High-Entropy Alloy for Thin Film Application: A Review
Abstract
:1. Introduction
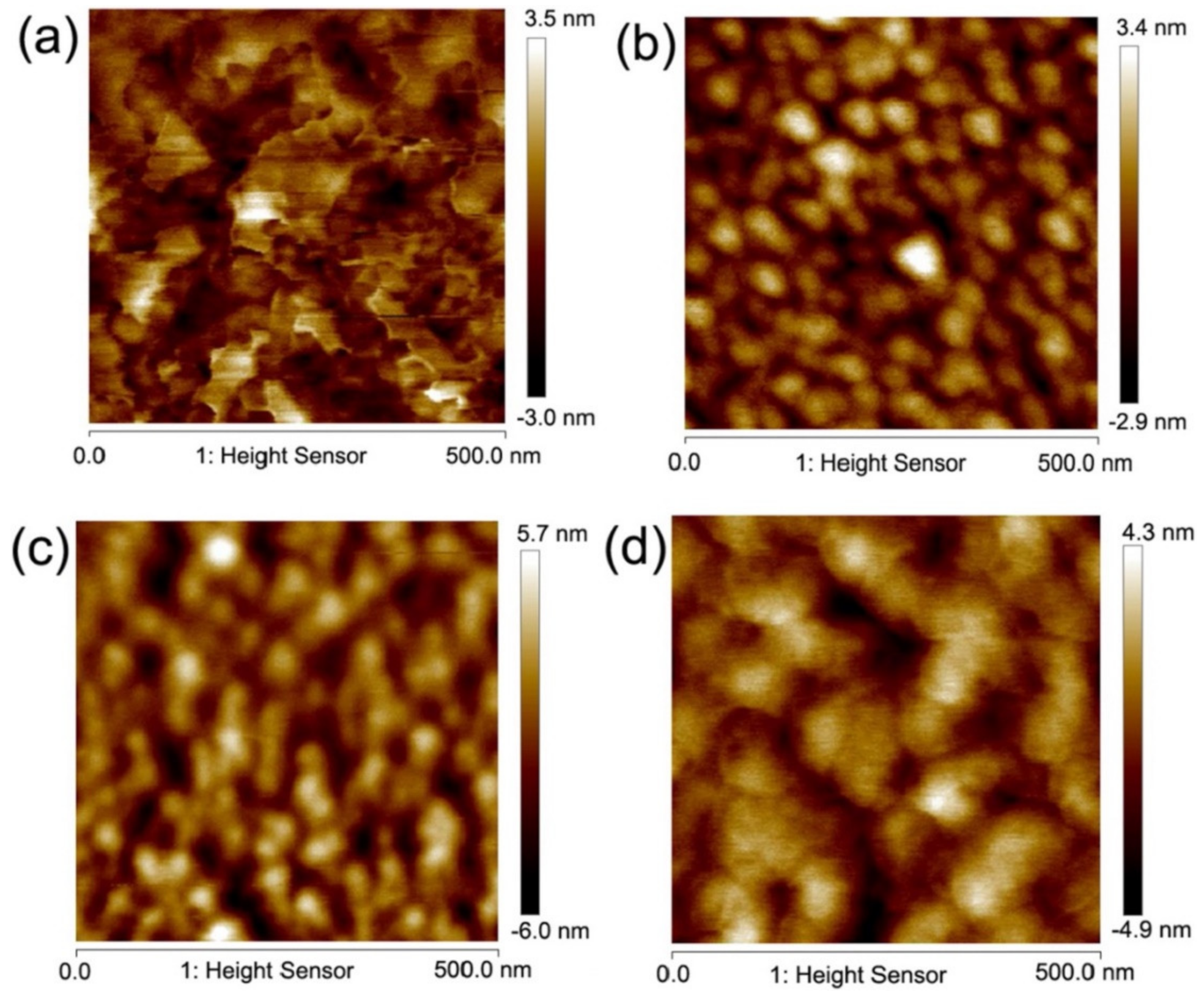
2. Bulk to Thin Films
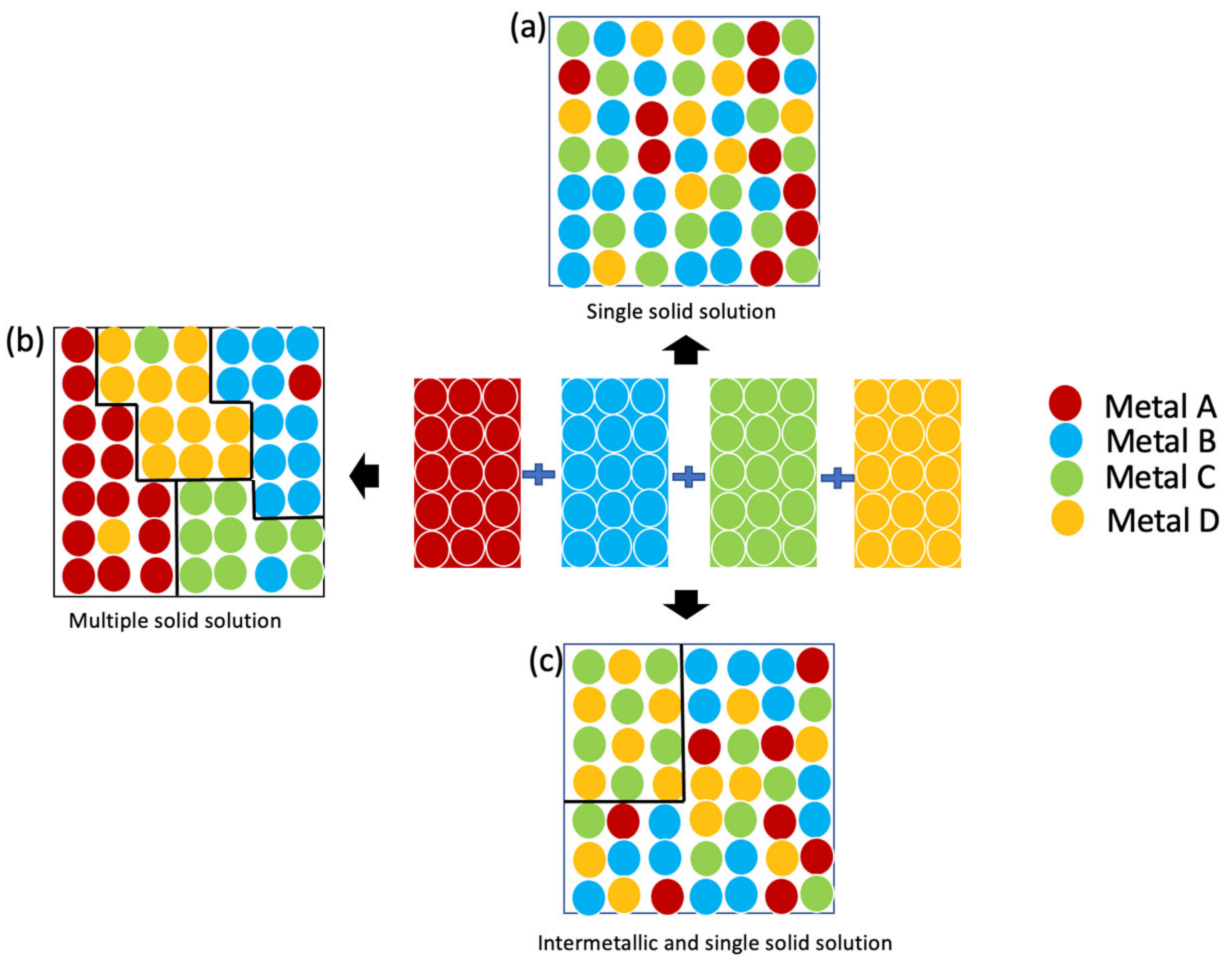
3. Thin-Film-Based High-Entropy Alloys
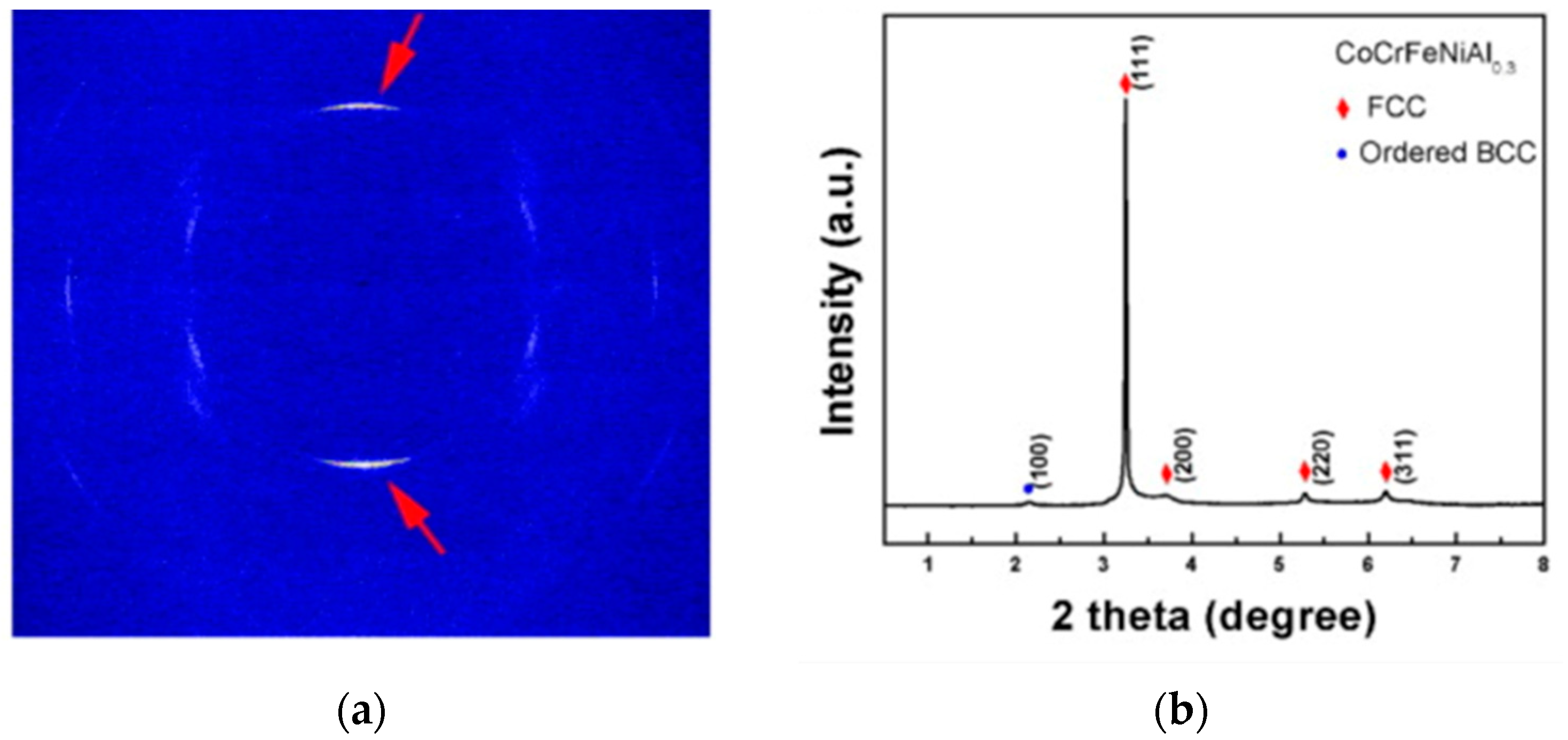
4. Phase and Crystal Structure
5. Mechanical Properties
5.1. Hardness
5.2. Interfacial Bonding
5.3. Corrosion Resistant
5.4. Tribological
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.-W. High-Entropy Alloys: Overview. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Oxford, UK, 2022; pp. 294–307. [Google Scholar]
- Senkov, O.; Rao, S.; Chaput, K.; Woodward, C. Compositional effect on microstructure and properties of NbTiZr-based complex concentrated alloys. Acta Mater. 2018, 151, 201–215. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Chen, S.; Wang, B.; Xiao, Y.; Wang, L.; Sun, S.; Xue, Y. High specific yield strength TiZrAlNbV high-entropy alloys via coherent nanoprecipitation strengthening. Mater. Sci. Eng. A 2022, 861, 144346. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. 2—High-entropy alloys: Basic concepts. In High-Entropy Alloys, 2nd ed.; Murty, B.S., Yeh, J.W., Ranganathan, S., Bhattacharjee, P.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–30. [Google Scholar] [CrossRef]
- Wu, P.; Liu, N.; Yang, W.; Zhu, Z.; Lu, Y.; Wang, X. Microstructure and solidification behavior of multicomponent CoCrCuxFeMoNi high-entropy alloys. Mater. Sci. Eng. A 2015, 642, 142–149. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.F. Microstructure and properties of a refractory NbCrMo0.5Ta0.5TiZr alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. 4—Alloy design and phase selection rules in high-entropy alloys. In High-Entropy Alloys, 2nd ed.; Murty, B.S., Yeh, J.W., Ranganathan, S., Bhattacharjee, P.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 51–79. [Google Scholar]
- Li, W.; Xie, D.; Li, D.; Zhang, Y.; Gao, Y.; Liaw, P.K. Mechanical behavior of high-entropy alloys. Prog. Mater. Sci. 2021, 118, 100777. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A brief review of high-entropy films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Wang, A.; Ramirez, M.O.; Caplovicova, M.; Vretenar, V.; Boettcher, J.; Hopfeld, M.; Kups, T.; Flock, D.; Schaaf, P. Formation of CuCrCoFeNiO high entropy alloy thin films by rapid thermal processing of Cu/CrNiO/FeCo multilayers. Surf. Coat. Technol. 2020, 405, 126563. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Roh, A.; Son, M.; Ham, M.-H.; Kim, J.-H.; Choi, H. Mechanical and electrical properties of NbMoTaW refractory high-entropy alloy thin films. Int. J. Refract. Met. Hard Mater. 2019, 80, 286–291. [Google Scholar] [CrossRef]
- Chu, J.P.; Greene, J.; Jang, J.S.; Huang, J.; Shen, Y.-L.; Liaw, P.K.; Yokoyama, Y.; Inoue, A.; Nieh, T. Bendable bulk metallic glass: Effects of a thin, adhesive, strong, and ductile coating. Acta Mater. 2012, 60, 3226–3238. [Google Scholar] [CrossRef]
- Soare, V.; Burada, M.; Constantin, I.; Mitrică, D.; Bădiliţă, V.; Caragea, A.; Târcolea, M. Electrochemical deposition and microstructural characterization of AlCrFeMnNi and AlCrCuFeMnNi high entropy alloy thin films. Appl. Surf. Sci. 2015, 358, 533–539. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Yang, H.; Dai, J.; Zhu, R.; Gong, J.; Peng, L.; Ding, W. Electrodeposition mechanism and characterization of Ni–Cu alloy coatings from a eutectic-based ionic liquid. Appl. Surf. Sci. 2014, 288, 530–536. [Google Scholar] [CrossRef]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Pavithra, C.L.P.; Janardhana, R.K.S.K.; Reddy, K.M.; Murapaka, C.; Joardar, J.; Sarada, B.V.; Tamboli, R.R.; Hu, Y.; Zhang, Y.; Wang, X.; et al. An advancement in the synthesis of unique soft magnetic CoCuFeNiZn high entropy alloy thin films. Sci. Rep. 2021, 11, 8836. [Google Scholar] [CrossRef] [PubMed]
- Kozak, R.; Steurer, W. High-entropy alloys. Acta Crystallogr. Sect. A Found. Crystallogr. 2013, 69, s497. [Google Scholar] [CrossRef]
- Tsai, M.-H. Physical Properties of High Entropy Alloys. Entropy 2013, 15, 5338–5345. [Google Scholar] [CrossRef] [Green Version]
- Quammen, R.N.; Rottmann, P.F. Investigation of low temperature oxidation behavior of MoNbTaW thin films. J. Alloys Compd. 2022, 900, 163373. [Google Scholar] [CrossRef]
- Han, J.; Su, B.; Lu, J.; Meng, J.; Zhang, A.; Wu, Y. Preparation of MoNbTaW refractory high entropy alloy powders by pressureless spark plasma sintering: Crystal structure and phase evolution. Intermetallics 2020, 123, 106382. [Google Scholar] [CrossRef]
- Gruber, G.C.; Lassnig, A.; Zak, S.; Gammer, C.; Cordill, M.J.; Franz, R. Synthesis and structure of refractory high entropy alloy thin films based on the MoNbTaW system. Surf. Coat. Technol. 2022, 439, 128446. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 13, 5049. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Dedoncker, R.; Glushko, O.; Cordill, M.J.; Depla, D.; Franz, R. Influence of the nitrogen content on the structure and properties of MoNbTaVW high entropy alloy thin films. J. Alloys Compd. 2020, 850, 156740. [Google Scholar] [CrossRef]
- Smeltzer, J.A.; Hornbuckle, B.C.; Giri, A.K.; Darling, K.A.; Harmer, M.P.; Chan, H.M.; Marvel, C.J. Nitrogen-induced hardening of refractory high entropy alloys containing laminar ordered phases. Acta Mater. 2021, 211, 116884. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Kucza, W.; Cieślak, G.; Kulik, T.; Danielewski, M.; Yeh, J.-W. Interdiffusion in the FCC-structured Al-Co-Cr-Fe-Ni high entropy alloys: Experimental studies and numerical simulations. J. Alloys Compd. 2016, 674, 455–462. [Google Scholar] [CrossRef]
- Lu, T.-W.; Feng, C.-S.; Wang, Z.; Liao, K.-W.; Liu, Z.-Y.; Xie, Y.-Z.; Hu, J.-G.; Liao, W.-B. Microstructures and mechanical properties of CoCrFeNiAl0.3 high-entropy alloy thin films by pulsed laser deposition. Appl. Surf. Sci. 2019, 494, 72–79. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Cao, Q.; Zhao, G.; Li, J.; Zhang, D.; Zhu, J.-J.; Jiang, J. Microstructure characterization of AlxCo1Cr1Cu1Fe1Ni1 (x = 0 and 2.5) high-entropy alloy films. J. Alloys Compd. 2014, 609, 137–142. [Google Scholar] [CrossRef]
- Sharma, A.; Oh, M.C.; Ahn, B. Microstructural evolution and mechanical properties of non-Cantor AlCuSiZnFe lightweight high entropy alloy processed by advanced powder metallurgy. Mater. Sci. Eng. A 2020, 797, 140066. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Wang, Q.; Lu, J.; Shi, S.-Q.; Liu, C.T. Phase stability and tensile properties of Co-free Al0.5CrCuFeNi2 high-entropy alloys. J. Alloys Compd. 2014, 584, 530–537. [Google Scholar] [CrossRef]
- WChang, S.-Y.; Lin, S.-Y.; Huang, Y.-C. Microstructures and mechanical properties of multi-component (AlCrTaTiZr)NxCy nanocomposite coatings. Thin Solid Films 2011, 519, 4865–4869. [Google Scholar] [CrossRef]
- Liao, W.; Lan, S.; Gao, L.; Zhang, H.; Xu, S.; Song, J.; Wang, X.; Lu, Y. Nanocrystalline high-entropy alloy (CoCrFeNiAl0.3) thin-film coating by magnetron sputtering. Thin Solid Films 2017, 638, 383–388. [Google Scholar] [CrossRef]
- Dolique, V.; Thomann, A.-L.; Brault, P.; Tessier, Y.; Gillon, P. Thermal stability of AlCoCrCuFeNi high entropy alloy thin films studied by in-situ XRD analysis. Surf. Coat. Technol. 2010, 204, 1989–1992. [Google Scholar] [CrossRef] [Green Version]
- Braeckman, B.; Boydens, F.; Hidalgo, H.; Dutheil, P.; Jullien, M.; Thomann, A.-L.; Depla, D. High entropy alloy thin films deposited by magnetron sputtering of powder targets. Thin Solid Films 2015, 580, 71–76. [Google Scholar] [CrossRef]
- Braeckman, B.; Depla, D. Structure formation and properties of sputter deposited Nbx-CoCrCuFeNi high entropy alloy thin films. J. Alloys Compd. 2015, 646, 810–815. [Google Scholar] [CrossRef]
- Xie, L.; Brault, P.; Thomann, A.-L.; Bauchire, J.-M. AlCoCrCuFeNi high entropy alloy cluster growth and annealing on silicon: A classical molecular dynamics simulation study. Appl. Surf. Sci. 2013, 285, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Thornton, J.A.; Hoffman, D. Stress-related effects in thin films. Thin Solid Films 1989, 171, 5–31. [Google Scholar] [CrossRef]
- Mahieu, S.; Ghekiere, P.; Depla, D.; De Gryse, R. Biaxial alignment in sputter deposited thin films. Thin Solid Films 2006, 515, 1229–1249. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Fu, W.; Zhang, J.; Zhao, J.; Li, J.; Wu, K.; Liu, G.; Sun, J. Effects of nanotwins on the mechanical properties of AlxCoCrFeNi high entropy alloy thin films. Scr. Mater. 2017, 139, 71–76. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Ustinov, A.; Demchenkov, S.; Melnychenko, T.; Skorodzievskii, V.; Polishchuk, S. Effect of structure of high entropy CrFeCoNiCu alloys produced by EB PVD on their strength and dissipative properties. J. Alloys Compd. 2021, 887, 161408. [Google Scholar] [CrossRef]
- Marshal, A.; Pradeep, K.G.; Music, D.; Wang, L.; Petracic, O.; Schneider, J. Combinatorial evaluation of phase formation and magnetic properties of FeMnCoCrAl high entropy alloy thin film library. Sci. Rep. 2019, 9, 7864. [Google Scholar] [CrossRef] [Green Version]
- Sha, C.; Zhou, Z.; Xie, Z.; Munroe, P. Extremely hard, α-Mn type high entropy alloy coatings. Scr. Mater. 2020, 178, 477–482. [Google Scholar] [CrossRef]
- Butler, T.M.; Alfano, J.P.; Martens, R.L.; Weaver, M.L. High-Temperature Oxidation Behavior of Al-Co-Cr-Ni-(Fe or Si) Multicomponent High-Entropy Alloys. Jom 2014, 67, 246–259. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.-Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Arfaoui, M.; Radnóczi, G.; Kis, V.K. Transformations in CrFeCoNiCu High Entropy Alloy Thin Films during In-Situ Annealing in TEM. Coatings 2020, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Carel, R.; Thompson, C.; Frost, H. Computer simulation of strain energy effects vs surface and interface energy effects on grain growth in thin films. Acta Mater. 1996, 44, 2479–2494. [Google Scholar] [CrossRef]
- Lee, D.N. A model for development of orientation of vapour deposits. J. Mater. Sci. 1989, 24, 4375–4378. [Google Scholar] [CrossRef]
- Choudhuri, D.; Gwalani, B.; Gorsse, S.; Mikler, C.; Ramanujan, R.; Gibson, M.; Banerjee, R. Change in the primary solidification phase from fcc to bcc -based B2 in high entropy or complex concentrated alloys. Scr. Mater. 2017, 127, 186–190. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.-H.; Liao, Y.-C.; Li, C.-L.; Jang, J.S.-C.; Hsueh, C.-H. Hardness and strength enhancements of CoCrFeMnNi high-entropy alloy with Nd doping. Mater. Sci. Eng. A 2019, 764. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, Y.; Han, W.; Jia, Q.; Deng, Y.; Wang, H. Identifying the high entropy effect on plastic dynamics in bulk metallic glasses: A nanoindentation study at room and elevated temperatures. Mater. Des. 2020, 189, 108500. [Google Scholar] [CrossRef]
- Kumar, K.; Van Swygenhoven, H.; Suresh, S. Mechanical behavior of nanocrystalline metals and alloys. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Dubey, D.k.; Yadav, R.A.K.; Lee, M.; Khan, S.; Liang, T.-W.; Jou, J.-H. Effect of molecular energy level of electron transport layer on recombination zone in OLED. In Proceedings of the 2018 25th International Workshop on Active-Matrix Flatpanel Displays and Devices (AM-FPD), Kyoto, Japan, 3–6 July 2018; pp. 1–3. [Google Scholar] [CrossRef]
- Gludovatz, B.; George, E.P.; Ritchie, R.O. Processing, Microstructure and Mechanical Properties of the CrMnFeCoNi High-Entropy Alloy. JOM 2015, 67, 2262–2270. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-high strength WNbMoTaV high-entropy alloys with fine grain structure fabricated by powder metallurgical process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Wei, Y.; Hutchinson, J.W. Nonlinear delamination mechanics for thin films. J. Mech. Phys. Solids 1997, 45, 1137–1159. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Chen, P.; Liu, H.; Peng, G.; Zhu, J.; Wu, Y. Interfacial behavior of thin film bonded to plastically graded substrate under tensile loading. Eur. J. Mech.—A/Solids 2023, 97, 104818. [Google Scholar] [CrossRef]
- Lee, C.; Song, G.; Gao, M.C.; Feng, R.; Chen, P.; Brechtl, J.; Chen, Y.; An, K.; Guo, W.; Poplawsky, J.D.; et al. Lattice distortion in a strong and ductile refractory high-entropy alloy. Acta Mater. 2018, 160, 158–172. [Google Scholar] [CrossRef]
- Jiang, M.; Xu, K.; Liao, N.; Zheng, B. Effect of sputtering power on piezoresistivity and interfacial strength of SiCN thin films prepared by magnetic sputtering. Ceram. Int. 2022, 48, 2112–2117. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, J.; Zhang, S.; Wang, G.; Han, J.; Zhang, C. Interfacial microstructure and mechanical properties of TiAl alloy/TC4 titanium alloy joints diffusion bonded with CoCuFeNiTiV0.6 high entropy alloy interlayer. J. Alloys Compd. 2023, 935, 167987. [Google Scholar] [CrossRef]
- Muftah, W.; Allport, J.; Vishnyakov, V. Corrosion performance and mechanical properties of FeCrSiNb amorphous equiatomic HEA thin film. Surf. Coat. Technol. 2021, 422, 127486. [Google Scholar] [CrossRef]
- Huang, K.; Zhou, X.; Guo, H.; Chen, Y. Thermal annealing effect on the corrosion resistance of AlCuNiTiZr high entropy alloy films in sulfuric acid solution. Thin Solid Films 2021, 730, 138707. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, L.; Zhang, W.; Yao, K.; Zhao, Z.; Shang, J.; Qi, J.; Chen, M.; Zhao, R.; Wu, F. Effects of electromagnetic pulse treatment on spinodal decomposed microstructure, mechanical and corrosion properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2021, 889, 161676. [Google Scholar] [CrossRef]
- Mohamed, O.; Hassan, M.; Egilmez, M.; Abuzaid, W.; Ibrahim, T.; Khamis, M. Corrosion behavior of CoCrNi/mild steel medium entropy alloy thin films. Mater. Today Commun. 2021, 30, 103015. [Google Scholar] [CrossRef]
- Lv, Y.; Lang, X.; Zhang, Q.; Liu, W.; Liu, Y. Study on corrosion behavior of (CuZnMnNi)100−xSnx high-entropy brass alloy in 5 wt% NaCl solution. J. Alloys Compd. 2022, 921, 166051. [Google Scholar] [CrossRef]
- Zhu, Z.-X.; Liu, X.-B.; Liu, Y.-F.; Zhang, S.-Y.; Meng, Y.; Zhou, H.-B. Effects of Cu/Si on the microstructure and tribological properties of FeCoCrNi high entropy alloy coating by laser cladding. Wear 2023, 512–513, 204533. [Google Scholar] [CrossRef]
- Sha, M.; Jia, C.; Qiao, J.; Feng, W.; Ai, X.; Jing, Y.-A.; Shen, M.; Li, S. Microstructure and Properties of High-Entropy AlxCoCrFe2.7MoNi Alloy Coatings Prepared by Laser Cladding. Metals 2019, 9, 1243. [Google Scholar] [CrossRef]
| Authors | Materials | Method | Target |
|---|---|---|---|
| Dolique et al., 2010 [34] | AlCoCrCuFeNi | Magnetron sputtering | Mosaic |
| Zhang et al., 2011 [47] | CrCoCuFeNi | Laser cladding | Powder |
| An et al., 2015 [41] | CrCoCuFeNi | Magnetron sputtering | Cast metal |
| Braeckman et al., 2015 [35] | AlCoCrCuFeNi | Magnetron sputtering | Cast metal |
| Liao et al., 2017 [33] | Al0.3CoCrCuFeNi | Magnetron sputtering | Cast metal |
| Marshal et al., 2019 [43] | AlCoCrFeMn | Co sputtering | Alloy target |
| Sha et al., 2020 [44] | AlCoCrFeMn | Magnetron sputtering | Alloy target |
| Arfaoui et al., 2020 [48] | CrCoCuFeNi | Magnetron sputtering | Cast metal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad Nadzri, N.I.; Halin, D.S.C.; Al Bakri Abdullah, M.M.; Joseph, S.; Mohd Salleh, M.A.A.; Vizureanu, P.; Burduhos-Nergis, D.-P.; Sandu, A.V. High-Entropy Alloy for Thin Film Application: A Review. Coatings 2022, 12, 1842. https://doi.org/10.3390/coatings12121842
Muhammad Nadzri NI, Halin DSC, Al Bakri Abdullah MM, Joseph S, Mohd Salleh MAA, Vizureanu P, Burduhos-Nergis D-P, Sandu AV. High-Entropy Alloy for Thin Film Application: A Review. Coatings. 2022; 12(12):1842. https://doi.org/10.3390/coatings12121842
Chicago/Turabian StyleMuhammad Nadzri, Nur Izzati, Dewi Suriyani Che Halin, Mohd Mustafa Al Bakri Abdullah, Sudha Joseph, Mohd Arif Anuar Mohd Salleh, Petrica Vizureanu, Diana-Petronela Burduhos-Nergis, and Andrei Victor Sandu. 2022. "High-Entropy Alloy for Thin Film Application: A Review" Coatings 12, no. 12: 1842. https://doi.org/10.3390/coatings12121842
APA StyleMuhammad Nadzri, N. I., Halin, D. S. C., Al Bakri Abdullah, M. M., Joseph, S., Mohd Salleh, M. A. A., Vizureanu, P., Burduhos-Nergis, D.-P., & Sandu, A. V. (2022). High-Entropy Alloy for Thin Film Application: A Review. Coatings, 12(12), 1842. https://doi.org/10.3390/coatings12121842










