Abstract
In this work, metal–organic frameworks (MOFs) were used as precursors to prepare Zn/Co oxide with a porous dodecahedral core-shell structure. Herein, a low-temperature self-assembly calcination and hydrothermal strategy of imidazole-based Zn-Co-MOF was used. As anode of lithium-ion batteries (LIBs), ZnO/Co3O4 has good cycling stability, the specific discharge capacity of ZnO/Co3O4 is stable at about 640 mAh g−1 after 200 cycles, and its coulombic efficiency (CE) is stable above 95% after the first 20 cycles. When the current density is 0.6 A/g, the discharge capacity is 420 mAh g−1. This excellent electrochemical performance is attributed to its unique porous hollow structure and unique heterojunction electrode interface, which improves the Li+ storage capacity, increases the contact area between the electrode and the electrolyte, and improves the overall electrochemical activity. In addition, the synergistic effect of ZnO and Co3O4 also plays an important role in improving the electrochemical performance.
1. Introduction
LIBs have significant advantages such as light weight, small size, high specific energy, small self-discharge, no memory effect, and good high-current discharge performance [1,2], whereas conventional graphite anodes are limited by their low theoretical capacity (372 mAh g−1) and safety concerns related to dendrite formation issues [3]. Therefore, finding high-performance anode materials is crucial for developing next-generation LIBs with higher energy density and high safety.
Transition metals/metal compounds (such as Co3O4 [4], TiO2 [5], and ZnS [6], among others) (TMOs) are considered as one of the most promising candidates thanks to their higher theoretical capacities. However, TMOs have inherent disadvantages such as large volume change and poor electrical conductivity [7]. To overcome the about-mentioned weak points, MOFs are introduced as precursors for the synthesis of multi-metal oxides [8]. For example, Li’s group reported an egg-shell ZnO/NiO microsphere based on Zn-Ni MOFs with a specific capacity of 1008.6 mAh g−1 after 200 cycles at 0.1 A/g [9]. The biphasic Co3O4/Fe3O4 hollow nanospheres prepared from Fe@Co-MOFs exhibited a specific capacity of 937 mAh g−1 after 150 cycles at 0.1 A/g, and their structures remained in good condition after 150 cycles [10]. The porous hollow nanostructures of MOF-derived material can provide 3D interconnected channels for the rapid transfer of Li+, increase the contact area between the electrode and the electrolyte, as well as provide additional internal space for volume change [11,12,13,14]. Based on the above studies, it is feasible and promising to synthesize multi-metal TMOs with regular shapes using MOFs as precursors.
This paper demonstrates a synthetic strategy for Zn/Co TMOs anode with porous core-shell nanocages. The synthesis process involves a low-temperature hydrothermal and calcination strategy. As a result of this strategy’s simplicity, adaptability, and low cost, mass production prospects are quite bright. Furthermore, the Zn and Co atoms are evenly distributed throughout the crystal structure and separated by organic linkers, which make it easier for ultrafine metal oxide nanocomposites to form during the calcination process in air.
The particular structure and distinctive electrode interface heterostructure of ZnO and Co3O4 provide this composite with a high electrochemical performance when tested as an anode for LIBs.
2. Experimental
2.1. Preparation of Zn-Co-MOF
Typically, C4H6O4·Zn·2H2O (3.28 mmol) and 2-methylimidazole (3.65 mmol) were added to 40 mL of deionized water and the solution was stirred and allowed to stand at room temperature for 24 h. The precipitate obtained by centrifugation was washed several times with methanol and deionized water and dried at 80 °C for 12 h to obtain Zn-MOF. Then, 0.08 g of Zn-MOF was weighed and dissolved in 10 mL of methanol to obtain a Zn-MOF solution. Then, Co(NO3)2·6H2O (0.74 mmol) and 2-methylimidazole (10.9 mmol) were then added to 6 mL of methanol and stirred for 5 min. Finally, the above solution was added to the Zn-MOF solution, stirred evenly, and reacted in the reactor at 120 °C for 4 h. The pellet obtained after centrifugation was washed several times with absolute ethanol and dried to obtain Zn-Co-MOF.
2.2. Preparation of ZnO/Co3O4
The Zn-Co-MOF precursor sample was annealed in a furnace with Ar atmosphere to 300 °C for 2 h with a heat-treatment rate of 2 °C min−1. Then, the obtained sample was stored in air at 300 °C for 1 h, and finally the ZnO/Co3O4 composite was obtained.
2.3. Material Characterization Test
An X-ray diffractometer (XRD, Bruker D8 Advance, Karlsruhe, Germany) with Cu Kα radiation (λ = 1.54056 Å) was used to analyze the sample crystals. The morphology of the sample was characterized by scanning electron microscope (SEM, Quanta200, Eindhoven, The Netherlands) and transmission electron microscope (TEM, JEOL 2001F, Tokyo, Japan).
2.4. Electrochemical Test
Dispersion of the TMOs (active material: 1 mg), PVDF, and acetylene black in the N,N-Dimethylpyrrolidone solution took place at a weight ratio of 8:1:1. After stirring, a slurry of the material was applied on the copper foil with a radius of 0.5 cm and dried in a vacuum oven at 60 °C for 6 h. The button battery (CR2032) was assembled in the glove box with the microporous polypropylene film as a separator, a metallic lithium foil as the counter electrode, and 1 M LiPF6 in ethylene carbonate (EC)–dimethyl carbonate (DMC)–ethylene methyl carbonate (EMC) with a volume ratio of 1:1:1 as the lithium-ion electrolyte. Finally, the Wuhan Lan Dian battery tester (BTRBTS CT2001A, Wuhan, China) was used to conduct constant current charge and discharge test of the battery in the voltage range of 0.01–3.00 V. The CHI608E electrochemical workstation was used to perform the volt-ampere cycle test (voltage range: 0.01–3.00 V, scan rate: 0.1 mV s−1) and electrochemical impedance spectroscopy test.
3. Results and Discussion
3.1. Analysis and Characterization
Figure 1a–c are the SEM images of Zn-Co-MOF. It can be clearly seen that the Zn-Co-MOFs precursors are uniformly distributed and the surface is very smooth. The precursor particles are rhombic polyhedrons with regular morphology. Besides, it can be observed that ZnO/Co3O4 (Figure 1d–f) presents a dodecahedral structure with an edge length of about 1.5 μm. This specific hollow structure enhances the storage capacity of Li+ [15,16,17]. During the growth and transformation of Zn-Co-MOF to ZnO/Co3O4, Zn-Co-MOF is self-assembled by internal metal ion centers and external imidazole ligands. During the low-temperature calcination process, the outer Co-MOFs shell is squeezed, the inner hollow section increases, and the Zn-MOFs particles are further decomposed. The surface of the particles is smooth with varying degrees of depressions and wrinkles at the boundaries, which can be seen from the scanned images.
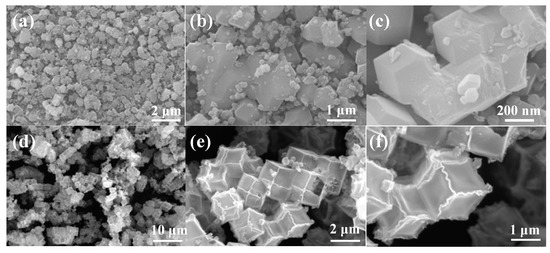
Figure 1.
SEM images of (a–c) Zn-Co-MOF and (d–f) ZnO/Co3O4.
The size and morphology of the ZnO/Co3O4 nanocomposite were further investigated by TEM. Figure 2a,b can clearly show that ZnO/Co3O4 presents a hollow dodecahedron porous core-shell structure. Figure 2c,d show the HRTEM of ZnO/Co3O4, where it can be seen that the material is crystalline. The crystal planes of ZnO are (100) and (002) and the measured lattice spacings are 0.28 nm and 0.27 nm, respectively. The SAED pattern of ZnO/Co3O4 (Figure 2c illustration) indicated the presence of ZnO and Co3O4, representing the predominant growth of ZnO and Co3O4 crystal planes, which is consistent with the XRD data.
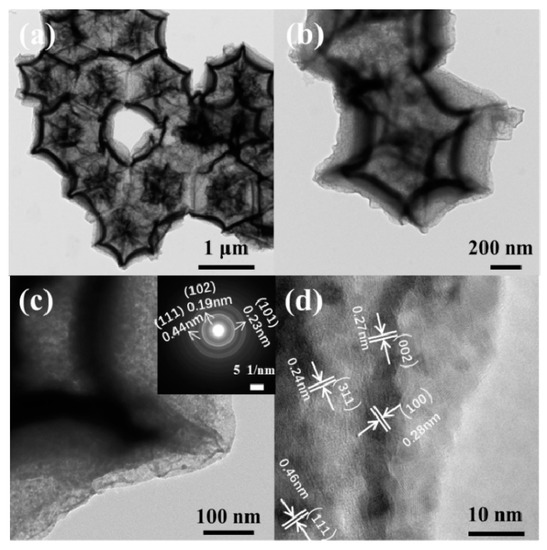
Figure 2.
TEM images of ZnO/Co3O4 (a,b) and HRTEM (d); selected area electron diffraction (illustration in (c).
Using X-ray diffraction (XRD), the crystal structure and phase analysis of the synthesized samples are performed. The red-star diffraction peaks represent the typical patterns of the spinel Co3O4’s face-centered-cubic phase. It can be seen from Figure 3 that the main diffraction peaks of Co3O4 in the ZnO/Co3O4 material are at 2θ of 18.975°, 36.845°, 44.762°, 59.271°, and 65.219°, corresponding to crystal planes of (111), (311), (400), and (511), respectively, with no impurity diffraction peaks (JCPDS Card No.43-1003). Besides, those marked with a green star can be indexed to the hexagonal ZnO (JCPDS Card No.36-1451). The diffraction peaks appear at the following positions: 2θ = 31.370°, 34.534°, 47.585°, and 56.711°, corresponding to the (100), (002), (102), and (110) crystal planes of ZnO, respectively.
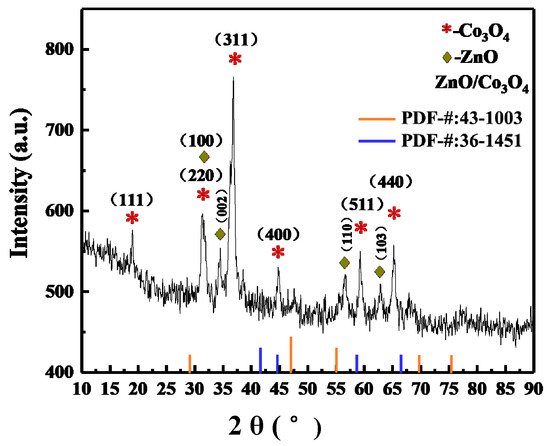
Figure 3.
The XRD image of ZnO/Co3O4.
3.2. Electrochemical Performance
It can be clearly seen from Figure 4 that, during the initial cycle of discharge, two distinct reduction peaks appear at 0.35 V and 0.7 V. The larger reduction peak at 0.7 V corresponds to the multi-step reduction of cobalt ions to Co. The smaller reduction peak at 0.35 V corresponds to the reduction of Zn2+ to Zn during the discharge process. Meanwhile, the reduction of Zn2+ to Zn is accompanied by the formation of a solid electrolyte interface film (SEI film) and the process is irreversible. During the initial cycle of charging, a relatively obvious oxidation peak appeared at 2.2 V and a very small oxidation peak appeared at 1.6 V. The oxidation peak at 2.2 V corresponds to the formation process of cobalt ions and the oxidation peak at 1.6 V corresponds to the oxidation of Zn to Zn2+. Compared with the initial cycle, the amplitudes of the oxidation and reduction peaks in the second cycle were significantly smaller, as well as the reduction peak shift to the right to a certain extent; the integral area was also significantly smaller than that in the initial cycle. This is mainly due to the formation of the SEI film, which leads to an irreversible decrease in the capacity of the battery [18,19,20]. During the second cycle of discharge, there is a decreased reduction peak at 0.78 V. In the following charge–discharge cycles, the amplitude of the reduction peak gradually became smaller and stabilized owing to the reconstruction of the SEI film and the consumption of lithium ions. This mainly correspond to the reduction of Zn2+ to Zn, accompanied by the reduction of Co2+ to Co, while the reduction peaks of cobalt ions in other valence states to cobalt are insignificant. The main reason for the reduction in the number of oxidation peaks and reduction peaks is that, in the process of calcination, when Co forms ions, cobalt elements in various valence states will be formed. Most of the valence states of Co ions are Co2+, so the redox peaks of Co ions in other valence states are insignificant during the charging process of the initial cycle and the charge-discharge process after the second cycle. The redox process of the third circle is similar to the redox process of the second circle. Both the oxidation peak and the reduction peak are close to those of the second circle, and the integral area does not decrease greatly. Therefore, the electrochemical reversibility of LIBs assembled with ZnO/Co3O4 is high.
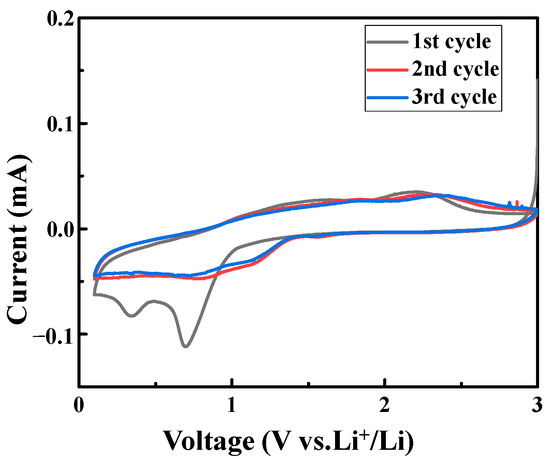
Figure 4.
CV curves of ZnO/Co3O4.
Figure 5 depicts the ZnO/Co3O4 electrode’s AC impedance spectrum. It is clear that it comprises a linear low-frequency region and an unmistakable semi-circular high-frequency portion. Particularly, the semicircle is associated with the charge transfer resistance at the electrode/electrolyte interface, while the intercept stands in for the ohmic resistance. The inset shows the equivalent circuit diagram fitted by Z-view software. The parameters of the fitting circuit components are shown in Table 1.
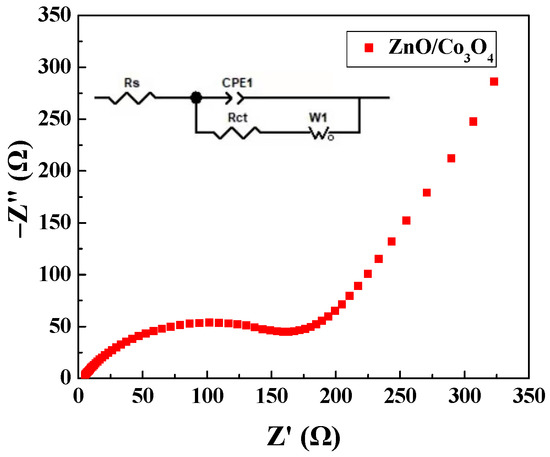
Figure 5.
AC impedance spectroscopy of ZnO/Co3O4.

Table 1.
Circuit element fitting parameters.
Figure 6 shows the discharge-charge voltage distributions of ZnO/Co3O4 at the 1st, 10th, and 20th cycles at a current density of 0.1 A g−1 between 0.01 and 3.0 V. The first discharge specific capacity is about 1250 mAh g−1, the charge specific capacity is about 880 mAh g−1, and the corresponding initial CE is 70.4%. The relatively low CE can be attributed to the irreversible capacity loss, including decomposition of electrolyte, formation of SEI film, and some undecomposed phases, which are common for most anode materials. In the following cycles, the specific capacity decays rapidly, and the discharge specific capacity is about 740 mAh g−1 and the charge specific capacity is about 700 mAh g−1 in the second cycle; its coulombic efficiency rapidly increases to 94.5%. ZnO/Co3O4 has a voltage plateau around 2.5 V for the first charge and a voltage plateau around 0.7 V for the first discharge, which is consistent with the cyclic voltammetry (CV) curve.
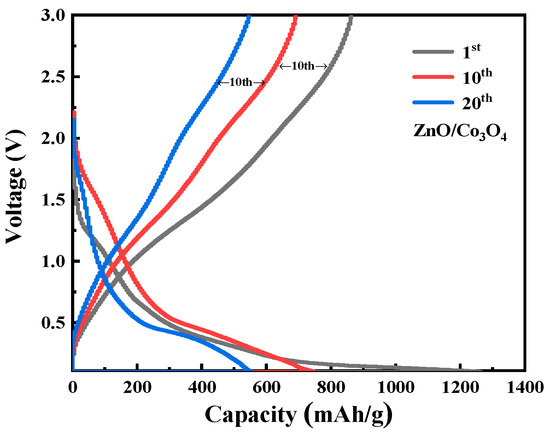
Figure 6.
Galvanostatic charge/discharge curves of ZnO/Co3O4.
Figure 7 shows the cycling performance of ZnO/Co3O4 at a current density of 0.1 A g−1. The initial discharge specific capacity reaches 1600 mAh g−1 and, after several cycles, the discharge specific capacity is stable at about 640 mAh g−1. This is because of the increased electrochemical access to the active material that occurs as cycling proceeds and large surface area provided by the hollow dodecahedral 3D core-shell structure of ZnO/Co3O4. The coulombic efficiency remains stabilized above 95% after multiple cycles.
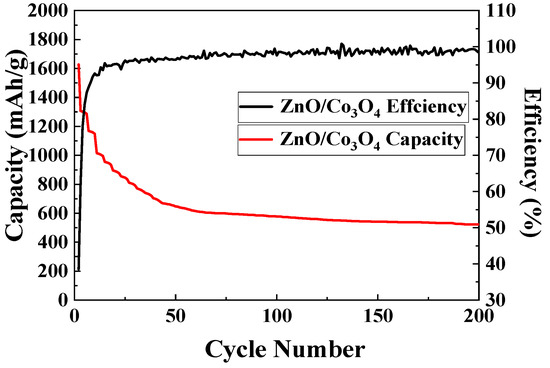
Figure 7.
Cyclic performance curve of ZnO/Co3O4.
In order to maintain the consistency of the testing electrode samples, thereby gaining insight into the durability of the electrode, the rate performance test was carried out after 200 cycles of the battery.
Figure 8 shows the rate capability of ZnO/Co3O4. It can be seen that its specific discharge capacity tends to be stable at high current densities, which again confirms that the hollow dodecahedral porous core-shell structure still has good performance at high current densities. When the current density is 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6 A/g, the specific capacity is 605, 582, 524, 513, 480, and 420 mAh/g respectively. When the current density drops to 0.1 A/g, the specific capacity can be recovered to 590 mAh/g, further indicating that the structure of the electrode material is stable and exhibits excellent rate performance.
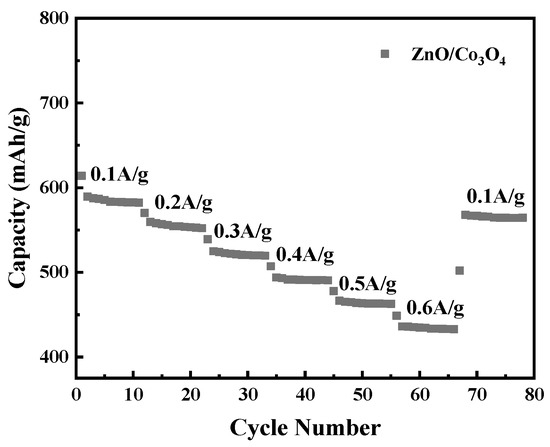
Figure 8.
The rate capability at various current densities of ZnO/Co3O4.
According to the above-mentioned discussions, ZnO/Co3O4 exhibits good rate performance and cycling stability, which are the most critical requirements for LIBs. For comparison, the morphological structures and electrochemical properties of the single metal oxides are listed in Table 2. Some single metal oxides such as ZnO [21] and Co3O4 [22] have high specific capacity, but their rate performance and cycling performance are poor.

Table 2.
Morphology and electrochemical properties of relevant metal oxides.
The excellent electrochemical performance can be attributed to the unique structure feature of porous hollow core-shell nanocages and Zn/Co heterogeneous interface, which can effectively improve the electrochemical kinetics, shorten the transport pathways of Li+ and electrons, and reduce the diffusion length and resistance for the transport of Li+ and electrolyte molecules. Finally, the synergetic effect of Co3O4 and ZnO may also contribute to the excellent electrochemical performance. Compared with the relevant reported papers, this paper reports a creative method combining low-temperature calcination (300 °C) and low-temperature hydrothermal (120 °C) strategy to realize the preparation of complex hollow nanocubic composite metal oxides. It provides a new strategy for subsequent novel energy storage multi-metal TMOs electrodes and catalytic materials.
4. Conclusions
In conclusion, we have successfully synthesized ZnO/Co3O4 TMOs with a porous dodecahedral structure by low-temperature calcination and hydrothermal strategy. Owing to the characteristics of the specific hollow porous core-shell nanocages structure and Zn/Co heterogeneous interface, ZnO/Co3O4 exhibits good cycling performance and high reversibility compared with the TMOs of ZnO or Co3O4. When used as anode for LIBs, ZnO/Co3O4 nanocomposites exhibit a high reversible capacity of 640 mAh g−1 after 100 cycles at a current density of 100 mA g−1. Its coulombic efficiency (CE) is stable above 95% after the first 20 cycles. This work may also provide a general method to design and prepare heterogeneous multi-metal oxides for extensive applications in high-performance LIBs, supercapacitors, and catalysis.
Author Contributions
Conceptualization, G.Z.; data curation, M.G. and W.C.; methodology, M.G.; project administration, M.S.; resources, W.C.; software, G.Z.; supervision, M.S.; writing—original draft, J.P.; writing—review and editing, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the research initiation fund of Hainan University (No. KYQD(ZR)20062), Hainan Provincial Natural Science Foundation of China (No. 620RC553).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Zhang, G.; Liu, Q.; Duan, H. Recent progress in Zn-based anodes for advanced lithium ion batteries. Mater. Chem. Front. 2018, 2, 1414–1435. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, C.; Qian, W.; Sui, X.; Teng, C.; Li, Q.; Lu, Z. Carbon coated porous Co3O4 polyhedrons as anode materials for highly reversible lithium-ion storage. J. Alloy. Compd. 2021, 855, 157387. [Google Scholar] [CrossRef]
- Sengottaiyan, S.; Shiva, G.; Ammar, H.; Fadl, A.; Zakaria, M.; Anjanapura, V. Investigation into the effects of SiO2/TiO2 nanolayer on the thermal performance of solar box type cooker. Energy Sources A Recovery Util. Environ. Eff. 2021, 43, 2724–2737. [Google Scholar]
- Jonnalagadda, M.; Prasad, V.; Kakarla, R.; Venkata, R.; Anjanapura, V. Facile synthesis of Ni-doped ZnS-CdS composite and their magnetic and photoluminescence properties. J. Environ. Chem. Eng. 2021, 6, 106335. [Google Scholar]
- Wu, Y.; Meng, J.; Li, Q.; Niu, C.; Wang, X.; Yang, W.; Li, W.; Mai, L. Interface-modulated fabrication of hierarchical yolk-shell Co3O4/C dodecahedrons as stable anodes for lithium and sodium storage. Nano Res. 2017, 10, 2364–2376. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Liu, H.; Zheng, H.; Gao, T.; Shen, M.; Qu, Q.; Zheng, H. Metal organic frameworks-derived Co3O4 hollow dodecahedrons with controllable interiors as outstanding anodes for Li storage. J. Mater. Chem. A 2014, 2, 12194–12200. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Hou, S.; Lu, T.; Yao, Y.; Chua, D.; Pan, L. Metal-organic frameworks derived yolk-shell ZnO/NiO microspheres as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 2018, 335, 579–589. [Google Scholar] [CrossRef]
- Nam, K.C.; Seon, Y.H.; Bandyopadhyay, P.; Cho, J.S.; Jeong, S.M. Porous nanofibers comprising hollow Co3O4/Fe3O4 nanospheres and nitrogen-doped carbon derived by Fe@ZIF-67 as anode materials for lithium-ion batteries. Int. J. Energy Res. 2022, 46, 8934–8948. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Li, Y.; Zhu, R.; Pang, H. Applications of Metal-Organic-Framework-Derived Carbon Materials. Adv. Mater. 2019, 31, 1804740. [Google Scholar] [CrossRef] [PubMed]
- Karthik, K.V.; Reddy, C.; Reddy, K.R.; Ravishankar, R.; Sanjeev, G.; Kulkarni, R.V.; Shetti, N.P.; Raghu, A.V. Barium titanate nanostructures for photocatalytic hydrogen generation and photodegradation of chemical pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 20646–20653. [Google Scholar] [CrossRef]
- Al, N.; Siddique, S.; Aisida, S.; Altowairqi, Y.; Fadhali, M.; Shariq, M.; Khan, M.; Qamar, M.; Shahid, T.; Shahzad, M.; et al. Structural, Magnetic, and Magnetothermal Properties of Co100−xNix Nanoparticles for Self-Controlled Hyperthermia. Coatings 2022, 12, 1272. [Google Scholar]
- Zhang, Y.; Li, Y.; Chen, J.; Zhao, P.; Li, D.; Mu, J.; Zhang, L. CoO/Co3O4/graphene nanocomposites as anode materials for lithium-ion batteries. J. Alloy. Compd. 2017, 699, 672–678. [Google Scholar] [CrossRef]
- Xiu, Z.L.; Alfaruqi, M.H.; Gim, J.; Song, J.; Kim, S.; Duong, P.T.; Baboo, J.P.; Mathew, V.; Kim, J. MOF-derived mesoporous anatase TiO2 as anode material for lithium-ion batteries with high rate capability and long cycle stability. J. Alloy. Compd. 2016, 674, 174–178. [Google Scholar] [CrossRef]
- Sun, D.; Tang, Y.; Ye, D.; Yan, J.; Zhou, H.; Wang, H. Tuning the Morphologies of MnO/C Hybrids by Space Constraint Assembly of Mn-MOFs for High Performance Li Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 5254–5262. [Google Scholar] [CrossRef]
- Gallo, M.; Polverino, P.; Mougin, J.; Morel, B.; Pianese, C. Coupling electrochemical impedance spectroscopy and model-based aging estimation for solid oxide fuel cell stacks lifetime prediction. Appl. Energy 2020, 279, 115718. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Huang, Y.; Zhang, X.; Wu, Q.; Gao, H.; Yang, J. Synthesis and electrochemical properties of nickel-manganese oxide on MWCNTs/CFP substrate as a supercapacitor electrode. Appl. Energy 2015, 153, 78–86. [Google Scholar] [CrossRef]
- Di Blasi, A.; Busaccaa, C.; Di Blasia, O.; Briguglioa, N.; Squadritoa, G.; Antonuccia, V. Synthesis of flexible electrodes based on electrospun carbon nanofibers with Mn3O4 nanoparticles for vanadium redox flow battery application. Appl. Energy 2017, 190, 165–171. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Shan, H.; Yan, B.; Li, D.; Langford, C.; Sun, X. Scalable synthesis of functionalized graphene as cathodes in Li-ion electrochemical energy storage devices. Appl. Energy 2016, 175, 512–521. [Google Scholar] [CrossRef]
- Kundu, S.; Sain, S.; Yoshio, M.; Kar, T.; Gunawardhana, N.; Pradhan, S.K. Structural interpretation of chemically synthesized ZnO nanorod and its application in lithium ion battery. Appl. Surf. Sci. 2015, 329, 206–211. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Yin, L.; Qi, Y. Prussion Blue-Supported Annealing Chemical Reaction Route Synthesized Double-Shelled Fe2O3/Co3O4 Hollow Microcubes as Anode Materials for Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 8098–8107. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).