Liquid Nanofilms’ Evaporation Inside a Heat Exchanger by Mixed Convection
Abstract
1. Introduction
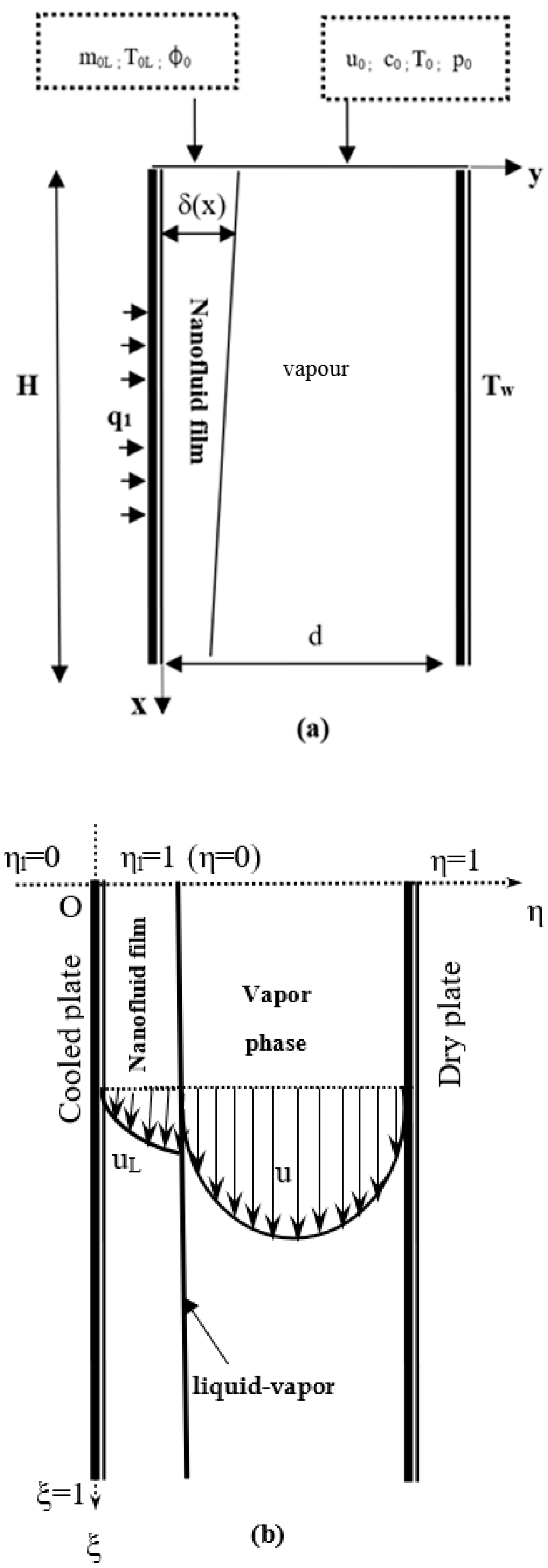
2. Numerical Model
2.1. Assumptions
- The gas is perfect;
- The nanoparticles and the liquid are in thermal equilibrium;
- Transfers and flows are two-dimensional, laminar and steady.
2.2. Basic Equations
2.2.1. For the Liquid Film
2.2.2. For the Gaseous Phase
2.3. Boundary Conditions
2.4. The Dimensionless Governing Equations
2.4.1. For the Liquid Phase
2.4.2. For the Gaseous Phase
2.4.3. Boundary Conditions
2.5. Solution Method
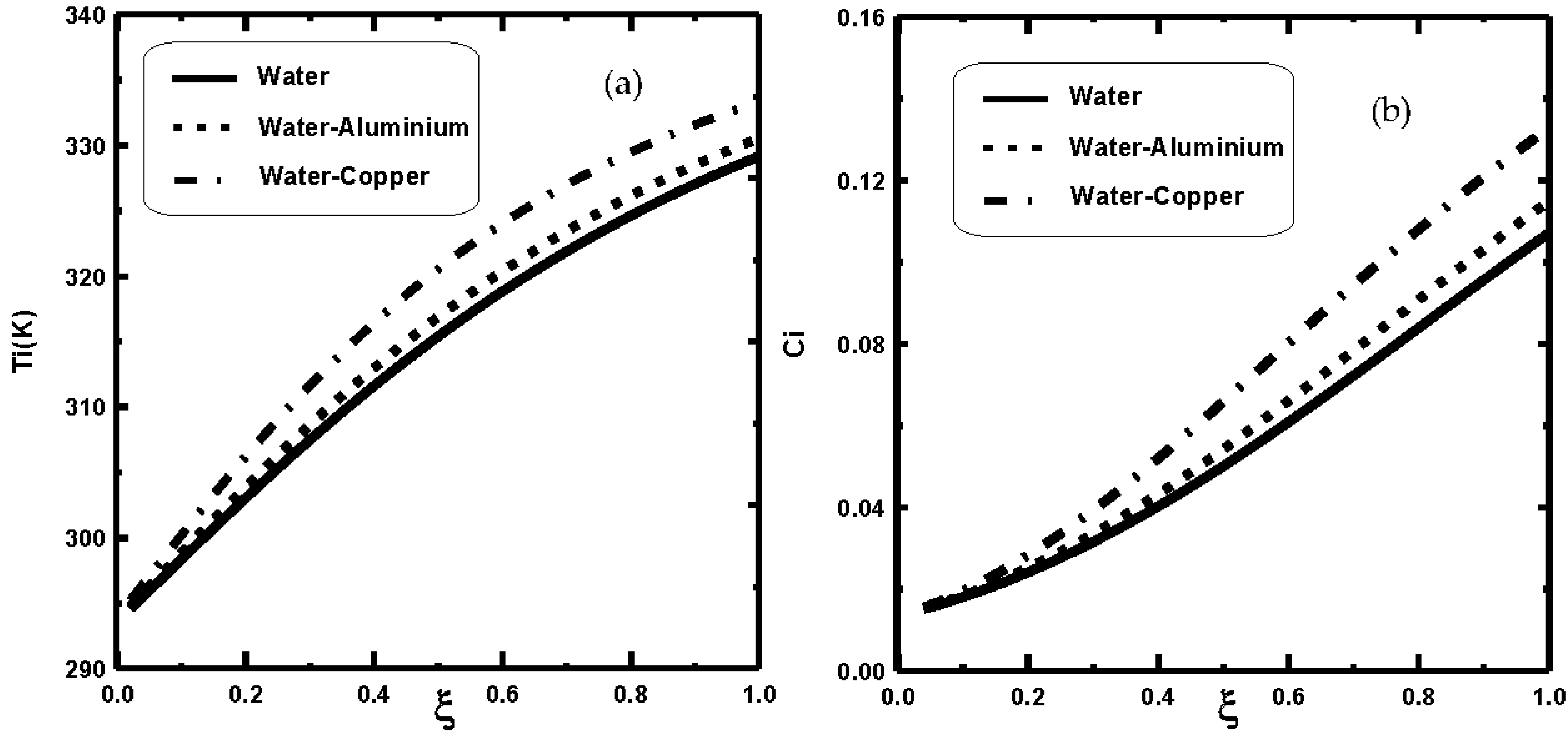
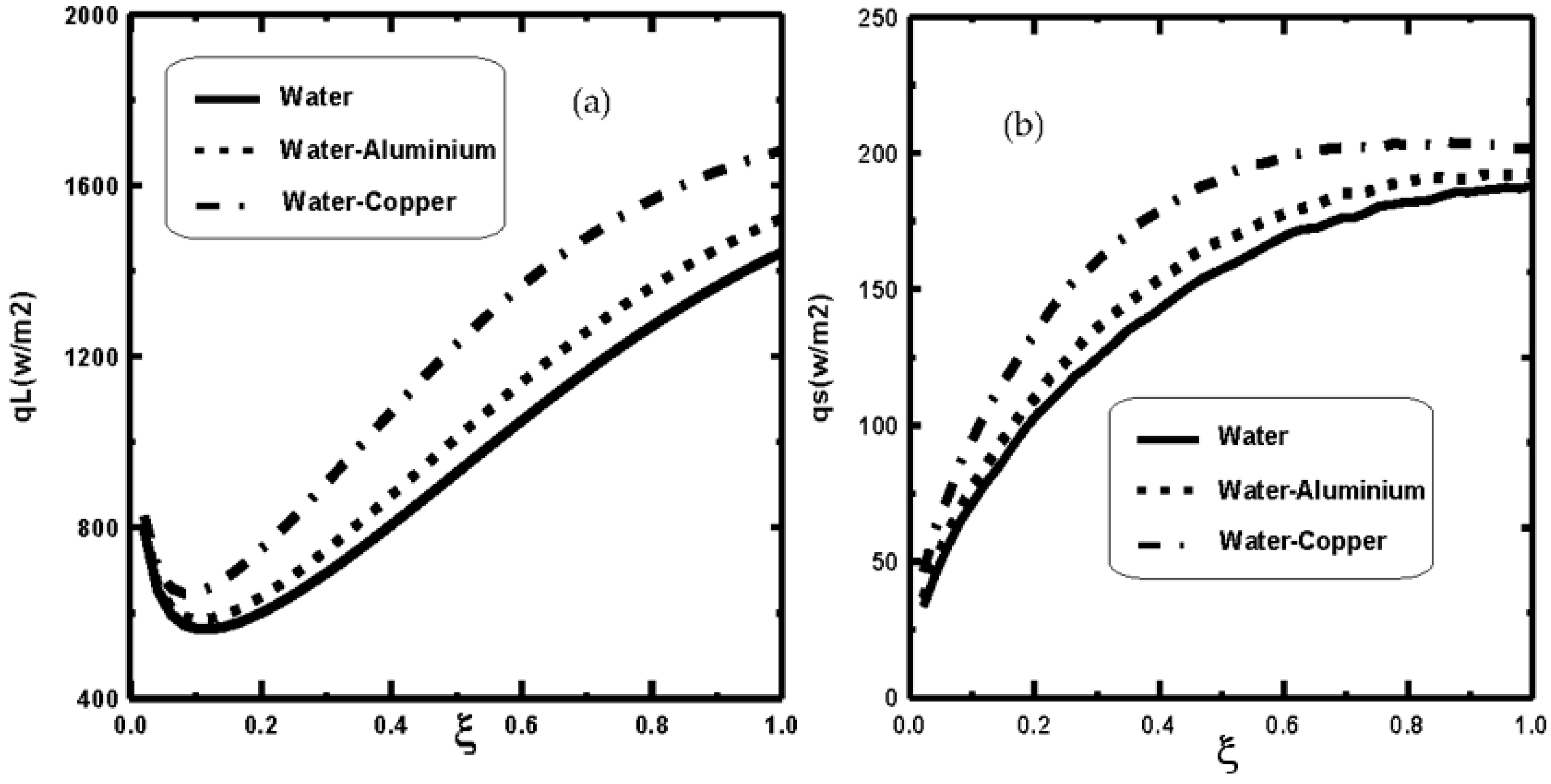
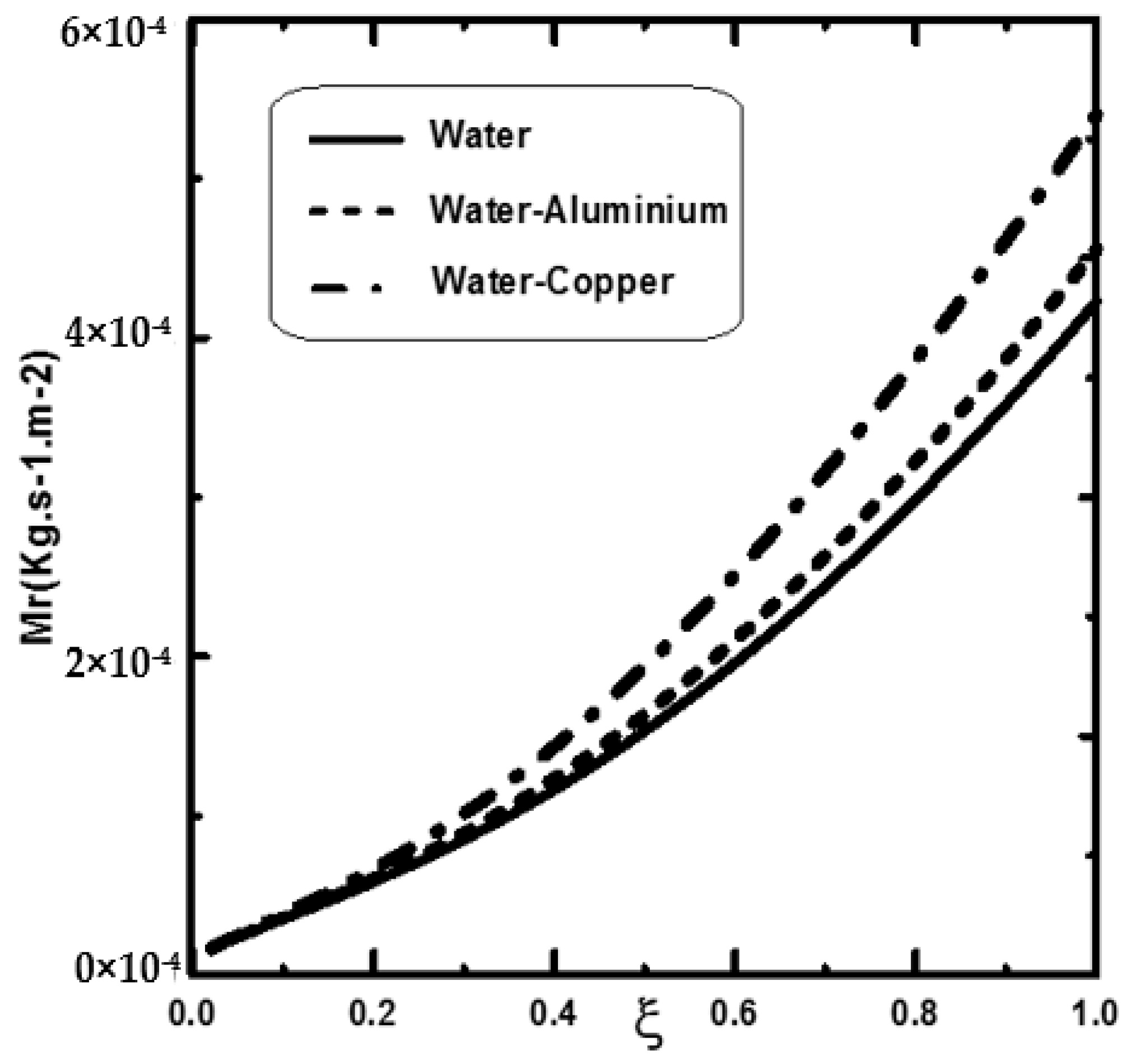
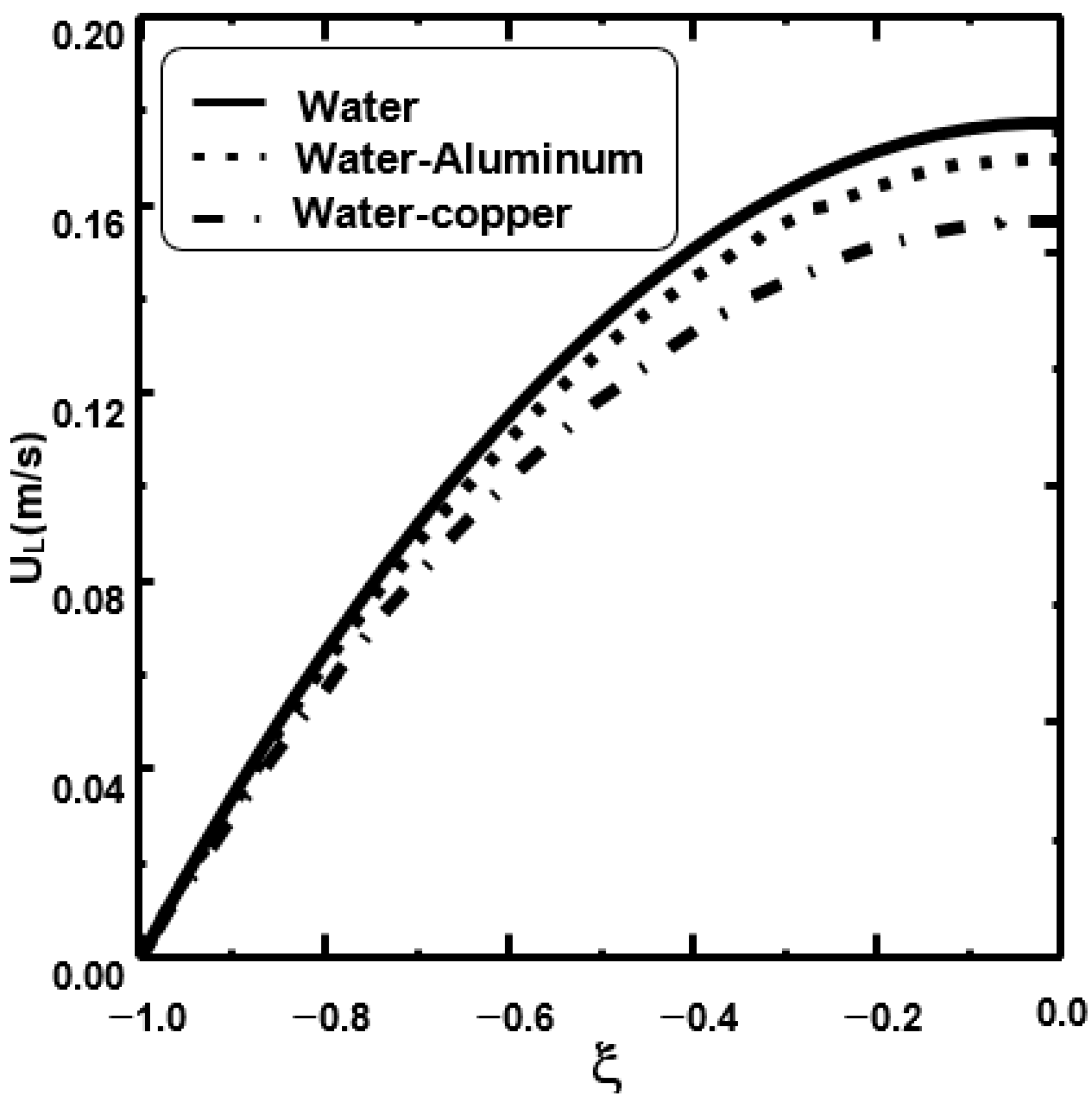
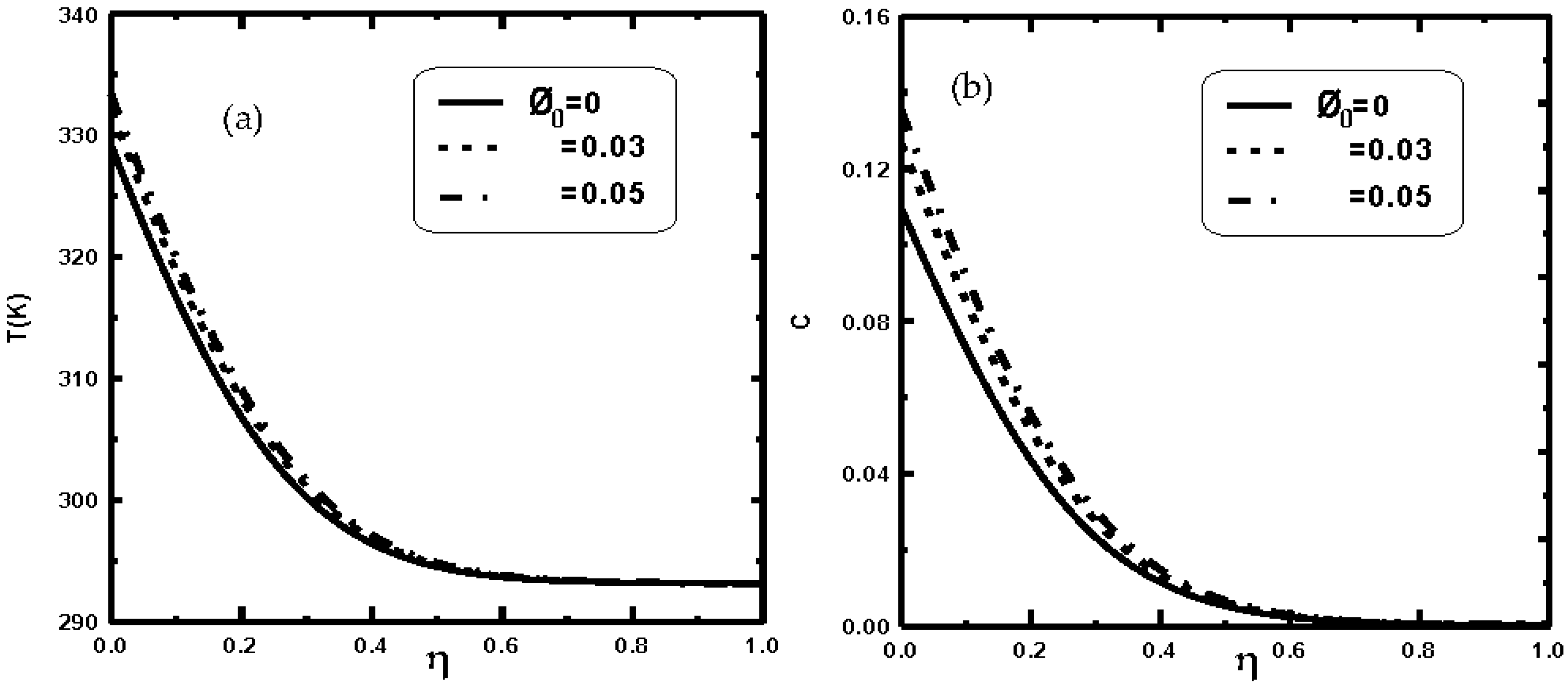
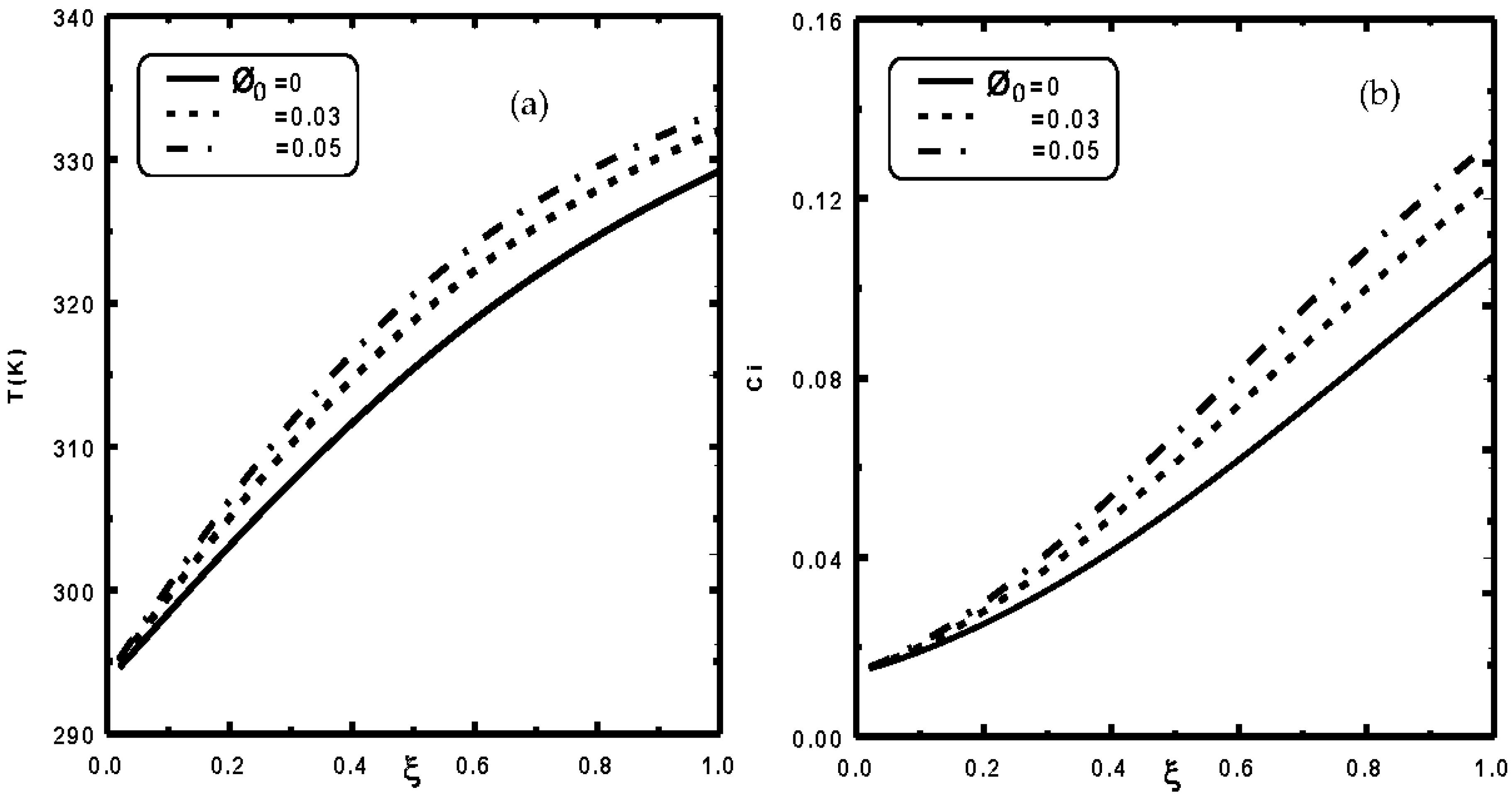
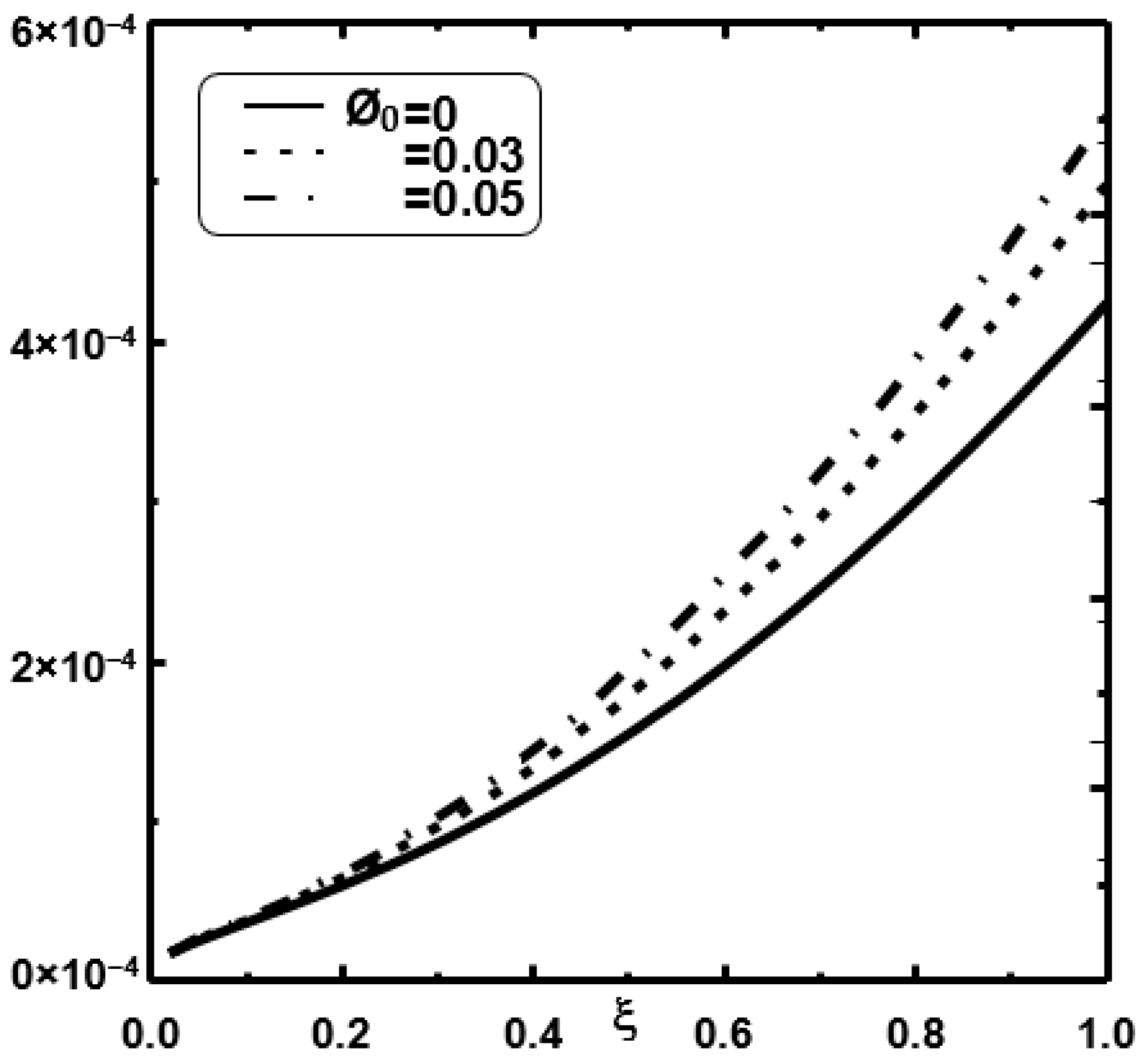
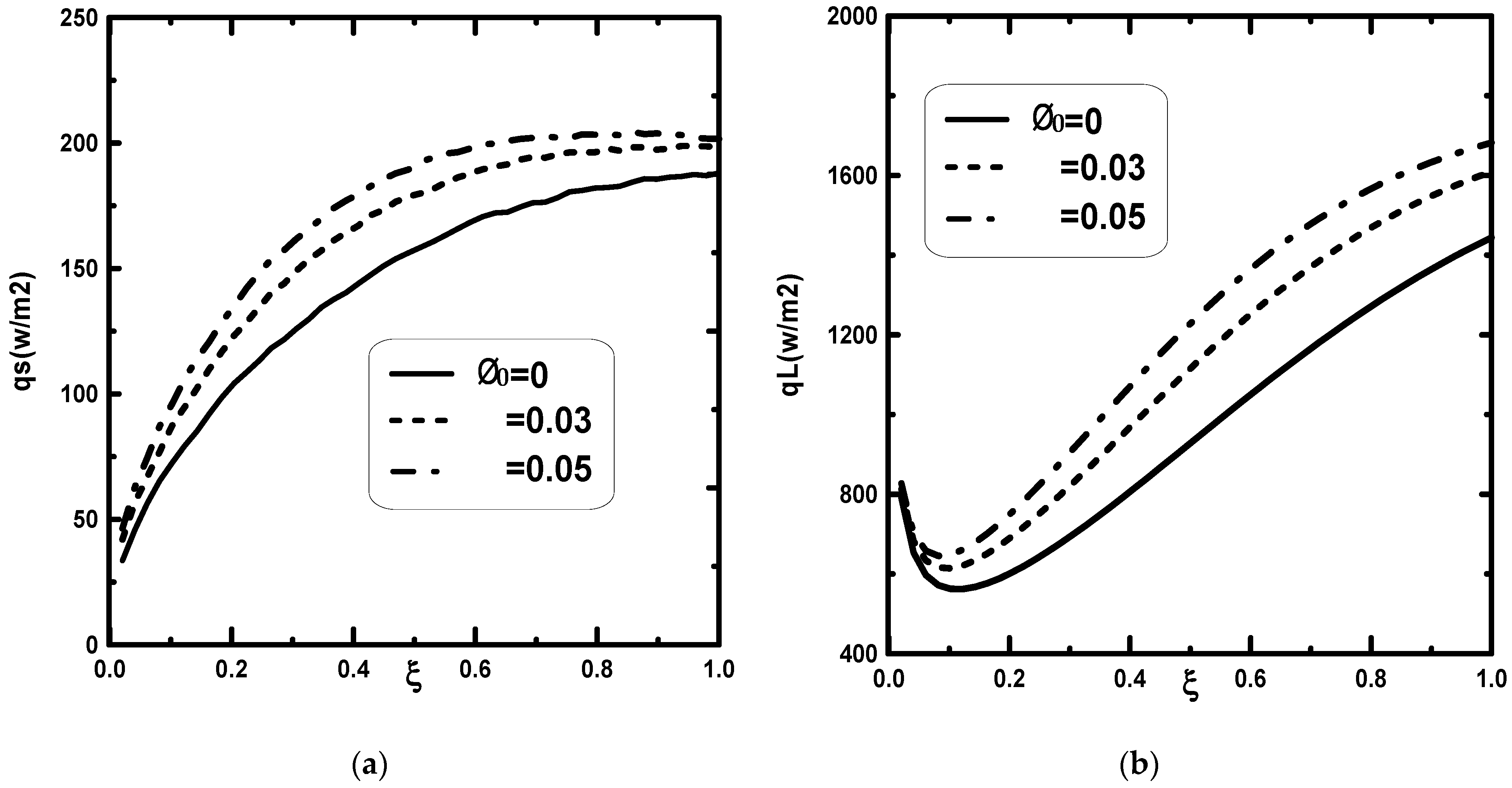
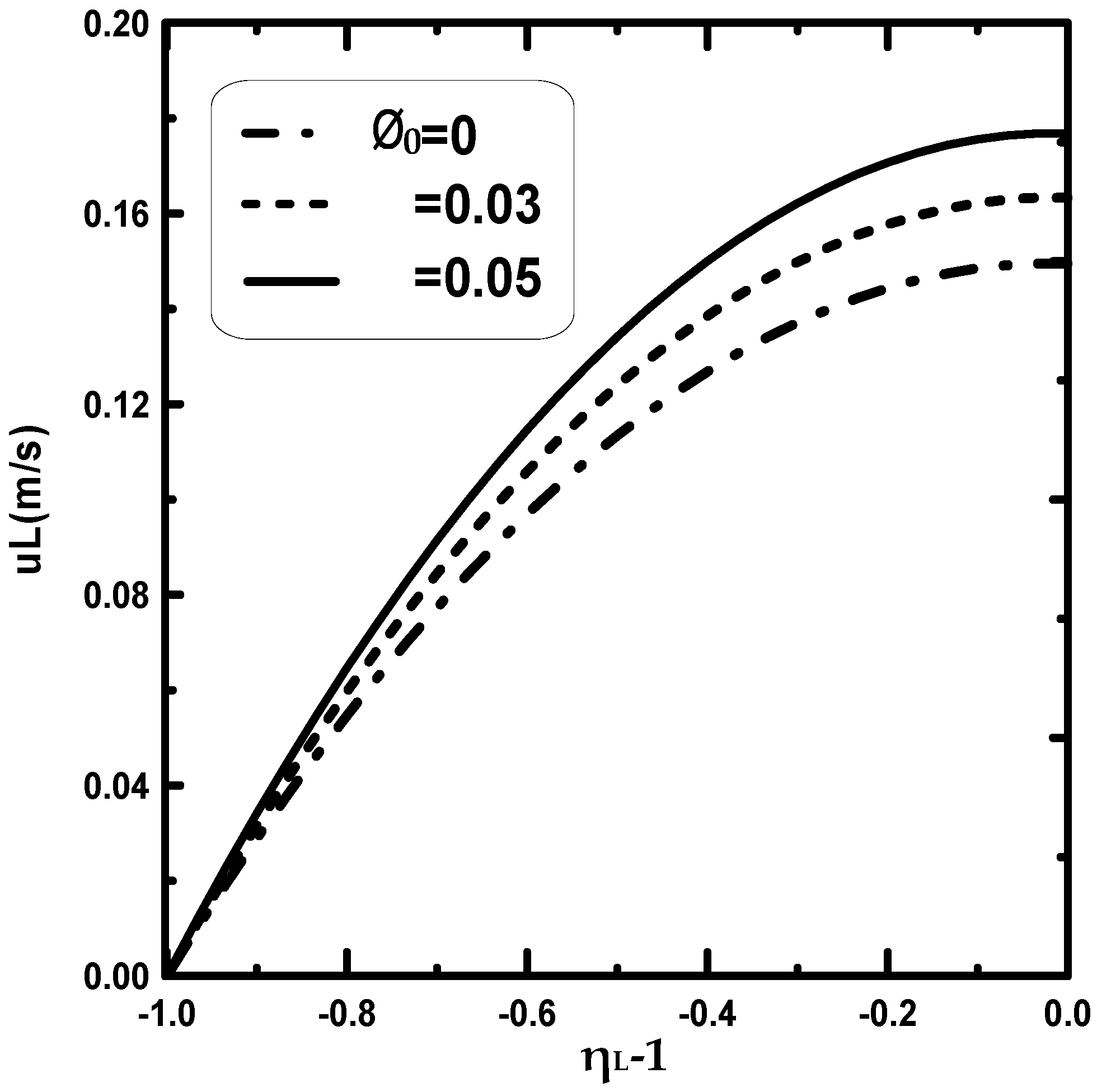
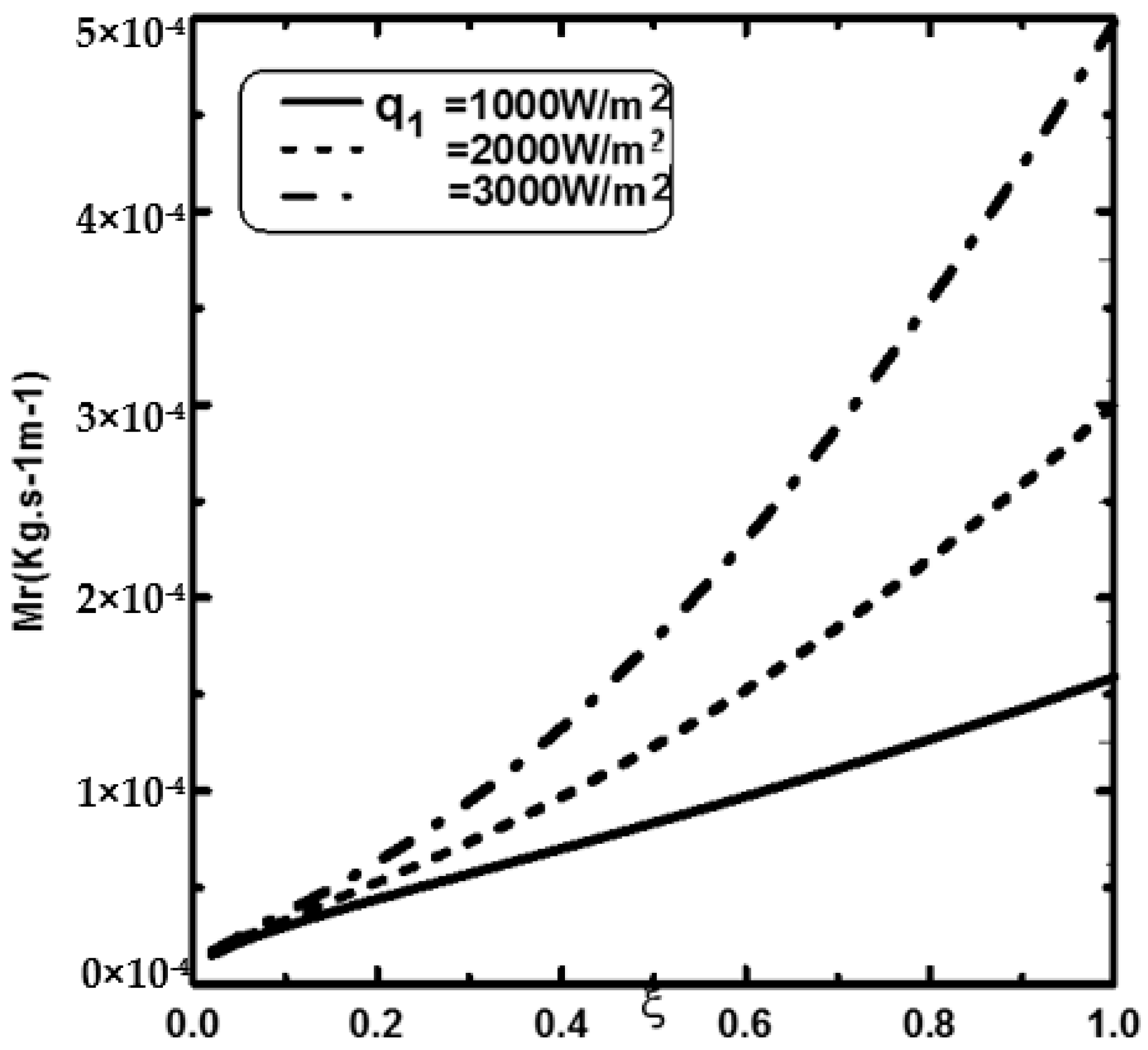
3. Results and Discussion
4. Conclusions
- (1).
- The dispersion of nanoparticles improves the mass and heat exchange during film evaporation.
- (2).
- It is shown that the dispersion of copper nanoparticles enhances liquid film evaporation more compared to aluminium nanoparticles and pure water.
- (3).
- It is shown that the liquid film evaporation rate is increased with an increase of nanoparticle volume fraction Φ0.
- (4).
- It is shown that an enhancement of 22% in evaporation rate has been recorded when the nanoparticle volume fraction Φ0 of copper is elevated by 5%.
- (5).
- It is observed that an increase in the inlet gas ameliorates the water film evaporation.
- (6).
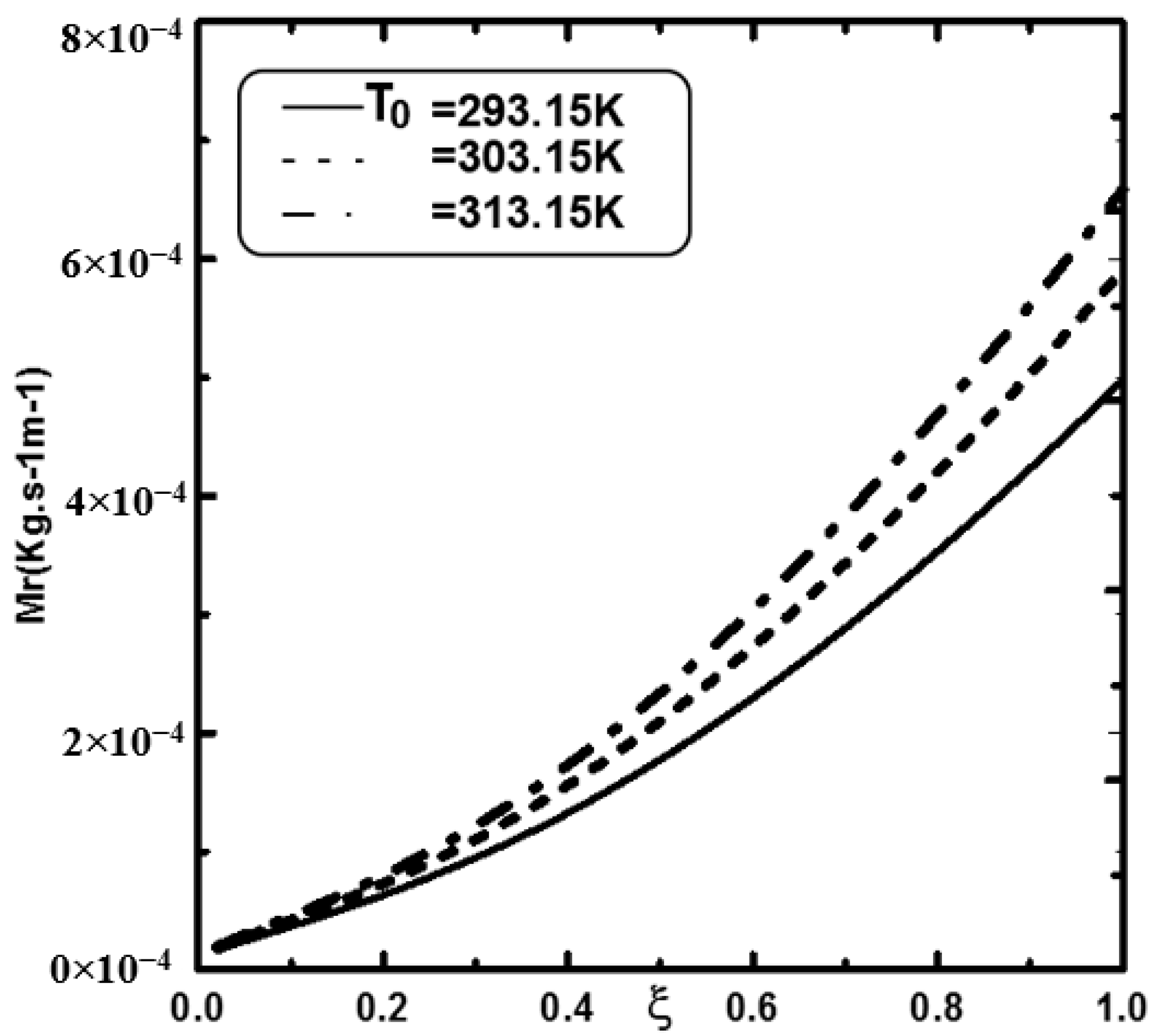
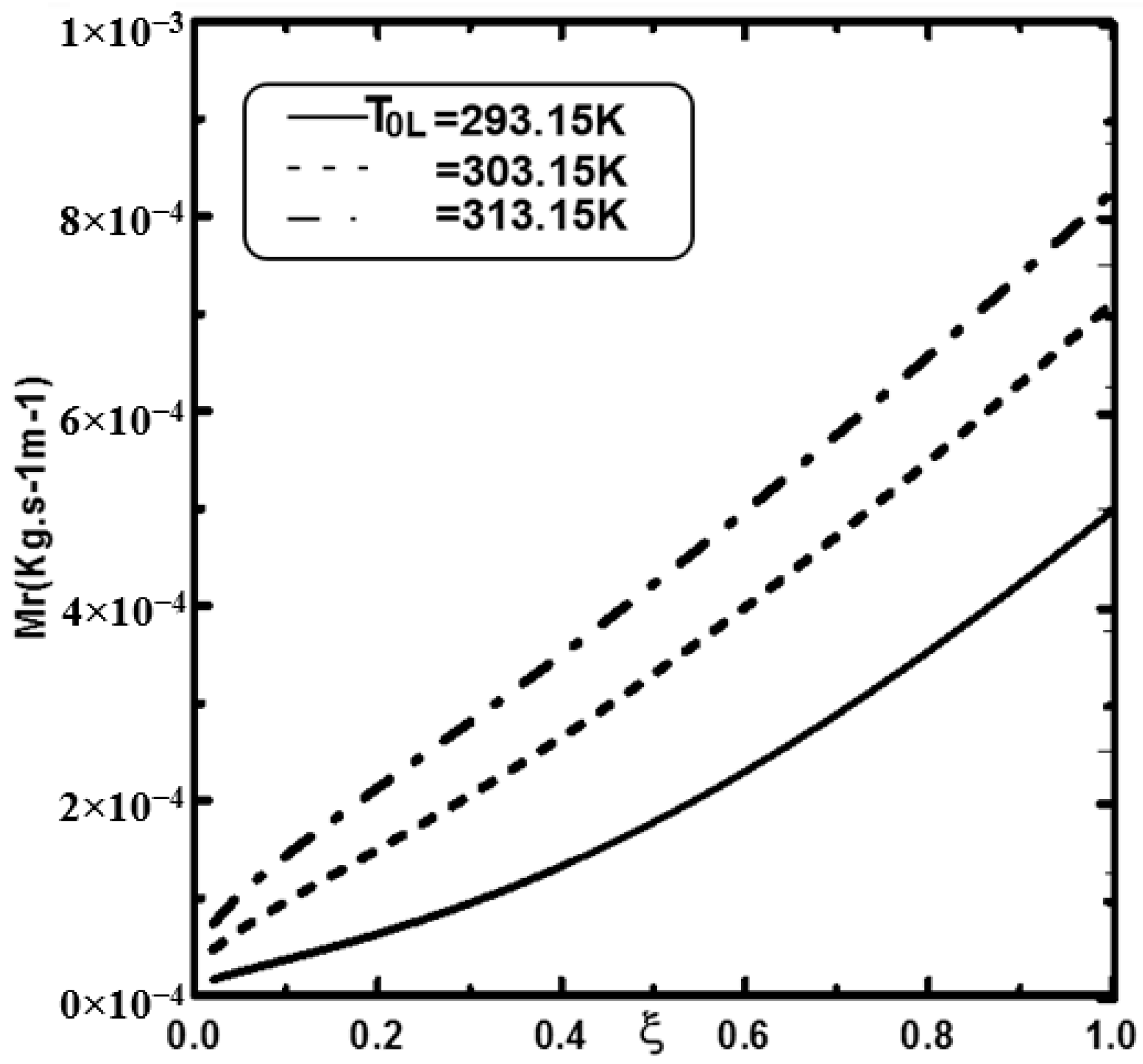
- An increase in the inlet liquid temperature improves the water film evaporation.
- (7).
- An increase in the supplied heat flux enhances the water film evaporation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Nomenclature | |
| c | mass fraction for water vapour |
| D | mass diffusivity [m2·s−1] |
| P | dynamic pressure in the channel [N·m−2] |
| T | absolute temperature [K] |
| u | axial velocity [m·s−1] |
| v | transverse velocity [m·s−1] |
| x | coordinate in the axial direction [m] |
| y | coordinate in the transverse direction [m] |
| Greek symbols | |
| φ | volume fraction of nanoparticles |
| λ | thermal conductivity of the fluid [W·m−1·K−1] |
| μ | dynamic viscosity of the fluid [kg·m−1·s−1] |
| ρ | density of the gas [kg·m−3] |
| η | dimensionless coordinate in the transverse direction |
| ξ | dimensionless coordinate in the flow direction |
| Subscripts | |
| n | nanoparticle |
| p | solid particle |
| f | Nanofluid |
| L | liquid |
References
- Do, K.H.; Jang, S.P. Effect of nanofluids on the thermal performance of a flat micro heat pipe with a rectangular grooved wick. Int. J. Heat Mass Transf. 2010, 53, 2183–2192. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Duan, Y.-Y.; Wang, X.-D.; Wang, B.-X. Effect of nanofluids on thin film evaporation in microchannels. J. Nanoparticle Res. 2011, 13, 5033–5047. [Google Scholar] [CrossRef]
- Namburu, P.K.; Das, D.K.; Tanguturi, K.M.; Vajjha, R.S. Numerical study of turbulent flow and heat transfer characteristics of nanofluids considering variable properties. Int. J. Therm. Sci. 2009, 48, 290–302. [Google Scholar] [CrossRef]
- Sefiane, K.; Bennacer, R. Nanofluids droplets evaporation kinetics and wetting dynamics on rough heated substrates. Adv. Colloid Interface Sci. 2009, 147–148, 263–271. [Google Scholar] [CrossRef]
- Gorjaei, A.R.; Soltani, M.; Bahiraei, M.; Kashkooli, F.M. Cfd simulation of nanofluid forced convection inside a three-dimensional annulus by two-phase mixture approach: Heat transfer and entropy generation analyses. Int. J. Mech. Sci. 2018, 146–147, 396–404. [Google Scholar] [CrossRef]
- Asirvatham, L.G.; Vishal, N.; Gangatharan, S.K.; Lal, D.M. Experimental Study on Forced Convective Heat Transfer with Low Volume Fraction of CuO/Water Nanofluid. Energies 2009, 2, 97–119. [Google Scholar] [CrossRef]
- Chen, R.-H.; Phuoc, T.X.; Martello, D. Effects of nanoparticles on nanofluid droplet evaporation. Int. J. Heat Mass Transf. 2010, 53, 3677–3682. [Google Scholar] [CrossRef]
- Siddiqa, S.; Begum, N.; Hossain, M.; Gorla, R.S.R.; Al-Rashed, A.A. Two-phase natural convection dusty nanofluid flow. Int. J. Heat Mass Transf. 2018, 118, 66–74. [Google Scholar] [CrossRef]
- Sheremet, M.A.; Cimpean, D.S.; Pop, I. Free convection in a partially heated wavy porous cavity filled with a nanofluid under the effects of Brownian diffusion and thermophoresis. Appl. Therm. Eng. 2017, 113, 413–418. [Google Scholar] [CrossRef]
- Malvandi, A.; Heysiattalab, S.; Ganji, D. Thermophoresis and Brownian motion effects on heat transfer enhancement at film boiling of nanofluids over a vertical cylinder. J. Mol. Liq. 2016, 216, 503–509. [Google Scholar] [CrossRef]
- Orejon, D.; Sefiane, K.; Shanahan, M.E.R. Evaporation of nanofluid droplets with applied DC potential. J. Colloid Interface Sci. 2013, 407, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Orejon, D.; Sefiane, K.; Shanahan, M.E.R. Stick–Slip of Evaporating Droplets: Substrate Hydrophobicity and Nanoparticle Concentration. Langmuir 2011, 27, 12834–12843. [Google Scholar] [CrossRef] [PubMed]
- Askounis, A.; Sefiane, K.; Koutsos, V.; Shanahan, M.E.R. The effect of evaporation kinetics on nanoparticle structuring within contact line deposits of volatile drops. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 855–866. [Google Scholar] [CrossRef]
- Perrin, L.; Pajor-Swierzy, A.; Magdassi, S.; Kamyshny, A.; Ortega, F.; Rubio, R.G. Evaporation of Nanosuspensions on Substrates with Different Hydrophobicity. ACS Appl. Mater. Interfaces 2018, 10, 3082–3093. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H.; Abdulmalik, A.A.; Eltaher, M.A.; Almitani, K.H.; Alnefaie, K.A.; Abusorrah, A.M.; Safaei, M.R. Implicit Finite Difference Simulation of Prandtl-Eyring Nanofluid over a Flat Plate with Variable Thermal Conductivity: A Tiwari and Das Model. Mathematics 2021, 9, 3153. [Google Scholar] [CrossRef]
- Mashhour, A.A.; Nidal, H.A.; Goodarzi, M. Entropy Optimization of First-Grade Viscoelastic Nanofluid Flow over a Stretching Sheet by Using Classical Keller-Box Scheme. Mathematics 2021, 9, 2563–2585. [Google Scholar]
- Kam, O.R.; Bakouan, C.; Zongo, I.; Guel, B. Removal of Thallium from Aqueous Solutions by Adsorption onto Alumina Nanoparticles. Processes 2022, 10, 1826. [Google Scholar] [CrossRef]
- Yan, W.M. Effects of film evaporation on laminar mixed heat and mass transfer in a vertical channel. Int. J. Heat Mass Transf. 1992, 12, 3419–3429. [Google Scholar]
- Huang, X.G.; Yang, Y.H.; Hu, P. Experimental study of falling film evaporation in large scale rectangular channel. Ann. Nucl. Energy 2015, 76, 237–242. [Google Scholar] [CrossRef]
- Palen, J.W.; Wang, Q.; Chen, J.C. Falling film evaporation of binary mixtures. AICHE J. 1994, 40, 207–214. [Google Scholar] [CrossRef]
- Brinkman, H.C. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: Oxford, UK, 1881; Volume 1. [Google Scholar]
- Rosenhain, W. The Properties of Some Copper Alloys (Classic Reprint) Paperback–October 27, 2017; Forgotten Books: London, UK, 2017. [Google Scholar]
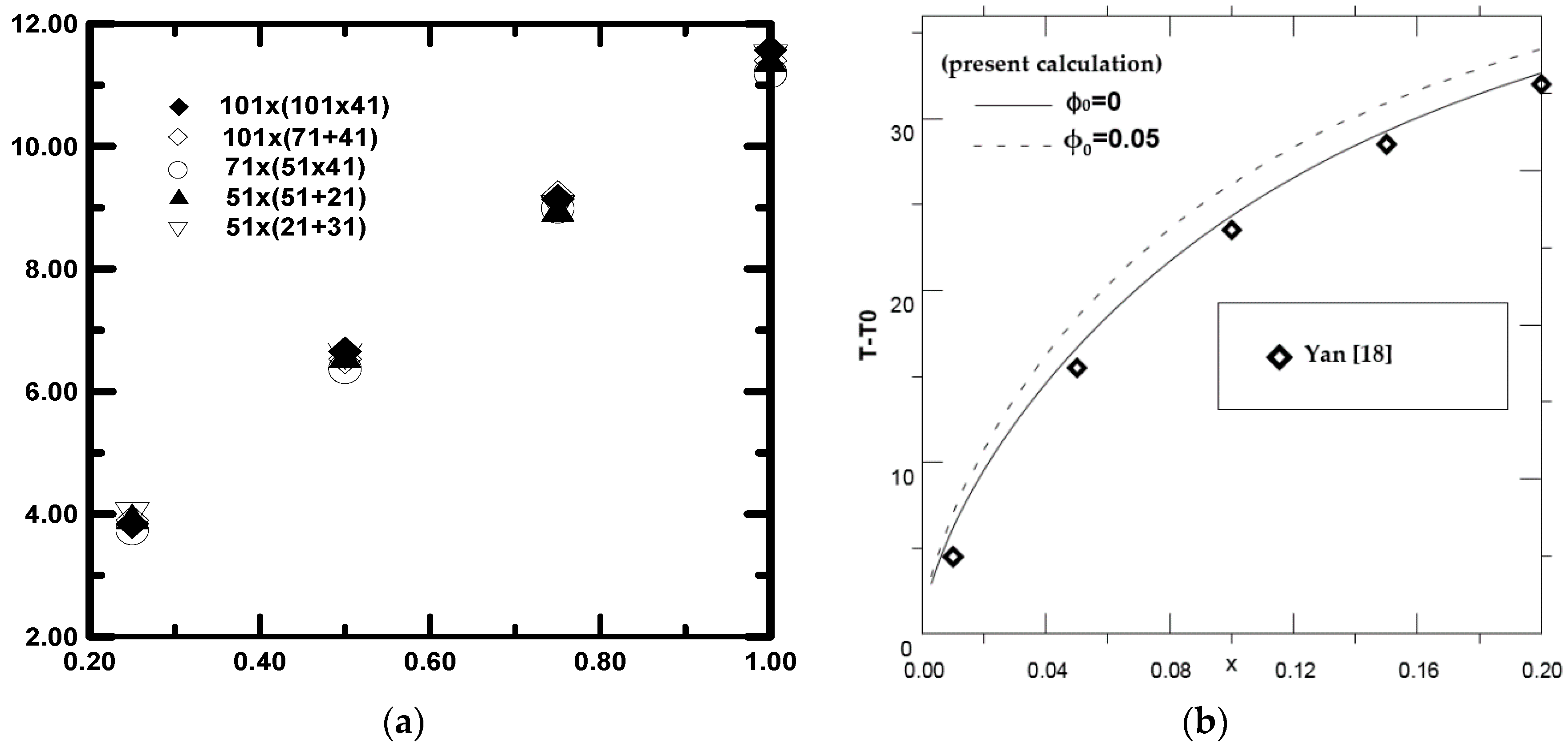
| I × (J + K) Grid Point | 101 × (101 + 41) | 101 × (71 + 41) | 71 × (51 + 41) | 51 × (51 + 21) | 51 × (21 + 31) |
|---|---|---|---|---|---|
| ξ = 0.25 | 3.841 | 3.900 | 3.973 | 3.959 | 3.736 |
| ξ = 0.50 | 6.651 | 6.530 | 6.572 | 6.593 | 6.367 |
| ξ = 0.75 | 9.140 | 9.200 | 8.984 | 8.986 | 8.959 |
| ξ = 1.00 | 11.573 | 11.400 | 11.442 | 11.425 | 11.195 |
| Thermo-Physical Properties | Aluminium | Copper (Cu) |
|---|---|---|
| ρ (kg·m−3) | 2700 | 8933 |
| Cp (J·kg−1·K−1) | 900 | 385 |
| λ (W·m−2·K−1) | 240 | 401 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasr, A.; Alzahrani, A.A. Liquid Nanofilms’ Evaporation Inside a Heat Exchanger by Mixed Convection. Coatings 2022, 12, 1564. https://doi.org/10.3390/coatings12101564
Nasr A, Alzahrani AA. Liquid Nanofilms’ Evaporation Inside a Heat Exchanger by Mixed Convection. Coatings. 2022; 12(10):1564. https://doi.org/10.3390/coatings12101564
Chicago/Turabian StyleNasr, Abdelaziz, and Abdullah A. Alzahrani. 2022. "Liquid Nanofilms’ Evaporation Inside a Heat Exchanger by Mixed Convection" Coatings 12, no. 10: 1564. https://doi.org/10.3390/coatings12101564
APA StyleNasr, A., & Alzahrani, A. A. (2022). Liquid Nanofilms’ Evaporation Inside a Heat Exchanger by Mixed Convection. Coatings, 12(10), 1564. https://doi.org/10.3390/coatings12101564







