Study on the Corrosion Resistance of Laser Clad Al0.7FeCoCrNiCux High-Entropy Alloy Coating in Marine Environment
Abstract
:1. Introduction
2. Aging and Failure Modes of Equipment Coatings in Marine Environments
3. Experimental Materials and Methods
3.1. Experimental Equipment and Materials
3.2. Experimental Process
3.2.1. Preparation of High-Entropy Alloy
3.2.2. Neutral Salt Spray Acceleration Test and Outdoor Atmospheric Exposure Test
4. Results and Analysis
4.1. XRD Diffraction Results and Analysis of Coating
4.2. Coating Microstructure
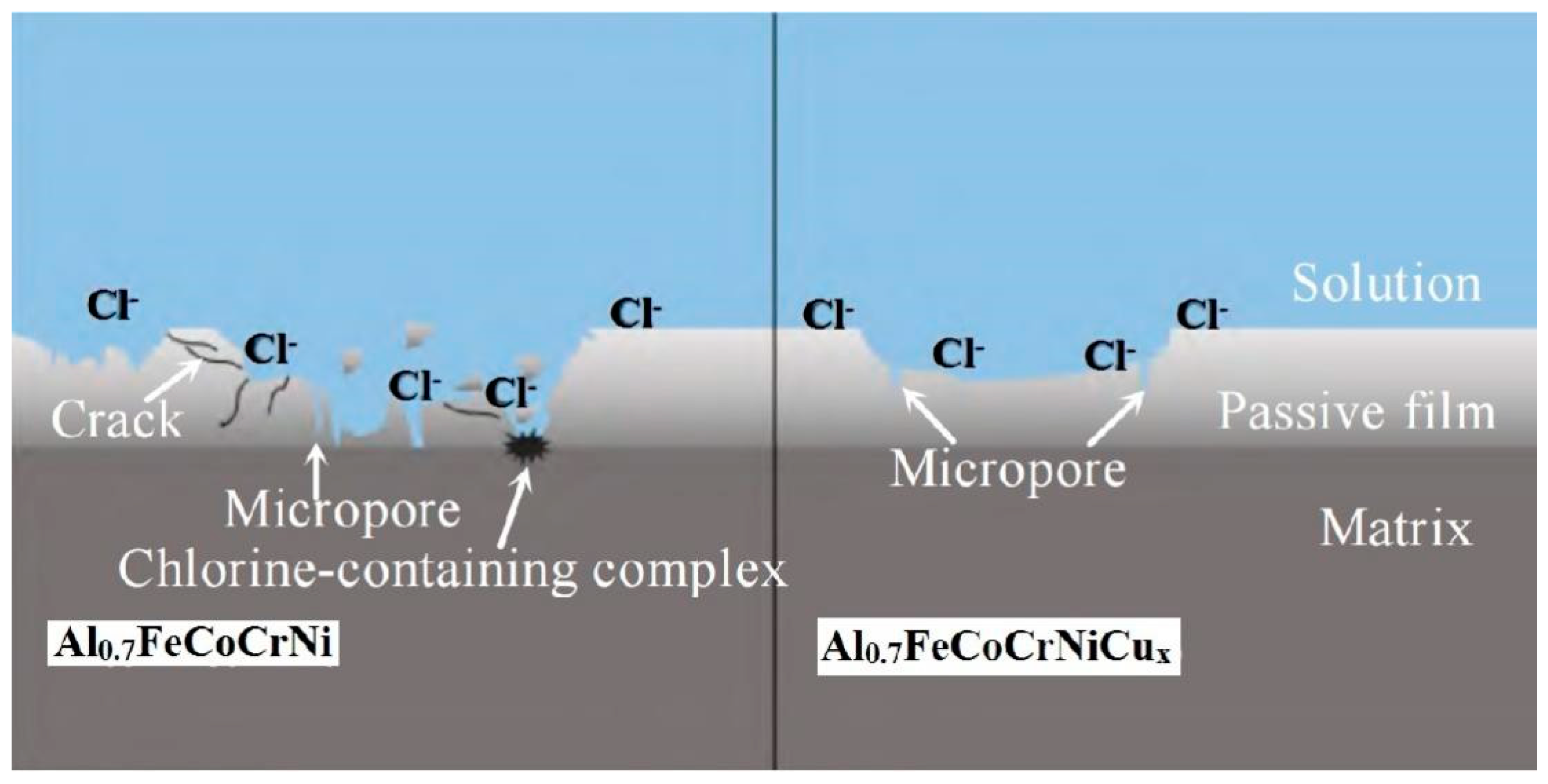
4.3. Corrosion Resistance of Coating
4.4. Electrochemical Properties of Coatings
4.4.1. Analysis of Dynamic Potential Polarization Curve
4.4.2. Analysis of Electrochemical AC Impedance Mapping
4.5. Neutral Salt Spray Acceleration Test
4.5.1. Qualitative Observation and Phenomenon Analysis of Accelerated Corrosion Test Pieces
4.5.2. Quantitative Analysis of Accelerated Corrosion Test Pieces
- (1)
- Determination of corrosion weight loss
- (2)
- Determination of mechanical properties of corrosion specimens
4.6. Outdoor Atmospheric Exposure Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; Zhao, J.; Cheng, X.; Huang, Y.; Lu, L.; Li, X. Galvanic corrosion of the anodized 7050 aluminum alloy coupled with the low hydrogen embrittlement Cd single bond Ti plated 300M steel in an industrial-marine atmospheric environment. Surf. Coat. Technol. 2020, 382, 125–131. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.; Das, S.; Ghosh, K.S.; Ghosh, M.M. Molecular Dynamics-Based Scheme of Designing Nanostructured Cu-Ni Alloy Thin Film for Coating on Advanced Structural Components in Naval Vessels. J. Mater. Eng. Perform. 2022, 7362–7369. [Google Scholar] [CrossRef]
- Wolfe, D.; Singh, J. Functionally gradient ceramic/metallic coatings for gas turbine components by high-energy beams for high-temperature applications. J. Mater. Sci. 1998, 33, 3677–3692. [Google Scholar] [CrossRef]
- de Jeer, L.T.H.; Ocelík, V.; de Hosson, J.T.M. Orientation Relationships in Al0.7CoCrFeNi High-Entropy Alloy. Microsc. Microanal. 2017, 23, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.; Singh, S.; Srivastava, M.; Chethan; Chakradhar, R.P.S.; Panigrahi, B.B. Effect of minute element addition on the oxidation resistance of FeCoCrNiAl and FeCoCrNi2Al high entropy alloy. Adv. Powder Technol. 2022, 33, 1034–1044. [Google Scholar] [CrossRef]
- Giwa, A.M.; Liaw, P.K.; Dahmen, K.A.; Greer, J.R. Microstructure and small-scale size effects in plasticity of individual phases of Al0.7CoCrFeNi High Entropy alloy. Extrem. Mech. Lett. 2016, 8, 220–228. [Google Scholar] [CrossRef]
- Nadaraia, K.V.; Suchkov, S.N.; Imshinetskiy, I.M.; Mashtalyar, D.V.; Sinebrykhov, S.L.; Gnedenkov, S.V. Some new aspects of the study of dependence of properties of PEO coatings on the parameters of current in potentiodynamic mode. Surf. Coat. Technol. 2021, 426, 127–136. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Imshinetskiy, I.M.; Sinebryukhov, S.L.; Gnedenkov, S.V. New approach to formation of coatings on Mg–Mn–Ce alloy using a combination of plasma treatment and spraying of fluoropolymers. J. Magnes. Alloy 2022, 10, 1033–1050. [Google Scholar] [CrossRef]
- Liu, C.; Lu, X.; Li, Y.; Chen, Q.; Zhang, T.; Wang, F. Influence of post-treatment process on corrosion and wear properties of PEO coatings on AM50 Mg alloy. J. Alloys Compd. 2021, 870, 159–168. [Google Scholar] [CrossRef]
- Gan, Y.; Liu, H.; Li, G.; Shi, Y.; Ma, L.; Zhao, Y.; Liu, M.; Zhang, J. Effect of Ni on Corrosion Resistance of Zr-Cu-Al Amorphous Alloys in NaCl Solution. Rare Met. Mater. Eng. 2022, 51, 712–718. [Google Scholar]
- Yang, X.; Zeng, R.; Fu, X.; Wang, X.; Zhou, J.; Yu, L. Influence of the Cu content on the electrochemical corrosion performances of Ni60 coating. Corros. Sci. 2022, 205, 1104–1112. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Lu, N.; Chen, Y.; Liang, X. Corrosion and Wear Behavior of AlNiZr Amorphous Nanocrystalline Composite Coatings. China Mech. Eng. 2022, 33, 1435–1443. [Google Scholar]
- Liu, N.; Wu, P.H.; Zhou, P.J.; Peng, Z.; Wang, X.J.; Lu, Y.P. Rapid solidification and liquid-phase separation of under cooled CoCrCuFex Ni high entropy alloys. Intermetallics 2016, 72, 44–52. [Google Scholar] [CrossRef]
- López-Ortega, A.; Bayón, R.; Arana, J.L. Evaluation of Protective Coatings for High-Corrosivity Category Atmospheres in Offshore Applications. Materials 2019, 12, 1325. [Google Scholar] [CrossRef]
- Marchetti, L.; Hulme-Smith, C. Flowability of steel and tool steel powders: A comparison between testing methods. Powder Technol. 2021, 384, 402–413. [Google Scholar] [CrossRef]
- Vaziri, M.R.R.; Hajiesmaeilbaigi, F.; Maleki, M.H. Monte Carlo simulation of the subsurface growth mode during pulsed laser deposition. J. Appl. Phys. 2011, 110, 043304. [Google Scholar] [CrossRef]
- Serres, N.; Portha, N.; Machi, F. Influence of salt fog aging tests on mechanical resistance of laser clad-coatings. Surf. Coat. Technol. 2011, 205, 5330–5337. [Google Scholar] [CrossRef]
- Agarwal, R.; Sonkusare, R.; Jha, S.R.; Gurao, N.P.; Biswas, K.; Nayan, N. Understanding the deformation behavior of CoCuFeMnNi high entropy alloy by investigating mechanical properties of binary ternary and quaternary alloy subsets. Mater. Des. 2018, 157, 539–550. [Google Scholar] [CrossRef]
- Xie, H.; Liu, G.; Guo, J.; Zhou, M.; Liu, D.; Mao, W. Effect of Ti addition on the microstructure and wear properties of AlFeCrCoCu high-entropy alloy. Rare Met. Mater. Eng. 2016, 45, 145–151. [Google Scholar]
- Bai, L.; Li, X.; Li, M.; Lyu, Y. Microstructure and Mechanical Properties of (Fe40Mn40Ni10Cr10)100-xCx High Entropy Alloy. J. Xi’an Technol. Univ. 2020, 40, 518–523. [Google Scholar]
- Karlsson, D.; Beran, P.; Riekehr, L.; Tseng, J.; Harlin, P.; Jansson, U.; Cedervall, J. Structure and phase transformations in gas atomized AlCoCrFeNi high entropy alloy powders. J. Alloys Compd. 2022, 893, 1620–1630. [Google Scholar] [CrossRef]
- Zarkadoula, E.; Yang, Y.; Borisevich, A.; George, E. Effects of precipitate size and spacing on deformation-induced fcc to bcc phase transformation. Mater. Res. Lett. 2022, 10, 585–592. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Prasad, A.; Murty, B.S. Phase prediction in high entropy alloys—A kinetic approach. Acta Mater. 2018, 153, 214–225. [Google Scholar] [CrossRef]
- Al-Rasheedi, A.; Wageh, S.; Al-Zhrani, E.; Al-Ghamdi, A. Structural and optical properties of CdZnTe quantum dots capped with a bifunctional Molecule. J. Mater. Sci. Mater. Electron. 2017, 28, 9114–9125. [Google Scholar] [CrossRef]
- Cui, W.; Zhou, L.; Luo, G.; Bian, W. Effect of Yttrium on Thermal Stability and Creep Behavior of Ti-1100 High Temperature Titanium Alloy. J. Chin. Soc. Rare Earths 1999, 1, 237–247. [Google Scholar]
- Peng, Z.; Meiners, T.; Gault, B.; Liebscher, C.; Raabe, D.; Lu, Y. A Methodology for Investigation of Grain-Boundary Diffusion and Segregation. Microsc. Microanal. 2017, 23, 656–657. [Google Scholar] [CrossRef]
- Peter, N.J.; Frolov, T.; Duarte, M.J.; Hadian, R.; Ophus, C.; Kirchlechner, C.; Liebscher, C.H.; Dehm, G. Segregation-Induced Nanofaceting Transition at an Asymmetric Tilt Grain Boundary in Copper. Phys. Rev. Lett. 2018, 12, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Zander, D.; Heisterkamp, B.; Gallino, I. Corrosion resistance of Cu–Zr–Al–Y and Zr–Cu–Ni–Al–Nb bulk metallic glasses. J. Alloys Compd. 2007, 434, 234–236. [Google Scholar] [CrossRef]
- Varshney, P.; Mishra, R.S.; Kumar, N. Understanding the nature of passivation film formed during corrosion of Fe39Mn20Co20Cr15Si5Al1 high entropy alloy in 3.5 wt.% NaCl solution. J. Alloys Compd. 2022, 904, 1641–1650. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.-H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Hahn, R.; Kirnbauer, A.; Bartosik, M.; Kolozsvári, S.; Mayrhofer, P.H. Toughness of Si alloyed high-entropy nitride coatings. Mater. Lett. 2019, 251, 238–240. [Google Scholar] [CrossRef]
- Jin, Z.H.; Ge, H.H.; Lin, W.W.; Zong, Y.W.; Liu, S.J.; Shi, J.M. Corrosion behavior of 316L stainless and anti-corrosion materials in a high acidified chloride solution. Appl. Surf. Sci. 2014, 322, 47–56. [Google Scholar] [CrossRef]
- Yin, X. The atmospheric corrosion test of common metal materials at hot region Qionghai in the province Hainan. Environ. Technol. 1997, 2, 5–11. [Google Scholar]
- Fuentes, M.; de la Fuente, D.; Chico, B.; Llorente, I.; Jiménez, J.A.; Morcillo, M. Atmospheric corrosion of zinc in coastal atmospheres. Mater. Corros. 2019, 70, 1005–1015. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J. Study of the accelerated corrosion tests of high-strength Aluminum alloys. Acta Aeronaut. Astronaut. Sin. 2000, 21, 89–92. [Google Scholar]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Processing Route Effects on the Mechanical and Corrosion Properties of Dual Phase Steel. Met. Mater. Int. 2020, 26, 882–890. [Google Scholar] [CrossRef]










| Equipment Type | Weak Link of Protection | Aging Failure Mode of Coating |
|---|---|---|
| Outdoor communication equipment | Fastener connection | Cracking, peeling of coating, and corrosion of base metal |
| Support gap | Coating peeling, substrate metal corrosion | |
| Protective cover | Surface coating pulverization | |
| Dissimilar material joint | Coating peeling, substrate metal corrosion | |
| Edges and welding parts of structural members | Coating stress concentration, spalling, and substrate metal corrosion | |
| Printed circuit board | Coating mildew, solder joint and line corrosion | |
| Component surface coating | Coating cracking and falling off | |
| Vehicle | Engine water tank | Coating stress concentration, spalling, and substrate metal corrosion |
| Component edge | Coating stress concentration, spalling, and substrate metal corrosion | |
| Fastener connection | Cracking and peeling of coating and corrosion of base metal |
| Equipment Name | Model | Brand | Manufacturer | Producing Country |
|---|---|---|---|---|
| Planar CO2 laser | DC050 | ROFIN | ROFIN | Germany |
| Vertical ball mill | AX-100 | Haibo | Wuxi Haibo powder equipment Co., Ltd. | China |
| Double-barrel powder feeder | DPSF-2 | Everest | Jiangsu Everest Laser Technology Co., Ltd. | China |
| X-ray diffractometer | Empyrean | Sharp image | PANalytical | Netherlands |
| Electrochemical workstation | Zennium X | Zennium | Zana company | Germany |
| Salt spray corrosion test chamber | Q-FOGCCT | Q-Lab | Q-Lab | USA |
| Element | Mg | Si | Cu | Zn | Mo | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|
| Content | 3.8 | 0.5 | 0.2 | 0.3 | 1.3 | 0.3 | 0.06 | 93.54 |
| Abbreviation | Al | Cr | Fe | Co | Ni | Cu |
|---|---|---|---|---|---|---|
| Cu0 | 15.59 | 20.56 | 19.33 | 19.66 | 19.84 | 0 |
| Cu0.30 | 14.78 | 18.95 | 18.76 | 18.75 | 18.79 | 4.73 |
| Cu0.60 | 14.93 | 18.77 | 17.35 | 17.88 | 17.68 | 8.75 |
| Cu0.80 | 13.95 | 17.95 | 17.44 | 17.59 | 17.05 | 12.56 |
| Cu1.00 | 12.88 | 16.70 | 16.37 | 16.34 | 16.77 | 16.32 |
| Location | Al | Fe | Co | Cr | Ni | Cu |
|---|---|---|---|---|---|---|
| GB1 | 17.31 | 21.25 | 17.40 | 20.15 | 18.32 | 0.18 |
| GM1 | 12.48 | 24.36 | 13.54 | 28.63 | 11.61 | 1.36 |
| GB2 | 14.95 | 18.59 | 19.21 | 18.35 | 22.87 | 3.54 |
| GM2 | 13.87 | 20.14 | 17.05 | 20.31 | 18.74 | 6.15 |
| GB3 | 11.37 | 18.65 | 16.68 | 21.32 | 18.68 | 9.32 |
| GM3 | 18.45 | 14.48 | 14.35 | 20.54 | 16.25 | 12.78 |
| GB4 | 7.75 | 13.50 | 21.20 | 21.46 | 19.36 | 15.85 |
| GM4 | 11.37 | 17.95 | 22.08 | 18.21 | 17.15 | 21.72 |
| HEAs | Ecoor(vs.SCE)/mV | Jcorr/(A·cm−2) |
|---|---|---|
| Al0.7FeCoCrNi | −0.517 | 5.33 × 10−6 |
| Al0.7FeCoCrNiCu0.30 | −0.486 | 4.40 × 10−7 |
| Al0.7FeCoCrNiCu0.60 | −0.411 | 2.54 × 10−7 |
| Al0.7FeCoCrNiCu0.80 | −0.401 | 2.03 × 10−7 |
| Al0.7FeCoCrNiCu1.00 | −0.388 | 1.05 × 10−7 |
| 5083 aluminum alloy | −0.522 | 5.73 × 10−5 |
| Alloy | Rs/(Ω·cm2) | Q/(mF/cm2) | Rp/(Ω·cm2) |
|---|---|---|---|
| Al0.7FeCoCrNi | 1.15 | 0.12 | 5740 |
| Al0.7FeCoCrNiCu0.30 | 0.65 | 0.15 | 6190 |
| Al0.7FeCoCrNiCu0.60 | 0.78 | 0.12 | 7771 |
| Al0.7FeCoCrNiCu0.80 | 0.91 | 0.09 | 11,115 |
| Al0.7FeCoCrNiCu1.00 | 0.79 | 0.06 | 12,640 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Lu, Y. Study on the Corrosion Resistance of Laser Clad Al0.7FeCoCrNiCux High-Entropy Alloy Coating in Marine Environment. Coatings 2022, 12, 1855. https://doi.org/10.3390/coatings12121855
Wu X, Lu Y. Study on the Corrosion Resistance of Laser Clad Al0.7FeCoCrNiCux High-Entropy Alloy Coating in Marine Environment. Coatings. 2022; 12(12):1855. https://doi.org/10.3390/coatings12121855
Chicago/Turabian StyleWu, Xuehong, and Yanjun Lu. 2022. "Study on the Corrosion Resistance of Laser Clad Al0.7FeCoCrNiCux High-Entropy Alloy Coating in Marine Environment" Coatings 12, no. 12: 1855. https://doi.org/10.3390/coatings12121855
APA StyleWu, X., & Lu, Y. (2022). Study on the Corrosion Resistance of Laser Clad Al0.7FeCoCrNiCux High-Entropy Alloy Coating in Marine Environment. Coatings, 12(12), 1855. https://doi.org/10.3390/coatings12121855






