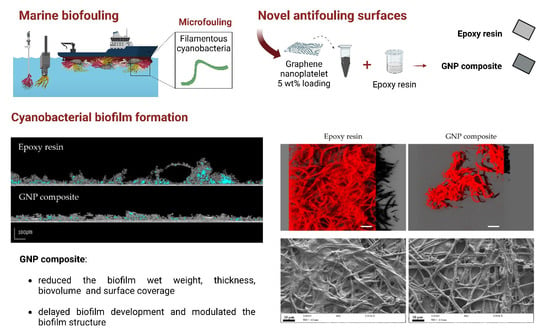
How do Graphene Composite Surfaces Affect the Development and Structure of Marine Cyanobacterial Biofilms?
Abstract
1. Introduction
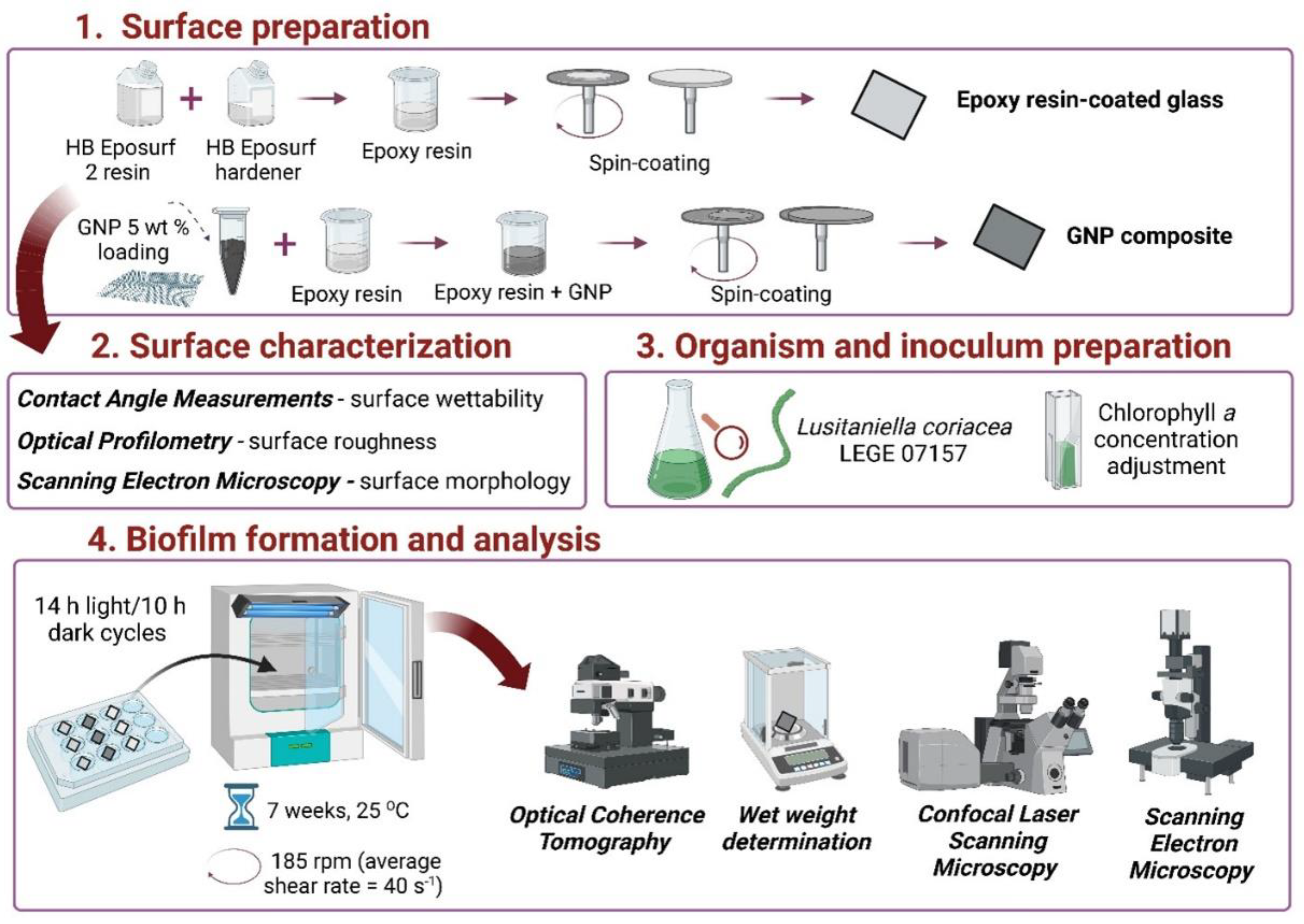
2. Materials and Methods
2.1. Surface Preparation
2.2. Surface Characterization
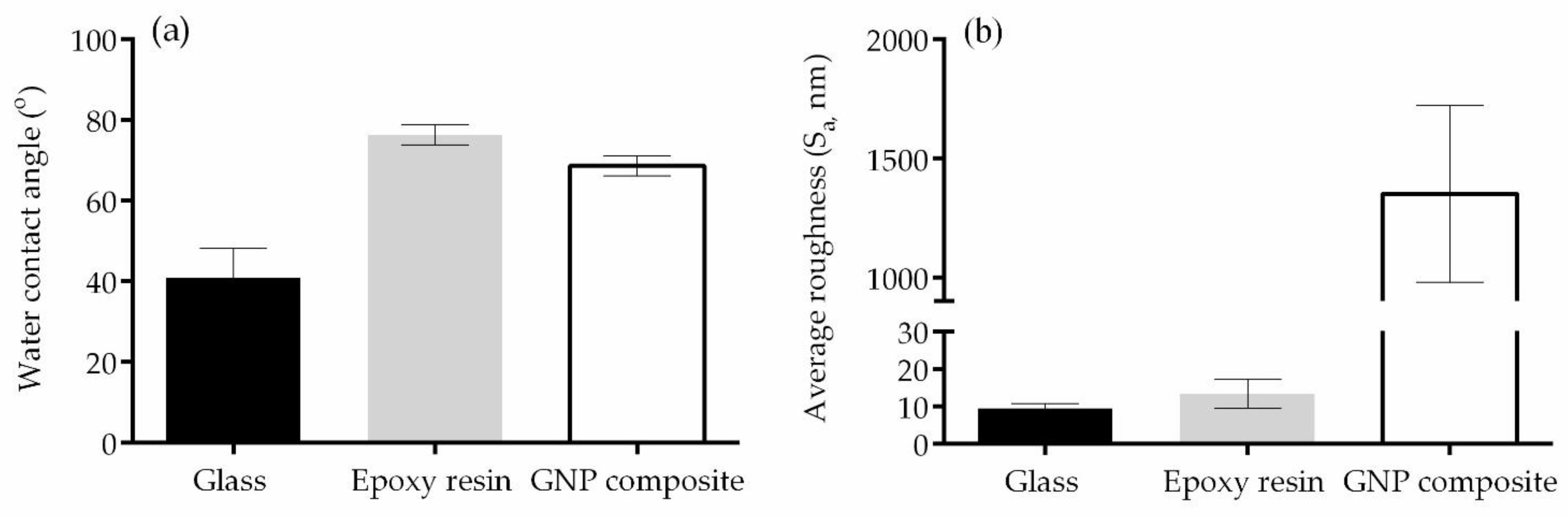
2.2.1. Wettability
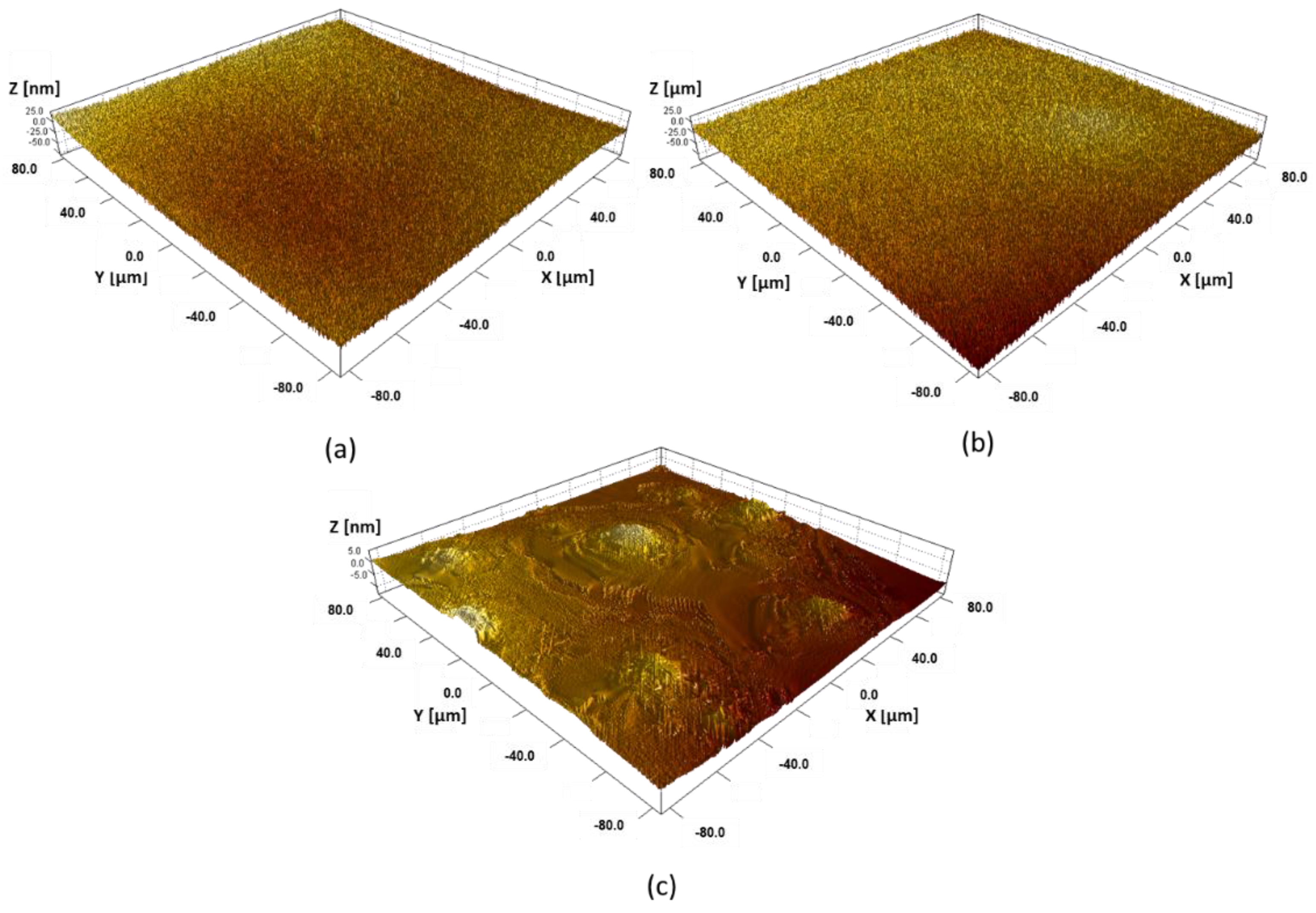
2.2.2. Optical Profilometry
2.2.3. Scanning Electron Microscopy (SEM)
2.3. Organism and Inoculum Preparation
2.4. Biofilm Formation
2.5. Biofilm Analysis
2.5.1. Optical Coherence Tomography (OCT)
2.5.2. Wet Weight Determination
2.5.3. Confocal Laser Scanning Microscopy (CLSM)
2.5.4. SEM
2.6. Statistical Analysis
3. Results and Discussion
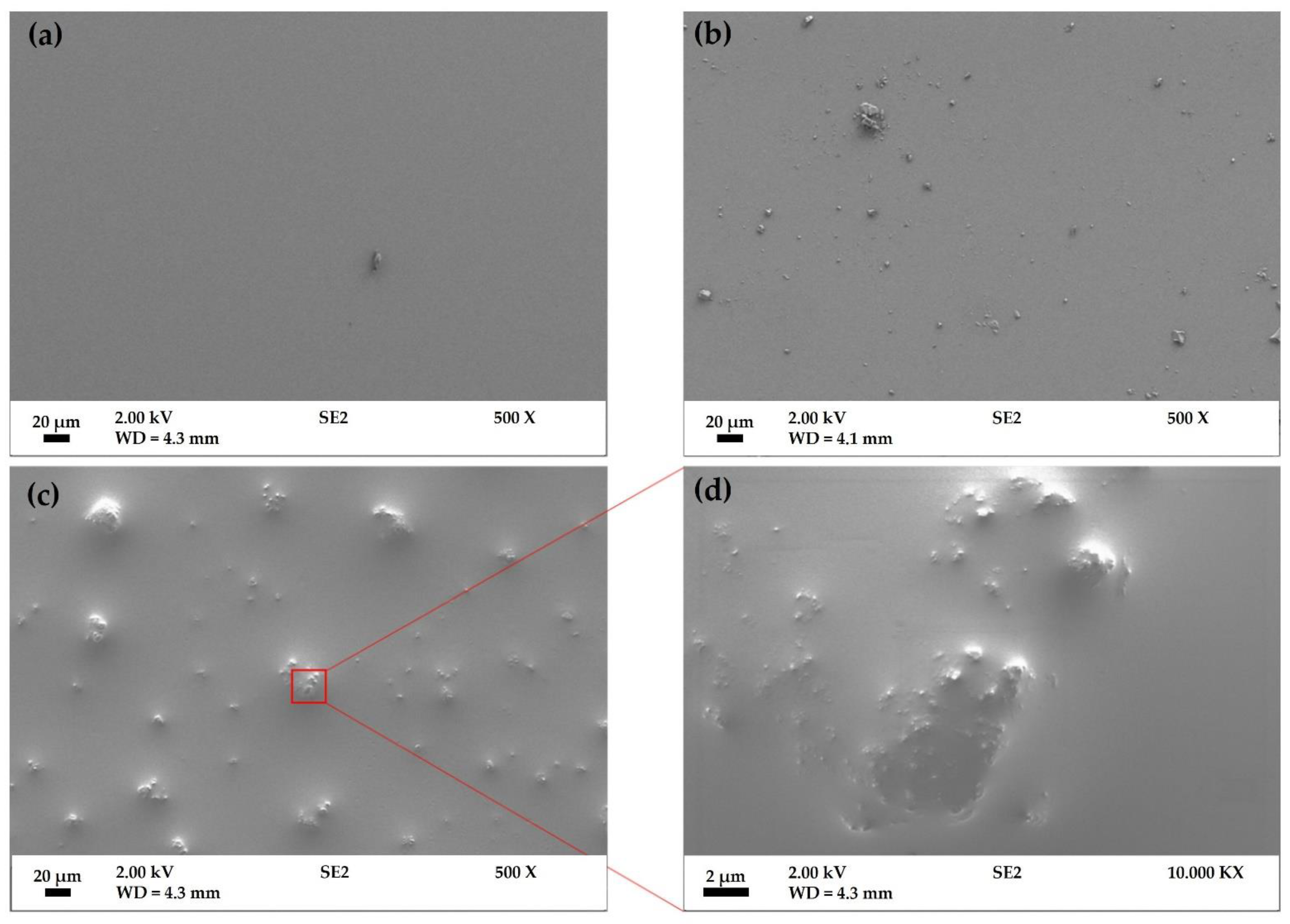
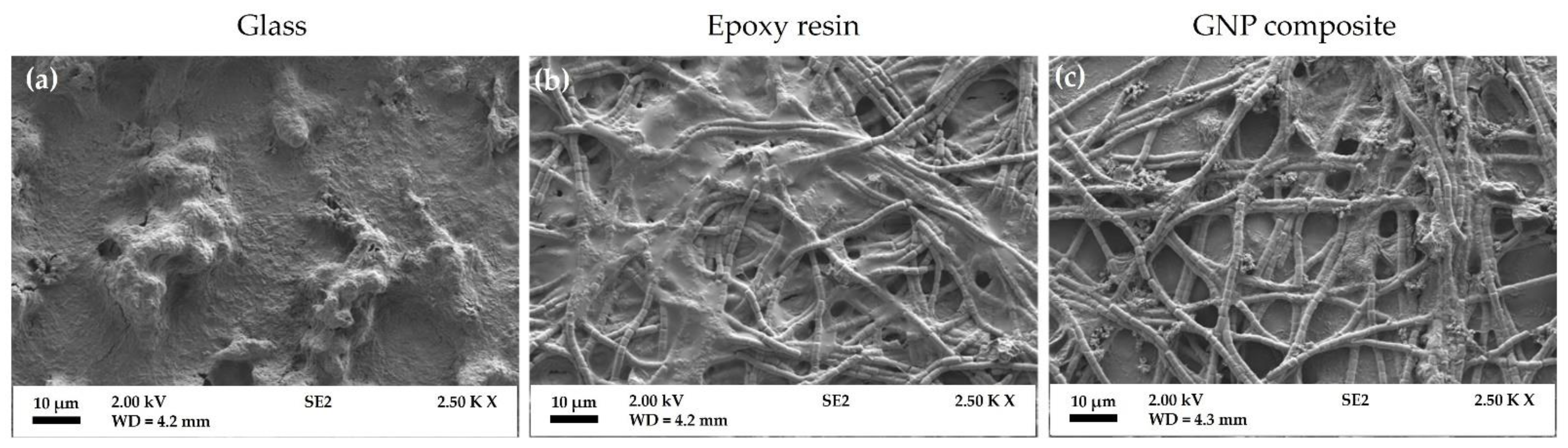
3.1. Surface Analysis
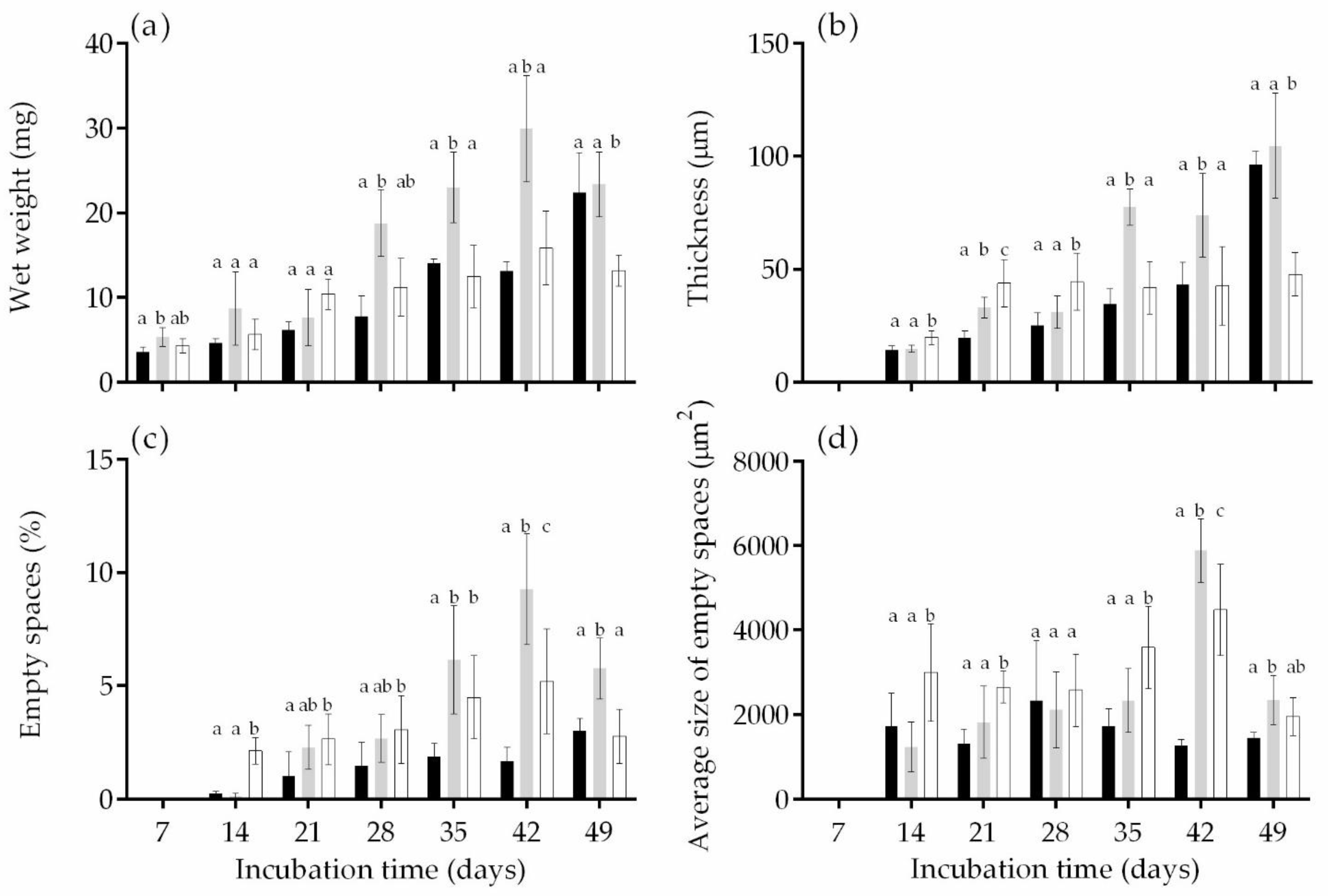
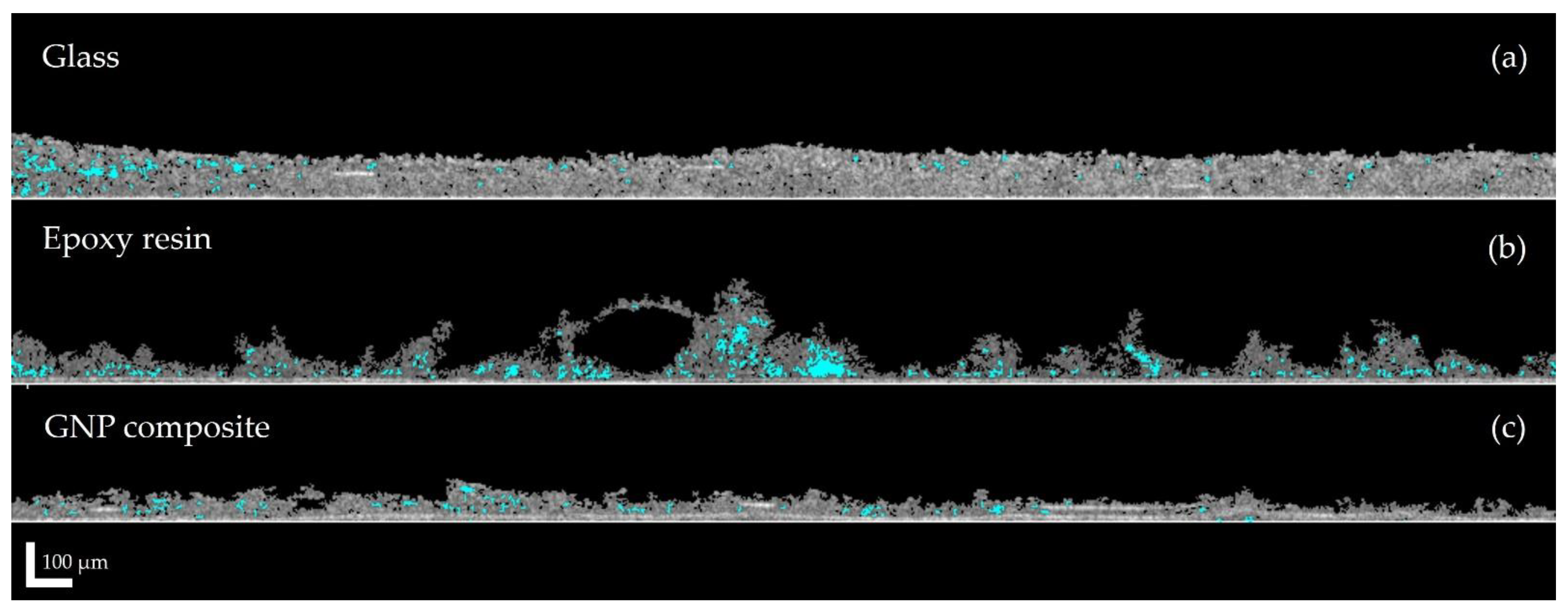
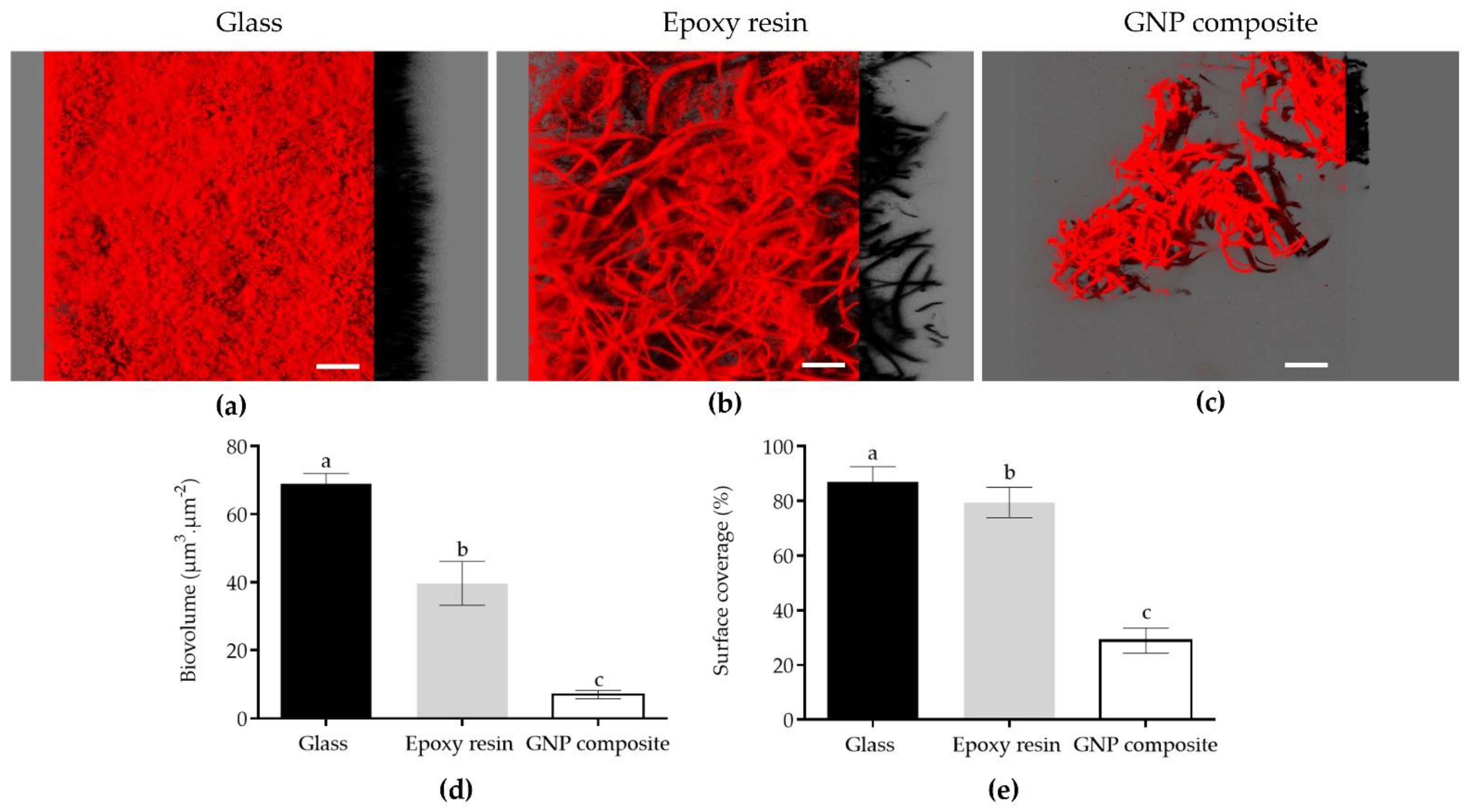
3.2. Biofilm Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Delauney, L.; Compère, C.; Lehaitre, M. Biofouling protection for marine environmental sensors. Ocean Sci. 2010, 6, 503–511. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.; Sievers, M.; Bush, F.; Bloecher, N. Biofouling in marine aquaculture: A review of recent research and developments. Biofouling 2019, 35, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhattacharya, W.; Singh, M.; Halder, D.; Mitra, A. Plant latex capped colloidal silver nanoparticles: A potent anti-biofilm and fungicidal formulation. J. Mol. Liq. 2017, 230, 705–713. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Wu, G.; Yang, Z.; Cui, Y.; Li, H.; Zhang, Y. Fluorinated carbon nanotube superamphiphobic coating for high-efficiency and long-lasting underwater antibiofouling surfaces. ACS Appl. Bio Mater. 2021, 4, 6351–6360. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, T.; Bing, W.; Dong, S.; Tian, L. Antifouling performance and mechanism of elastic graphene-silicone rubber composite membranes. J. Mater. Chem. B 2018, 7, 488–497. [Google Scholar] [CrossRef]
- Kumar, S.; Boro, J.C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 2019, 5, e01867. [Google Scholar] [CrossRef]
- Kim, J.; Baek, Y.; Hong, S.P.; Yoon, H.; Kim, S.; Kim, C.; Kim, J.; Yoon, J. Evaluation of thin-film nanocomposite RO membranes using TiO2 nanotubes and TiO2 nanoparticles: A comparative study. Desalin. Water Treat. 2016, 57, 24674–24681. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the antimicrobial properties of carbon nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-doped graphene: Synthesis, characterizations and energy applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Henríquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing bacterial interaction with copper surfaces through graphene and hexagonal-boron nitride coatings. ACS Appl. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef]
- Naddaf, A.; Heris, S.Z.; Pouladi, B. An experimental study on heat transfer performance and pressure drop of nanofluids using graphene and multi-walled carbon nanotubes based on diesel oil. Powder Technol. 2019, 352, 369–380. [Google Scholar] [CrossRef]
- Smaradhana, D.F.; Prabowo, A.R.; Ganda, A.N.F. Exploring the potential of graphene materials in marine and shipping industries—A technical review for prospective application on ship operation and material-structure aspects. J. Ocean Eng. Sci. 2021, 6, 299–316. [Google Scholar] [CrossRef]
- Pan, L.; Liu, S.; Oderinde, O.; Li, K.; Yao, F.; Fu, G. Facile fabrication of graphene-based aerogel with rare earth metal oxide for water purification. Appl. Surf. Sci. 2018, 427, 779–786. [Google Scholar] [CrossRef]
- Hu, M.; Cui, Z.; Li, J.; Zhang, L.; Mo, Y.; Dlamini, D.S.; Wang, H.; He, B.; Li, J.; Matsuyama, H. Ultra-low graphene oxide loading for water permeability, antifouling and antibacterial improvement of polyethersulfone/sulfonated polysulfone ultrafiltration membranes. J. Colloid Interface Sci. 2019, 552, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Balaure, P.C. Special Issue: Advances in engineered nanostructured antibacterial surfaces and coatings. Coatings 2022, 12, 1041. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A. Silicone/graphene oxide sheet-alumina nanorod ternary composite for superhydrophobic antifouling coating. Prog. Org. Coatings 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Selim, M.S.; Fatthallah, N.A.; Higazy, S.A.; Hao, Z.; Mo, P.J. A comparative study between two novel silicone/graphene-based nanostructured surfaces for maritime antifouling. J. Colloid Interface Sci. 2022, 606, 367–383. [Google Scholar] [CrossRef]
- Gu, J.; Li, L.; Huang, D.; Jiang, L.; Liu, L.; Li, F.; Pang, A.; Guo, X.; Tao, B. In situ synthesis of graphene@cuprous oxide nanocomposite incorporated marine antifouling coating with elevated antifouling performance. Open J. Org. Polym. Mater. 2019, 9, 47–62. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Wang, F.; Liang, W.; Yang, H.; Wu, D. Fabrication of acrylic acid modified graphene oxide (AGO)/acrylate composites and their synergistic mechanisms of anticorrosion and antifouling properties. Prog. Org. Coatings 2022, 168, 106910. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, R.; Song, D.; Yu, J.; Sun, G.; Liu, Q.; Han, S.; Liu, J.; Zhang, H.; Wang, J. Guanidine-functionalized graphene to improve the antifouling performance of boron acrylate polymer. Prog. Org. Coatings 2021, 159, 106396. [Google Scholar] [CrossRef]
- Jin, H.; Bing, W.; Tian, L.; Wang, P.; Zhao, J. Combined effects of color and elastic modulus on antifouling performance: A study of graphene oxide/silicone rubber composite membranes. Materials 2019, 12, 2608. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, S.; Li, Q.; Wang, J.; Pu, J.; Wang, G.; Zhao, W.; Feng, F.; Qin, J.; Ren, L. Integrated dual-functional ormosil coatings with AgNPs@rGO nanocomposite for corrosion resistance and antifouling applications. ACS Sustain. Chem. Eng. 2020, 8, 6786–6797. [Google Scholar] [CrossRef]
- Zhang, X.; Mikkelsen, Ø. Graphene oxide/silver nanocomposites as antifouling coating on sensor housing materials. J. Clust. Sci. 2021, 33, 627–635. [Google Scholar] [CrossRef]
- Sousa-Cardoso, F.; Teixeira-Santos, R.; Mergulhão, F.J.M. Antifouling performance of carbon-based coatings for marine applications: A systematic review. Antibiotics 2022, 11, 1102. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Khiew, P.-S.; Chiu, W.S.; Tan, Y.F.; Kok, Y.-Y.; Leong, C.-O. Green synthesis of graphene-silver nanocomposites and its application as a potent marine antifouling agent. Colloids Surf. B Biointerfaces 2016, 148, 392–401. [Google Scholar] [CrossRef]
- Jiang, T.; Qi, L.; Qin, W. Improving the environmental compatibility of marine sensors by surface functionalization with graphene oxide. Anal. Chem. 2019, 91, 13268–13274. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Dorta, F.; Jimenez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. A nanomolecular approach to decrease adhesion of biofouling-producing bacteria to graphene-coated material. J. Nanobiotechnol. 2015, 13, 82. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Jeyasubramanian, K.; Premanathan, M.; Subbiah, G.; Shin, H.S.; Kim, S.-J. Graphene oxide nanopaint. Carbon N. Y. 2014, 72, 328–337. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Jena, G.; George, R.P.; Philip, J. Polydimethylsiloxane-graphene oxide nanocomposite coatings with improved anti-corrosion and anti-biofouling properties. Environ. Sci. Pollut. Res. 2020, 28, 7404–7422. [Google Scholar] [CrossRef]
- Manderfeld, E.; Kleinberg, M.N.; Thamaraiselvan, C.; Koschitzki, F.; Gnutt, P.; Plumere, N.; Arnusch, C.J.; Rosenhahn, A. Electrochemically activated laser-induced graphene coatings against marine biofouling. Appl. Surf. Sci. 2021, 569, 150853. [Google Scholar] [CrossRef]
- Fazli-Shokouhi, S.; Nasirpouri, F.; Khatamian, M. Epoxy-matrix polyaniline/p-phenylenediamine-functionalised graphene oxide coatings with dual anti-corrosion and anti-fouling performance. RSC Adv. 2021, 11, 11627–11641. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef]
- Mostafaei, A.; Nasirpouri, F. Preparation and characterization of a novel conducting nanocomposite blended with epoxy coating for antifouling and antibacterial applications. J. Coatings Technol. Res. 2013, 10, 679–694. [Google Scholar] [CrossRef]
- Hoge, J.; Leach, C. Epoxy resin infused boat hulls. Reinf. Plast. 2016, 60, 221–223. [Google Scholar] [CrossRef]
- Faria, S.I.; Teixeira-Santos, R.; Gomes, L.C.; Silva, E.R.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J.M. Experimental assessment of the performance of two marine coatings to curb biofilm formation of microfoulers. Coatings 2020, 10, 893. [Google Scholar] [CrossRef]
- Oliveira, I.M.; Gomes, M.; Gomes, L.C.; Pereira, M.F.R.; Soares, O.S.G.P.; Mergulhão, F.J. Performance of graphene/polydimethylsiloxane surfaces against S. aureus and P. aeruginosa single- and dual-species biofilms. Nanomaterials 2022, 12, 355. [Google Scholar] [CrossRef]
- Faria, S.I.; Teixeira-Santos, R.; Romeu, M.J.; Morais, J.; Vasconcelos, V.; Mergulhão, F.J. The relative importance of shear forces and surface hydrophobicity on biofilm formation by coccoid cyanobacteria. Polymers 2020, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.J.; Lima, M.; Gomes, L.C.; de Jong, E.D.; Vasconcelos, V.; Pereira, M.F.R.; Soares, O.S.G.P.; Sjollema, J.; Mergulhão, F.J. The use of 3D optical coherence tomography to analyze the architecture of cyanobacterial biofilms formed on a carbon nanotube composite. Polymers 2022, 14, 4410. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.; Kelly, P.; Li, H.; Verran, J. Surface topography and physicochemistry of silver containing titanium nitride nanocomposite coatings. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2010, 28, 180. [Google Scholar] [CrossRef]
- Skovager, A.; Whitehead, K.; Siegumfeldt, H.; Ingmer, H.; Verran, J.; Arneborg, N. Influence of flow direction and flow rate on the initial adhesion of seven Listeria monocytogenes strains to fine polished stainless steel. Int. J. Food Microbiol. 2012, 157, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial diversity held in microbial biological resource centers as a biotechnological asset: The case study of the newly established LEGE culture collection. J. Appl. Phycol. 2018, 30, 1437–1451. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for the Preparation of Modified Nutrient Solution Z8 for Algae. Nor. Inst. Water Res. 1972, 11, 5. [Google Scholar]
- Romeu, M.J.; Alves, P.; Morais, J.; Miranda, J.M.; de Jong, E.D.; Sjollema, J.; Ramos, V.; Vasconcelos, V.; Mergulhão, F.J.M. Biofilm formation behaviour of marine filamentous cyanobacterial strains in controlled hydrodynamic conditions. Environ. Microbiol. 2019, 21, 4411–4424. [Google Scholar] [CrossRef]
- Boyer, J.N.; Kelble, C.R.; Ortner, P.B.; Rudnick, D.T. Phytoplankton bloom status: Chlorophyll a biomass as an indicator of water quality condition in the southern estuaries of Florida, USA. Ecol. Indic. 2009, 9, S56–S67. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Bakker, D.P.; Van der Plaats, A.; Verkerke, G.J.; Busscher, H.J.; Mei, H.C. Van der comparison of velocity profiles for different flow chamber designs used in studies of microbial adhesion to surfaces. Appl. Environ. Microbiol. 2003, 69, 6280–6287. [Google Scholar] [CrossRef]
- Silva, E.R.; Tulcidas, A.V.; Ferreira, O.; Bayón, R.; Igartua, A.; Mendoza, G.; Mergulhão, F.J.; Faria, S.I.; Gomes, L.C.; Carvalho, S.; et al. Assessment of the environmental compatibility and antifouling performance of an innovative biocidal and foul-release multifunctional marine coating. Environ. Res. 2021, 198, 111219. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.J.; Domínguez-Pérez, D.; Almeida, D.; Morais, J.; Araújo, M.J.; Osório, H.; Campos, A.; Vasconcelos, V.; Mergulhão, F.J. Hydrodynamic conditions affect the proteomic profile of marine biofilms formed by filamentous cyanobacterium. NPJ Biofilms Microbiomes 2022, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.; Gomes, L.C.; Teixeira-Santos, R.; Morais, J.; Vasconcelos, V.; Mergulhão, F. Developing new marine antifouling surfaces: Learning from single-strain laboratory tests. Coatings 2021, 11, 90. [Google Scholar] [CrossRef]
- Romeu, M.J.L.; Domínguez-Pérez, D.; Almeida, D.; Morais, J.; Campos, A.; Vasconcelos, V.; Mergulhão, F.J.M. Characterization of planktonic and biofilm cells from two filamentous cyanobacteria using a shotgun proteomic approach. Biofouling 2020, 36, 631–645. [Google Scholar] [CrossRef]
- Faria, S.; Teixeira-Santos, R.; Romeu, M.J.; Morais, J.; Jong, E.; Sjollema, J.; Vasconcelos, V.; Mergulhão, F.J. Unveiling the antifouling performance of different marine surfaces and their effect on the development and structure of cyanobacterial biofilms. Microorganisms 2021, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.J.; Domínguez-Pérez, D.; Almeida, D.; Morais, J.; Araújo, M.J.; Osório, H.; Campos, A.; Vasconcelos, V.; Mergulhão, F. Quantitative proteomic analysis of marine biofilms formed by filamentous cyanobacterium. Environ. Res. 2021, 201, 111566. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Alves, P.; Gomes, L.C.; Rodríguez-Emmenegger, C.; Mergulhão, F.J. Efficacy of a poly (MeOEGMA) brush on the prevention of Escherichia coli biofilm formation and susceptibility. Antibiotics 2020, 9, 216. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. In Surface Science Techniques; Springer Series in surface sciences 51; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. ISBN 978-3-642-34242-4. [Google Scholar]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Rafiee, J.; Mi, X.; Gullapalli, H.; Thomas, A.V.; Yavari, F.; Shi, Y.; Ajayan, P.M.; Koratkar, N.A. Wetting transparency of graphene. Nat. Mater. 2012, 11, 217–222. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nüesch, F.A.; Chu, B.T.T. Comparing carbon nanotubes and graphene nanoplatelets as reinforcements in polyamide 12 composites. Nanotechnology 2011, 22, 275714. [Google Scholar] [CrossRef]
- Santos, C.M.; Mangadlao, J.; Ahmed, F.; Leon, A.; Advincula, R.C.; Rodrigues, D. Graphene nanocomposite for biomedical applications: Fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 2012, 23, 395101. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial mechanisms and effectiveness of graphene and graphene-functionalized biomaterials. A scope review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Staneva, A.D.; Dimitrov, D.K.; Gospodinova, D.N.; Vladkova, T.G. Antibiofouling activity of graphene materials and graphene-based antimicrobial coatings. Microorganisms 2021, 9, 1839. [Google Scholar] [CrossRef] [PubMed]
- Sjollema, J.; Rustema-Abbing, M.; van der Mei, H.C.; Busscher, H.J. Generalized relationship between numbers of bacteria and their viability in biofilms. Appl. Environ. Microbiol. 2011, 77, 5027–5029. [Google Scholar] [CrossRef]
- Song, C.; Yang, C.-M.; Sun, X.-F.; Xia, P.-F.; Qin, J.; Guo, B.-B.; Wang, S.-G. Influences of graphene oxide on biofilm formation of gram-negative and gram-positive bacteria. Environ. Sci. Pollut. Res. 2017, 25, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Ameen, F.; Rizvi, A.; Ali, K.; Sonbol, H.; Zaidi, A.; Khan, M.S.; Musarrat, J. Destruction of cell topography, morphology, membrane, inhibition of respiration, biofilm formation, and bioactive molecule production by nanoparticles of Ag, ZnO, CuO, TiO2, and Al2O3 toward beneficial soil bacteria. ACS Omega 2020, 5, 7861–7876. [Google Scholar] [CrossRef]
- Fatima, N.; Qazi, U.Y.; Mansha, A.; Bhatti, I.A.; Javaid, R.; Abbas, Q.; Nadeem, N.; Rehan, Z.A.; Noreen, S.; Zahid, M. Recent developments for antimicrobial applications of graphene-based polymeric composites: A review. J. Ind. Eng. Chem. 2021, 100, 40–58. [Google Scholar] [CrossRef]
- Radhi, A.; Mohamad, D.; Rahman, F.S.A.; Abdullah, A.M.; Hasan, H. Mechanism and factors influence of graphene-based nanomaterials antimicrobial activities and application in dentistry. J. Mater. Res. Technol. 2021, 11, 1290–1307. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm viability checker: An open-source tool for automated biofilm viability analysis from confocal microscopy images. NPJ Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Van den Driessche, F.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing risk of failure from ceramic-on-ceramic total hip prosthesis by selecting ceramic materials based on tresca stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Dingreville, R.; Karnesky, R.A.; Puel, G.; Schmitt, J.-H. Synergies between computational modeling and experimental characterization of materials across length scales. J. Mater. Sci. 2015, 51, 1176–1177. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeu, M.J.; Gomes, L.C.; Sousa-Cardoso, F.; Morais, J.; Vasconcelos, V.; Whitehead, K.A.; Pereira, M.F.R.; Soares, O.S.G.P.; Mergulhão, F.J. How do Graphene Composite Surfaces Affect the Development and Structure of Marine Cyanobacterial Biofilms? Coatings 2022, 12, 1775. https://doi.org/10.3390/coatings12111775
Romeu MJ, Gomes LC, Sousa-Cardoso F, Morais J, Vasconcelos V, Whitehead KA, Pereira MFR, Soares OSGP, Mergulhão FJ. How do Graphene Composite Surfaces Affect the Development and Structure of Marine Cyanobacterial Biofilms? Coatings. 2022; 12(11):1775. https://doi.org/10.3390/coatings12111775
Chicago/Turabian StyleRomeu, Maria J., Luciana C. Gomes, Francisca Sousa-Cardoso, João Morais, Vítor Vasconcelos, Kathryn A. Whitehead, Manuel F. R. Pereira, Olívia S. G. P. Soares, and Filipe J. Mergulhão. 2022. "How do Graphene Composite Surfaces Affect the Development and Structure of Marine Cyanobacterial Biofilms?" Coatings 12, no. 11: 1775. https://doi.org/10.3390/coatings12111775
APA StyleRomeu, M. J., Gomes, L. C., Sousa-Cardoso, F., Morais, J., Vasconcelos, V., Whitehead, K. A., Pereira, M. F. R., Soares, O. S. G. P., & Mergulhão, F. J. (2022). How do Graphene Composite Surfaces Affect the Development and Structure of Marine Cyanobacterial Biofilms? Coatings, 12(11), 1775. https://doi.org/10.3390/coatings12111775