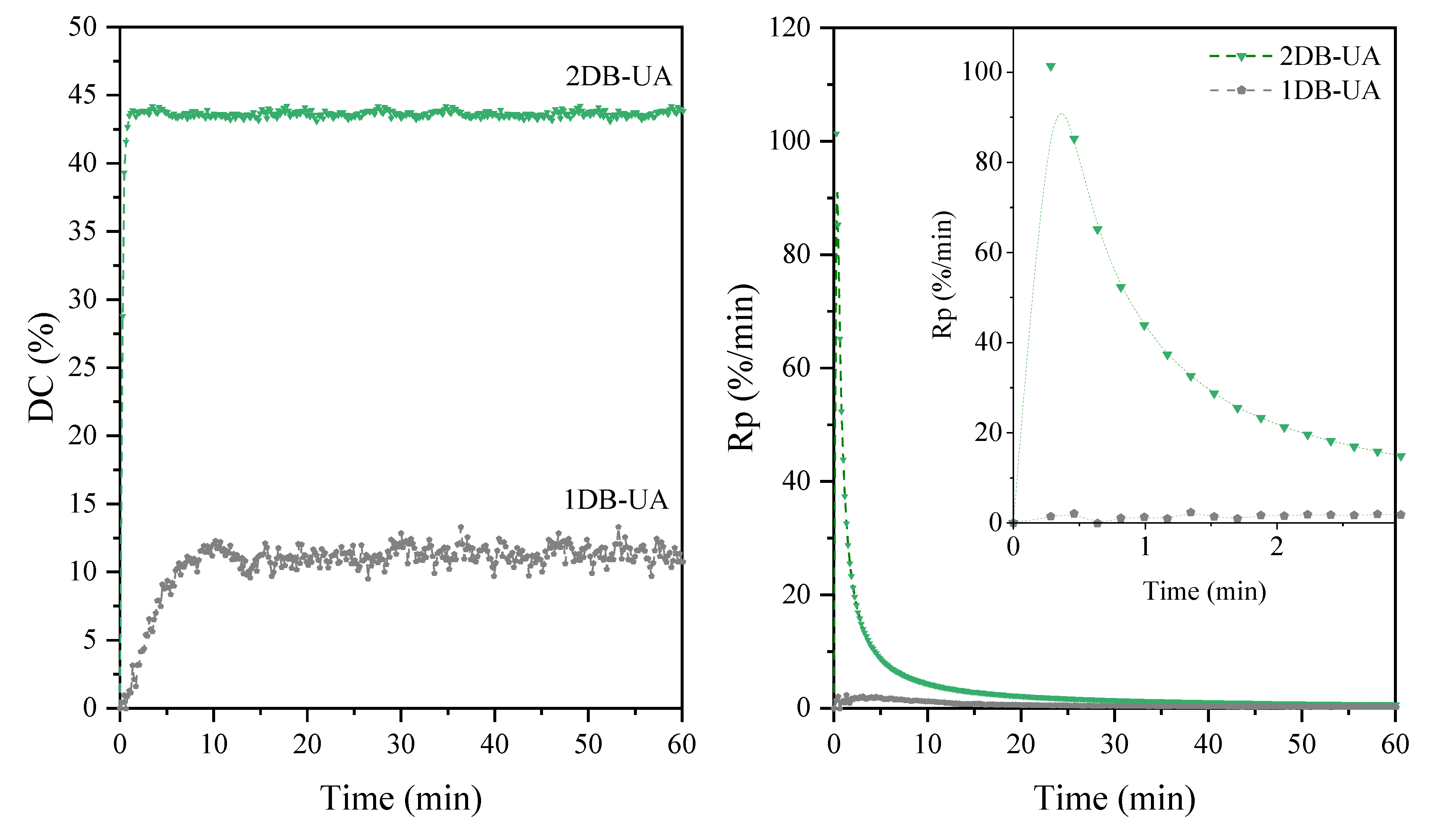
| Sample | DCmax (1) (%) | Rpmax (2) (%/min) | Hardness | Adhesion | Gloss (GU) | Yellowness Index |
|---|---|---|---|---|---|---|
| 2DB-UA- AC-UV-PI | 44 | 101 | 65 | 0 | 100 | 3.70 |
| 1DB-UA- AC-UV-PI | 12 | 2 | 25 | 4 | 85 | 3.99 |
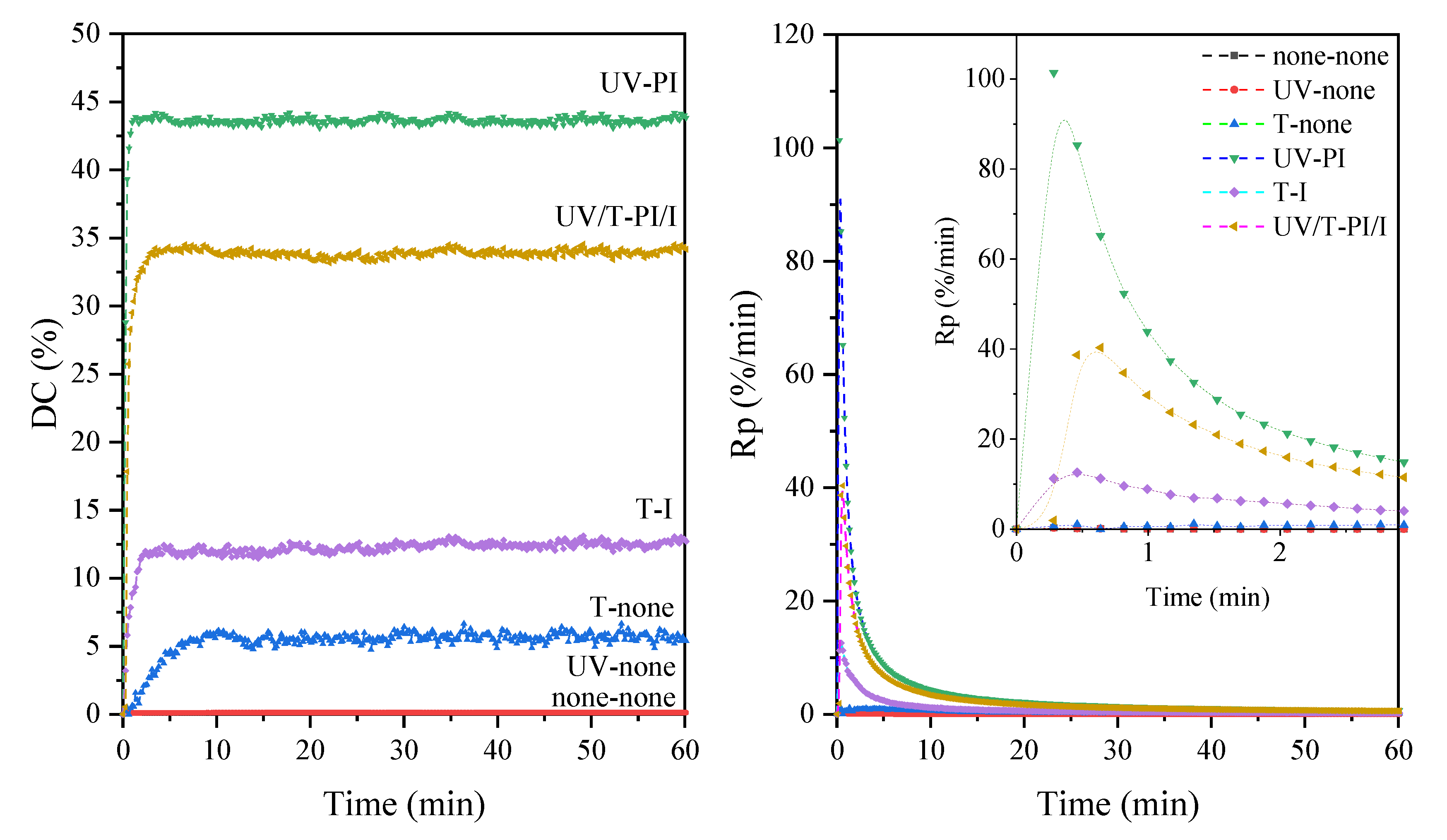
| Sample | DCmax (1) (%) | Rpmax (2) (%/min) | Hardness | Adhesion | Gloss (GU) | Yellowness Index |
|---|---|---|---|---|---|---|
| 2DB-UA- UV-PI | 44 | 101 | 65 | 0 | 100 | 3.70 |
| 2DB-UA- T-I | 13 | 13 | 20 | 0 | 85 | 3.87 |
| 2DB-UA- UV/T-PI/I | 34 | 40 | 45 | 0 | 92 | 3.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, M.; Bednarczyk, P.; Mozelewska, K.; Czech, Z. Synthesis and Characterization of Urethane Acrylate Resin Based on 1,3-Propanediol for Coating Applications. Coatings 2022, 12, 1860. https://doi.org/10.3390/coatings12121860
Nowak M, Bednarczyk P, Mozelewska K, Czech Z. Synthesis and Characterization of Urethane Acrylate Resin Based on 1,3-Propanediol for Coating Applications. Coatings. 2022; 12(12):1860. https://doi.org/10.3390/coatings12121860
Chicago/Turabian StyleNowak, Małgorzata, Paulina Bednarczyk, Karolina Mozelewska, and Zbigniew Czech. 2022. "Synthesis and Characterization of Urethane Acrylate Resin Based on 1,3-Propanediol for Coating Applications" Coatings 12, no. 12: 1860. https://doi.org/10.3390/coatings12121860
APA StyleNowak, M., Bednarczyk, P., Mozelewska, K., & Czech, Z. (2022). Synthesis and Characterization of Urethane Acrylate Resin Based on 1,3-Propanediol for Coating Applications. Coatings, 12(12), 1860. https://doi.org/10.3390/coatings12121860