Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review
Abstract
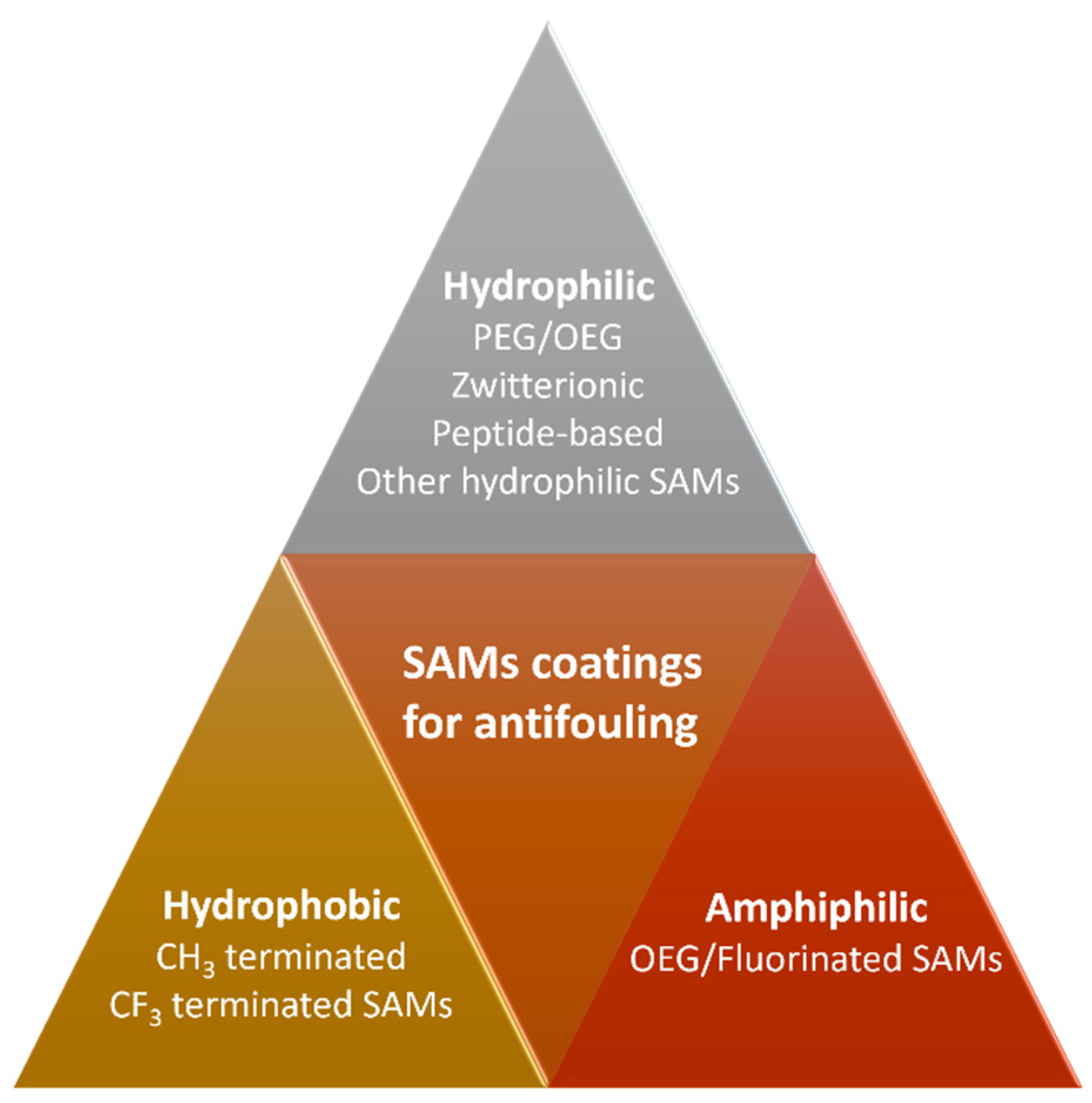
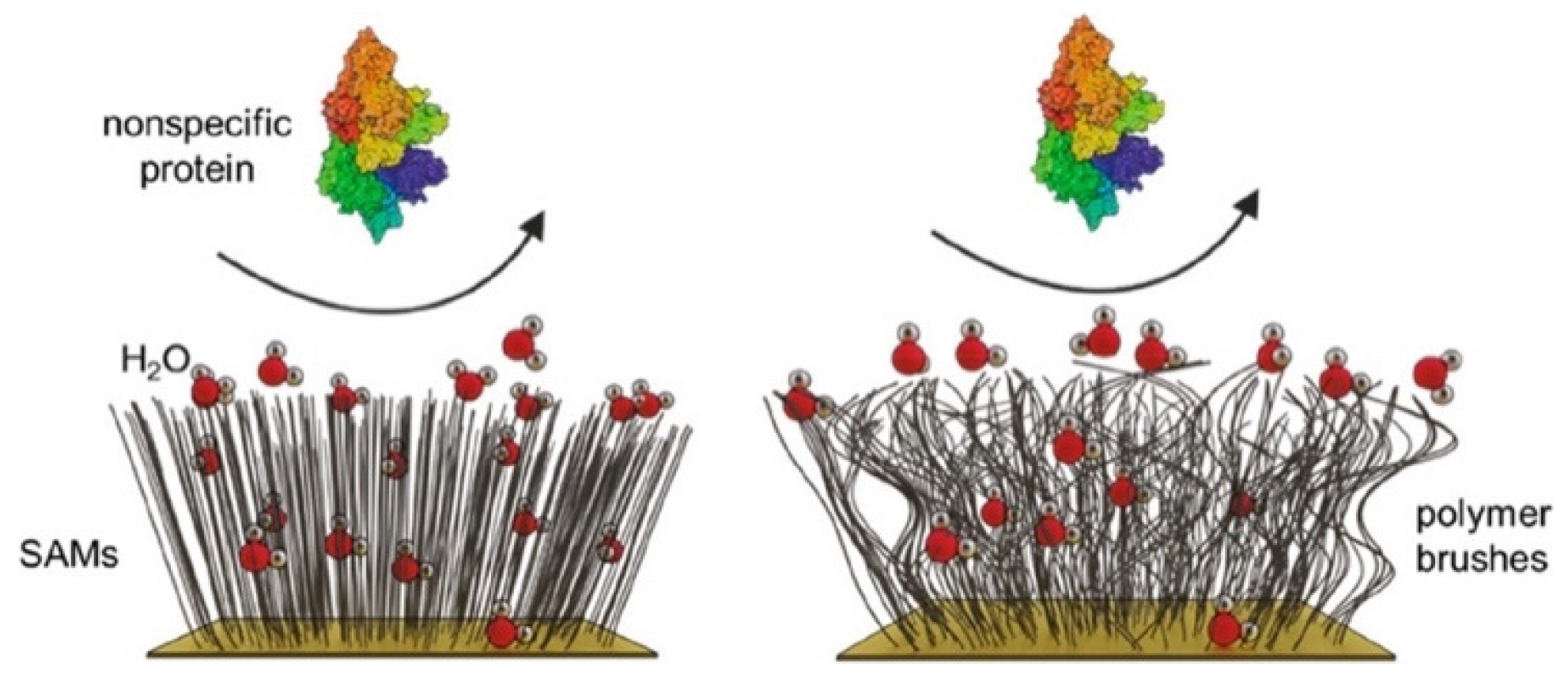
1. Introduction
2. Hydrophilic Antifouling SAMs
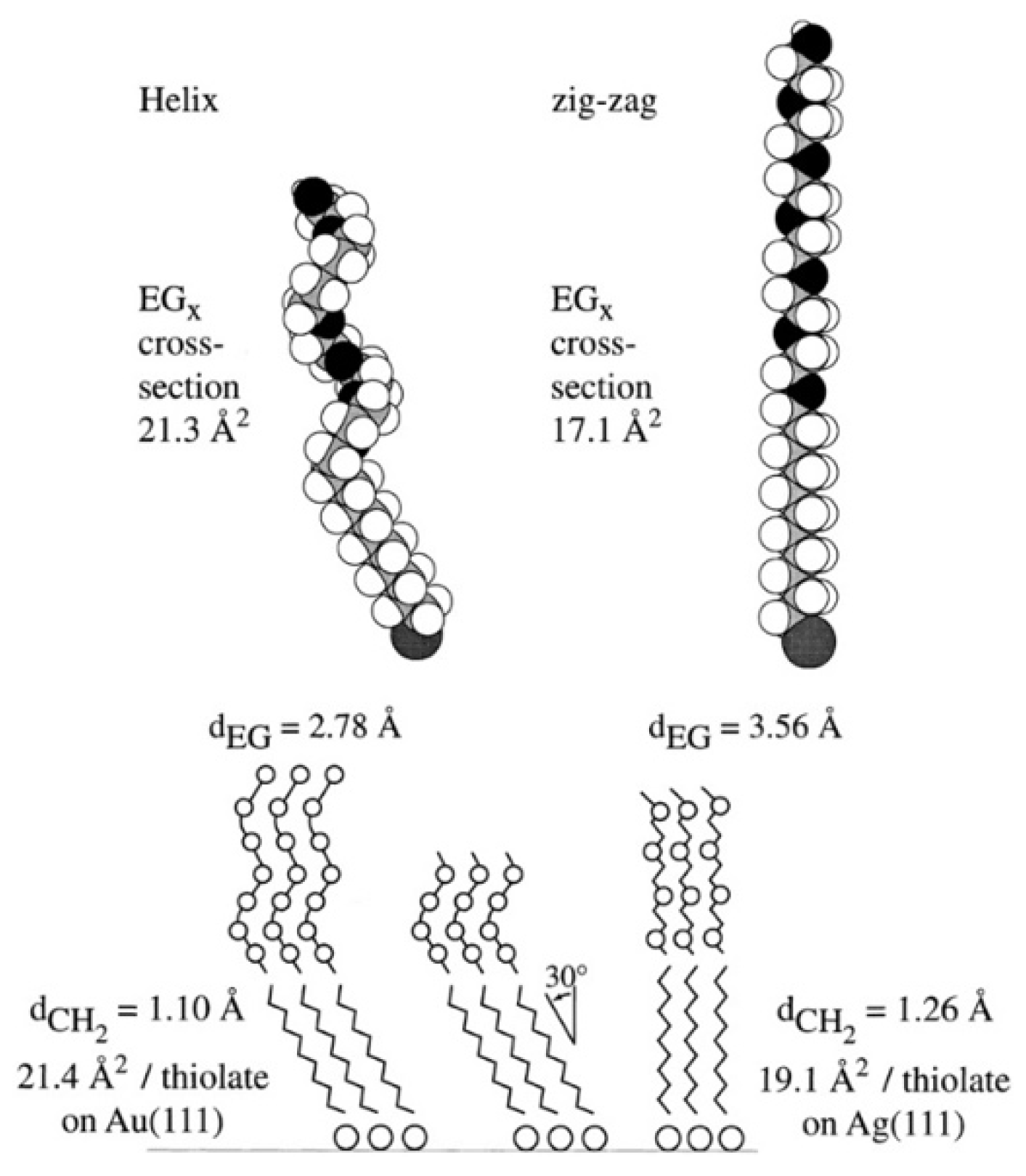
2.1. PEG and OEG SAMs
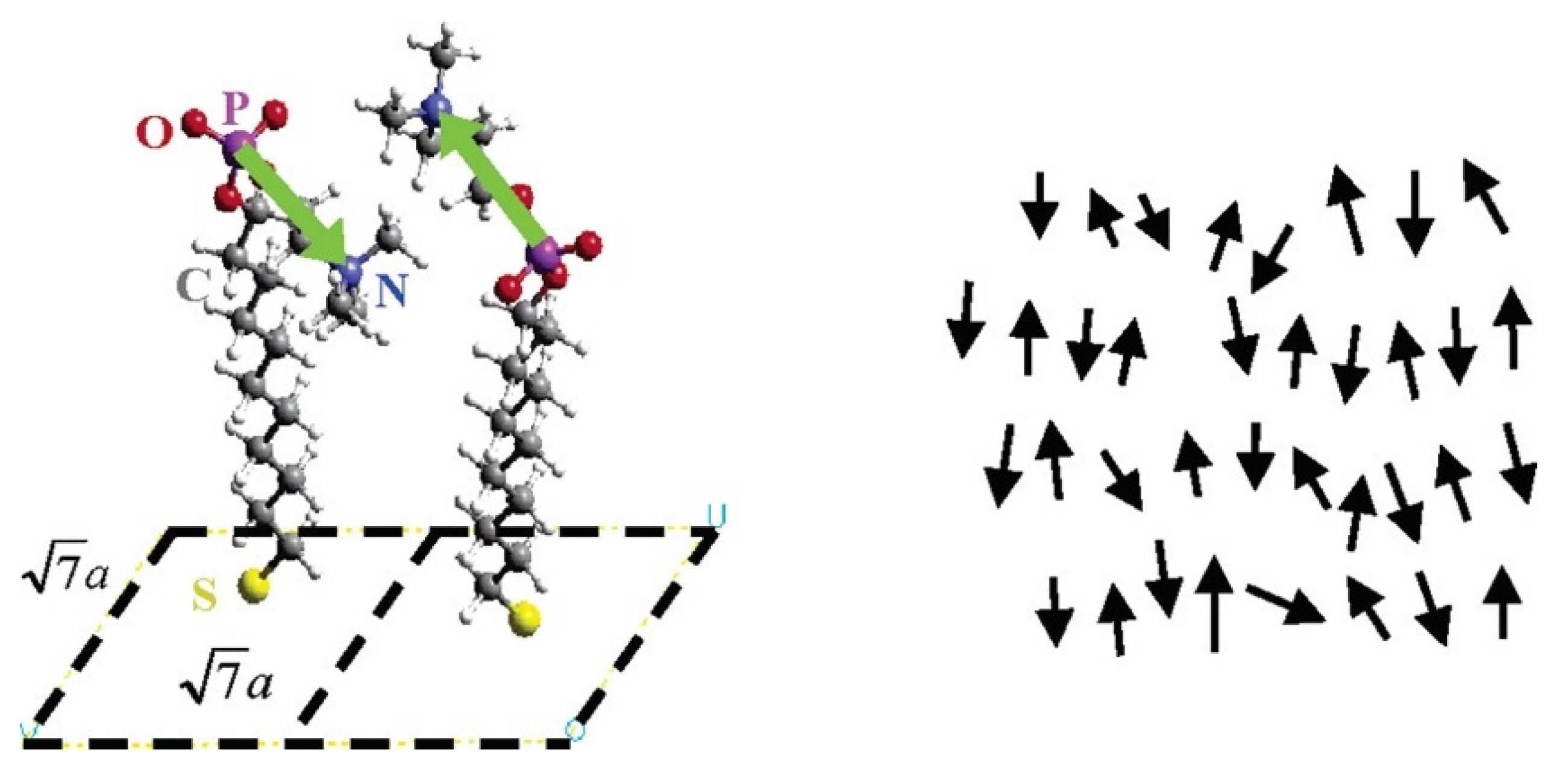
2.2. Zwitterionic SAMs
2.3. Peptide-Based SAMs
2.4. Other Hydrophilic SAMs
3. Hydrophobic Antifouling SAMs
4. Amphiphilic Antifouling SAMs
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, F. Antifouling Surfaces and Materials: From Land to Marine Environment; Springer: Berlin, Germany, 2015; pp. 31–54. [Google Scholar]
- Ahn, H.S.; Kim, K.M.; Lim, S.T.; Lee, C.H.; Han, S.W.; Choi, H.; Koo, S.; Kim, N.; Jerng, D.-W.; Wongwises, S. Anti-Fouling Performance of Chevron Plate Heat Exchanger by the Surface Modification. Int. J. Heat Mass Transf. 2019, 144, 118634. [Google Scholar] [CrossRef]
- Scurria, A.; Scolaro, C.; Sfameni, S.; Di Carlo, G.; Pagliaro, M.; Visco, A.; Ciriminna, R. Towards AquaSun Practical Utilization: Strong Adhesion and Lack of Ecotoxicity of Solar-Driven Antifouling Sol-Gel Coating. Prog. Org. Coat. 2022, 165, 106771. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Ma, S. Preparation of an Anti-Fouling Silicone Polyacrylate/SiO2/HDPE Composite for Industrial Wastewater Pipe. Mater. Lett. 2021, 304, 130661. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, L.; Liu, C.; Yang, C.; Lin, G.; Liu, S.; Jia, K.; Liu, X. Interfacial Coordination Mediated Surface Segregation of Halloysite Nanotubes to Construct a High-Flux Antifouling Membrane for Oil-Water Emulsion Separation. J. Membr. Sci. 2021, 620, 118828. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Y.; Chen, M.; Ambrosi, A.; Ding, C.; Luo, X. Electrochemical Biosensor with Enhanced Antifouling Capability for COVID-19 Nucleic Acid Detection in Complex Biological Media. Anal. Chem. 2021, 93, 5963–5971. [Google Scholar] [CrossRef]
- Lee, D.U.; Kim, D.W.; Lee, S.Y.; Choi, D.Y.; Choi, S.Y.; Moon, K.-S.; Shon, M.Y.; Moon, M.J. Amino Acid-Mediated Negatively Charged Surface Improve Antifouling and Tribological Characteristics for Medical Applications. Colloids Surf. B Biointerfaces 2022, 211, 112314. [Google Scholar] [CrossRef]
- Yeginbayeva, I.A.; Atlar, M. An Experimental Investigation into the Surface and Hydrodynamic Characteristics of Marine Coatings with Mimicked Hull Roughness Ranges. Biofouling 2018, 34, 1001–1019. [Google Scholar] [CrossRef]
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional Polymer Materials for Modern Marine Biofouling Control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-Inspired Strategies for Designing Antifouling Biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Zhang, S.; Geryak, R.; Geldmeier, J.; Kim, S.; Tsukruk, V.V. Synthesis, Assembly, and Applications of Hybrid Nanostructures for Biosensing. Chem. Rev. 2017, 117, 12942–13038. [Google Scholar] [CrossRef] [PubMed]
- Krsmanovic, M.; Biswas, D.; Ali, H.; Kumar, A.; Ghosh, R.; Dickerson, A.K. Hydrodynamics and Surface Properties Influence Biofilm Proliferation. Adv. Colloid Interface Sci. 2021, 288, 102336. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Kumar, S.; Mohanty, S.; Nayak, S.K. Environmentally Benign Fouling-Resistant Marine Coatings: A Review. Polym.-Plast. Technol. Mater. 2019, 58, 498–518. [CrossRef]
- Zhang, H.; Chiao, M. Anti-Fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Brash, J.L.; Horbett, T.A.; Latour, R.A.; Tengvall, P. The Blood Compatibility Challenge. Part 2: Protein Adsorption Phenomena Governing Blood Reactivity. Acta Biomater. 2019, 94, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.F.; Susarrey-Arce, A. Strategies Applied to Modify Structured and Smooth Surfaces: A Step Closer to Reduce Bacterial Adhesion and Biofilm Formation. Colloids Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Schøyen, M.; Green, N.W.; Hjermann, D.Ø.; Tveiten, L.; Beylich, B.; Øxnevad, S.; Beyer, J. Levels and Trends of Tributyltin (TBT) and Imposex in Dogwhelk (Nucella lapillus) along the Norwegian Coastline from 1991 to 2017. Mar. Environ. Res. 2019, 144, 1–8. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling Processes and Toxicity Effects of Antifouling Paints on Marine Environment. A Review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Shi, S.; Huang, R.; Su, R.; Qi, W.; He, Z. Design and Mechanisms of Antifouling Materials for Surface Plasmon Resonance Sensors. Acta Biomater. 2016, 40, 100–118. [Google Scholar] [CrossRef]
- Martins, S.E.; Fillmann, G.; Lillicrap, A.; Thomas, K.V. Review: Ecotoxicity of Organic and Organo-Metallic Antifouling Co-Biocides and Implications for Environmental Hazard and Risk Assessments in Aquatic Ecosystems. Biofouling 2018, 34, 34–52. [Google Scholar] [CrossRef]
- Tian, L.; Yin, Y.; Bing, W.; Jin, E. Antifouling Technology Trends in Marine Environmental Protection. J. Bionic Eng. 2021, 18, 239–263. [Google Scholar] [CrossRef]
- Sakunkaewkasem, S.; Marquez, M.D.; Lee, H.J.; Lee, T.R. Mixed Phase-Incompatible Monolayers: Toward Nanoscale Anti-Adhesive Coatings. ACS Appl. Nano Mater. 2020, 3, 4091–4101. [Google Scholar] [CrossRef]
- Lee, H.J.; Jamison, A.C.; Lee, T.R. Two Are Better than One: Bidentate Adsorbates Offer Precise Control of Interfacial Composition and Properties. Chem. Mater. 2016, 28, 5356–5364. [Google Scholar] [CrossRef]
- Chinwangso, P.; Lee, H.J.; Jamison, A.C.; Marquez, M.D.; Park, C.S.; Lee, T.R. Structure, Wettability, and Thermal Stability of Organic Thin-Films on Gold Generated from the Molecular Self-Assembly of Unsymmetrical Oligo(ethylene glycol) Spiroalkanedithiols. Langmuir 2017, 33, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-Assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Fu, S.; Zhang, S.; Chen, S.; Zou, X. Designing and Fabricating of Single and Double Alkyl-Chain Indazole Derivatives Self-Assembled Monolayer for Corrosion Inhibition of Copper. Corros. Sci. 2018, 140, 111–121. [Google Scholar] [CrossRef]
- Yoon, H.J.; Liao, K.-C.; Lockett, M.R.; Kwok, S.W.; Baghbanzadeh, M.; Whitesides, G.M. Rectification in Tunneling Junctions: 2,2′-Bipyridyl-Terminated n-Alkanethiolates. J. Am. Chem. Soc. 2014, 136, 17155–17162. [Google Scholar] [CrossRef]
- Macchia, E.; Tiwari, A.; Manoli, K.; Holzer, B.; Ditaranto, N.; Picca, R.A.; Cioffi, N.; Di Franco, C.; Scamarcio, G.; Palazzo, G.; et al. Label-Free and Selective Single-Molecule Bioelectronic Sensing with a Millimeter-Wide Self-Assembled Monolayer of Anti-Immunoglobulins. Chem. Mater. 2019, 31, 6476–6483. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]
- Acosta, S.; Quintanilla, L.; Alonso, M.; Aparicio, C.; Rodríguez-Cabello, J.C. Recombinant AMP/Polypeptide Self-Assembled Monolayers with Synergistic Antimicrobial Properties for Bacterial Strains of Medical Relevance. ACS Biomater. Sci. Eng. 2019, 5, 4708–4716. [Google Scholar] [CrossRef]
- Jakobi, V.; Schwarze, J.; Finlay, J.A.; Nolte, K.A.; Spöllmann, S.; Becker, H.-W.; Clare, A.S.; Rosenhahn, A. Amphiphilic Alginates for Marine Antifouling Applications. Biomacromolecules 2018, 19, 402–408. [Google Scholar] [CrossRef]
- Crudden, C.M.; Horton, J.H.; Ebralidze, I.I.; Zenkina, O.V.; McLean, A.B.; Drevniok, B.; She, Z.; Kraatz, H.-B.; Mosey, N.J.; Seki, T.; et al. Ultra Stable Self-Assembled Monolayers of N-Heterocyclic Carbenes on Gold. Nat. Chem. 2014, 6, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Narouz, M.R.; Munro, K.; Hao, B.; Crudden, C.M.; Horton, J.H.; Hao, H. Carboxymethylated Dextran-Modified N-Heterocyclic Carbene Self-Assembled Monolayers on Gold for Use in Surface Plasmon Resonance Biosensing. ACS Appl. Mater. Interfaces 2017, 9, 39223–39234. [Google Scholar] [CrossRef] [PubMed]
- St. Hill, L.R.; Craft, J.W.; Chinwangso, P.; Tran, H.-V.; Marquez, M.D.; Lee, T.R. Antifouling Coatings Generated from Unsymmetrical Partially Fluorinated Spiroalkanedithiols. ACS Appl. Bio Mater. 2021, 4, 1563–1572. [Google Scholar] [CrossRef]
- St. Hill, L.R.; Tran, H.-V.; Chinwangso, P.; Lee, H.J.; Marquez, M.D.; Craft, J.W.; Lee, T.R. Antifouling Studies of Unsymmetrical Oligo(ethylene glycol) Spiroalkanedithiol Self-Assembled Monolayers. Micro 2021, 1, 12. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Yau, S.; Chau, L.-K.; Mohamed, A.; Huang, C.-J. Functional Biointerfaces Based on Mixed Zwitterionic Self-Assembled Monolayers for Biosensing Applications. Langmuir 2019, 35, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Tabata, M.; Sanjoh, M.; Uchimura, M.; Iwasaki, Y.; Miyahara, Y. Thiolated 2-Methacryloyloxyethyl Phosphorylcholine for an Antifouling Biosensor Platform. Chem. Commun. 2013, 49, 8683–8685. [Google Scholar] [CrossRef] [PubMed]
- Koc, J.; Schardt, L.; Nolte, K.; Beyer, C.; Eckhard, T.; Schwiderowski, P.; Clarke, J.L.; Finlay, J.A.; Clare, A.S.; Muhler, M.; et al. Effect of Dipole Orientation in Mixed, Charge-Equilibrated Self-Assembled Monolayers on Protein Adsorption and Marine Biofouling. ACS Appl. Mater. Interfaces 2020, 12, 50953–50961. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Yan, L.; Whitesides, G.M. The Interaction of Proteins and Cells with Self-Assembled Monolayers of Alkanethiolates on Gold and Silver. Colloids Surf. B Biointerfaces 1999, 15, 3–30. [Google Scholar] [CrossRef]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; et al. A Comparative Study of Protein Adsorption on Titanium Oxide Surfaces Using in Situ Ellipsometry, Optical Waveguide Lightmode Spectroscopy, and Quartz Crystal Microbalance/Dissipation. Colloids Surf. B Biointerfaces 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Elwing, H. Protein Absorption and Ellipsometry in Biomaterial Research. Biomaterials 1998, 19, 397–406. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Liang, M.N.; Meluleni, G.; Pier, G.; Ingber, D.E.; Whitesides, G.M. Self-Assembled Monolayers that Resist the Adsorption of Proteins and the Adhesion of Bacterial and Mammalian Cells. Langmuir 2001, 17, 6336–6343. [Google Scholar] [CrossRef]
- Chapman, R.G.; Ostuni, E.; Yan, L.; Whitesides, G.M. Preparation of Mixed Self-Assembled Monolayers (SAMs) that Resist Adsorption of Proteins Using the Reaction of Amines with a SAM that Presents Interchain Carboxylic Anhydride Groups. Langmuir 2000, 16, 6927–6936. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Self-Assembled Organic Monolayers: Model Systems for Studying Adsorption of Proteins at Aurfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Harder, P.; Grunze, M.; Dahint, R.; Whitesides, G.M.; Laibinis, P.E. Molecular Conformation in Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers on Gold and Silver Surfaces Determines their Ability To Resist Protein Adsorption. J. Phys. Chem. B 1998, 102, 426–436. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Zheng, J.; Ratner, B.D.; Jiang, S. Protein Adsorption on Oligo(ethylene glycol)-Terminated Alkanethiolate Self-Assembled Monolayers: The Molecular Basis for Nonfouling Behavior. J. Phys. Chem. B 2005, 109, 2934–2941. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Adsorption of Proteins onto Surfaces Containing End-Attached Oligo(ethylene oxide): A Model System Using Self-Assembled Monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that Determine the Protein Resistance of Oligoether Self-Assembled Monolayers-Internal Hydrophilicity, Terminal Hydrophilicity, and Lateral Packing Density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that Resist Nonspecific Adsorption of Protein from Aqueous Buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chu, S.-H.; Li, C.-H.; Lee, T.R. Surface Modification with Zwitterionic Cysteine Betaine for Nanoshell-Assisted Near-Infrared Plasmonic Hyperthermia. Colloids Surf. B Biointerfaces 2016, 145, 291–300. [Google Scholar] [CrossRef]
- Luk, Y.-Y.; Kato, M.; Mrksich, M. Self-Assembled Monolayers of Alkanethiolates Presenting Mannitol Groups are Inert to Protein Adsorption and Cell Attachment. Langmuir 2000, 16, 9604–9608. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure-Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Chen, S.; Yu, F.; Yu, Q.; He, Y.; Jiang, S. Strong Resistance of a Thin Crystalline Layer of Balanced Charged Groups to Protein Adsorption. Langmuir 2006, 22, 8186–8191. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, J.; Li, L.; Jiang, S. Strong Resistance of Phosphorylcholine Self-Assembled Monolayers to Protein Adsorption: Insights into Nonfouling Properties of Zwitterionic Materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chu, S.-H.; Wang, L.-C.; Li, C.-H.; Lee, T.R. Bioinspired Zwitterionic Surface Coatings with Robust Photostability and Fouling Resistance. ACS Appl. Mater. Interfaces 2015, 7, 23776–23786. [Google Scholar] [CrossRef]
- Humblot, V.; Yala, J.-F.; Thebault, P.; Boukerma, K.; Héquet, A.; Berjeaud, J.-M.; Pradier, C.-M. The Antibacterial Activity of Magainin I Immobilized onto Mixed Thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef]
- Nowinski, A.K.; Sun, F.; White, A.D.; Keefe, A.J.; Jiang, S. Sequence, Structure, and Function of Peptide Self-Assembled Monolayers. J. Am. Chem. Soc. 2012, 134, 6000–6005. [Google Scholar] [CrossRef]
- Fyrner, T.; Lee, H.-H.; Mangone, A.; Ekblad, T.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Conlan, S.L.; Mutton, R.; Clare, A.S.; et al. Saccharide-Functionalized Alkanethiols for Fouling-Resistant Self-Assembled Monolayers: Synthesis, Monolayer Properties, and Antifouling Behavior. Langmuir 2011, 27, 15034–15047. [Google Scholar] [CrossRef]
- Siegers, C.; Biesalski, M.; Haag, R. Self-Assembled Monolayers of Dendritic Polyglycerol Derivatives on Gold that Resist the Adsorption of Proteins. Chem. Eur. J. 2004, 10, 2831–2838. [Google Scholar] [CrossRef]
- Callow, J.A.; Callow, M.E.; Ista, L.K.; Lopez, G.; Chaudhury, M.K. The Influence of Surface Energy on the Wetting Behaviour of the Spore Adhesive of the Marine Alga Ulva linza (Synonym Enteromorpha linza). J. R. Soc. Interface 2005, 2, 319–325. [Google Scholar] [CrossRef]
- Khan, M.M.T.; Ista, L.K.; Lopez, G.P.; Schuler, A.J. Experimental and Theoretical Examination of Surface Energy and Adhesion of Nitrifying and Heterotrophic Bacteria Using Self-Assembled Monolayers. Environ. Sci. Technol. 2011, 45, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Lehnfeld, J.; Dukashin, Y.; Mark, J.; White, G.D.; Wu, S.; Katzur, V.; Müller, R.; Ruhl, S. Saliva and Serum Protein Adsorption on Chemically Modified Silica Surfaces. J. Dent. Res. 2021, 100, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Lee, J.M.; Park, J.-G.; Chung, B.G. A Self-Assembled Monolayer-Based Micropatterned Array for Controlling Cell Adhesion and Protein Adsorption. Biotechnol. Bioeng. 2011, 108, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Pranzetti, A.; Salaün, S.; Mieszkin, S.; Callow, M.E.; Callow, J.A.; Preece, J.A.; Mendes, P.M. Model Organic Surfaces to Probe Marine Bacterial Adhesion Kinetics by Surface Plasmon Resonance. Adv. Funct. Mater. 2012, 22, 3672–3681. [Google Scholar] [CrossRef]
- Faucheux, N.; Schweiss, R.; Lützow, K.; Werner, C.; Groth, T. Self-Assembled Monolayers with Different Terminating Groups as Model Substrates for Cell Adhesion Studies. Biomaterials 2004, 25, 2721–2730. [Google Scholar] [CrossRef]
- Patel, K.R.; Tang, H.; Grever, W.E.; Simon Ng, K.Y.; Xiang, J.; Keep, R.F.; Cao, T.; McAllister, J.P. Evaluation of Polymer and Self-Assembled Monolayer-Coated Silicone Surfaces to Reduce Neural Cell Growth. Biomaterials 2006, 27, 1519–1526. [Google Scholar] [CrossRef]
- Li, S.; Yang, D.; Tu, H.; Deng, H.; Du, D.; Zhang, A. Protein Adsorption and Cell Adhesion Controlled by the Surface Chemistry of Binary Perfluoroalkyl/Oligo(ethylene glycol) Self-Assembled Monolayers. J. Colloid Interface Sci. 2013, 402, 284–290. [Google Scholar] [CrossRef]
- Yang, Z.; Galloway, J.A.; Yu, H. Protein Interactions with Poly(ethylene glycol) Self-Assembled Monolayers on Glass Substrates: Diffusion and Adsorption. Langmuir 1999, 15, 8405–8411. [Google Scholar] [CrossRef]
- Feldman, K.; Hähner, G.; Spencer, N.D.; Harder, P.; Grunze, M. Probing Resistance to Protein Adsorption of Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers by Scanning Force Microscopy. J. Am. Chem. Soc. 1999, 121, 10134–10141. [Google Scholar] [CrossRef]
- Lan, S.; Veiseh, M.; Zhang, M. Surface Modification of Silicon and Gold-Patterned Silicon Surfaces for Improved Biocompatibility and Cell Patterning Selectivity. Biosens. Bioelectron. 2005, 20, 1697–1708. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; De Gennes, P.G. Protein—Surface Interactions in the Presence of Polyethylene Oxide: I. Simplified Theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Latour, R.A. Fundamental Principles of the Thermodynamics and Kinetics of Protein Adsorption to Material Surfaces. Colloids Surf. B Biointerfaces 2020, 191, 110992. [Google Scholar] [CrossRef] [PubMed]
- Pertsin, A.J.; Grunze, M. Computer Simulation of Water near the Surface of Oligo(ethylene glycol)-Terminated Alkanethiol Self-Assembled Monolayers. Langmuir 2000, 16, 8829–8841. [Google Scholar] [CrossRef]
- Pertsin, A.J.; Hayashi, T.; Grunze, M. Grand Canonical Monte Carlo Simulations of the Hydration Interaction between Oligo(ethylene glycol)-Terminated Alkanethiol Self-Assembled Monolayers. J. Phys. Chem. B 2002, 106, 12274–12281. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface Hydration: Principles and Applications toward Low-Fouling/Nonfouling Biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent Advances in Zwitterionic Hydrogels: Preparation, Property, and Biomedical Application. Gels 2022, 8, 46. [Google Scholar] [CrossRef]
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why Do Phospholipid Polymers Reduce Protein Adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Influence of Charged Groups on the Properties of Zwitterionic Moieties: A Molecular Simulation Study. J. Phys. Chem. B 2014, 118, 7630–7637. [Google Scholar] [CrossRef]
- Amit, M.; Yuran, S.; Gazit, E.; Reches, M.; Ashkenasy, N. Tailor-Made Functional Peptide Self-Assembling Nanostructures. Adv. Mater. 2018, 30, 1707083. [Google Scholar] [CrossRef]
- Hower, J.C.; He, Y.; Bernards, M.T.; Jiang, S. Understanding the Nonfouling Mechanism of Surfaces through Molecular Simulations of Sugar-Based Self-Assembled Monolayers. J. Chem. Phys. 2006, 125, 214704. [Google Scholar] [CrossRef] [PubMed]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-Toxic, Non-Biocide-Release Antifouling Coatings Based on Molecular Structure Design for Marine Applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [PubMed]
- Baier, R.E. Surface Behaviour of Biomaterials: The Theta Surface for Biocompatibility. J. Mater. Sci.: Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Shafrin, E.G.; Zisman, W.A. Critical Surface Tension for Spreading on a Liquid Substrate. J. Phys. Chem. 1967, 71, 1309–1316. [Google Scholar] [CrossRef]
- Schrader, M.E. On Adhesion of Biological Substances to Low Energy Solid Surfaces. J. Colloid Interface Sci. 1982, 88, 296–297. [Google Scholar] [CrossRef]
- Ostuni, E.; Grzybowski, B.A.; Mrksich, M.; Roberts, C.S.; Whitesides, G.M. Adsorption of Proteins to Hydrophobic Sites on Mixed Self-Assembled Monolayers. Langmuir 2003, 19, 1861–1872. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.H.; Li, X.S.; Yang, J.Y.; Que, G.H. Influence of Surface Free Energy on the Adhesion of Marine Benthic Diatom Nitzschia Closterium MMDL533. Colloids Surf. B Biointerfaces 2010, 75, 550–556. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Chen, S.; Horbett, T.A.; Ratner, B.D.; Jiang, S. Blood Compatibility of Surfaces with Superlow Protein Adsorption. Biomaterials 2008, 29, 4285–4291. [Google Scholar] [CrossRef]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of Bacterial Adhesion and Biofilm Formation on Zwitterionic Surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef]
- Sperling, C.; Schweiss, R.B.; Streller, U.; Werner, C. In Vitro Hemocompatibility of Self-Assembled Monolayers Displaying Various Functional Groups. Biomaterials 2005, 26, 6547–6557. [Google Scholar] [CrossRef]
- Brady, R.F., Jr.; Singer, I.L. Mechanical Factors Favoring Release from Fouling Release Coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, C.S.; Finlay, J.A.; Callow, J.A.; Callow, M.E.; Wooley, K.L. The Antifouling and Fouling-Release Performance of Hyperbranched Fluoropolymer (HBFP)−Poly(ethylene glycol) (PEG) Composite Coatings Evaluated by Adsorption of Biomacromolecules and the Green Fouling Alga Ulva. Langmuir 2005, 21, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Chinwangso, P.; Lee, H.J.; Lee, T.R. Self-Assembled Monolayers Generated from Unsymmetrical Partially Fluorinated Spiroalkanedithiols. Langmuir 2015, 31, 13341–13349. [Google Scholar] [CrossRef] [PubMed]
- Marquez, M.D.; Zenasni, O.; Jamison, A.C.; Lee, T.R. Homogeneously Mixed Monolayers: Emergence of Compositionally Conflicted Interfaces. Langmuir 2017, 33, 8839–8855. [Google Scholar] [CrossRef]
- Chinwangso, P.; St. Hill, L.R.; Marquez, M.D.; Lee, T.R. Unsymmetrical Spiroalkanedithiols Having Mixed Fluorinated and Alkyl Tailgroups of Varying Length: Film Structure and Interfacial Properties. Molecules 2018, 23, 2632. [Google Scholar] [CrossRef]
- Choi, Y.; Park, C.S.; Tran, H.-V.; Li, C.-H.; Crudden, C.M.; Lee, T.R. Functionalized N-Heterocyclic Carbene Monolayers on Gold for Surface-Initiated Polymerizations. ACS Appl. Mater. Interfaces 2022. [Google Scholar] [CrossRef]

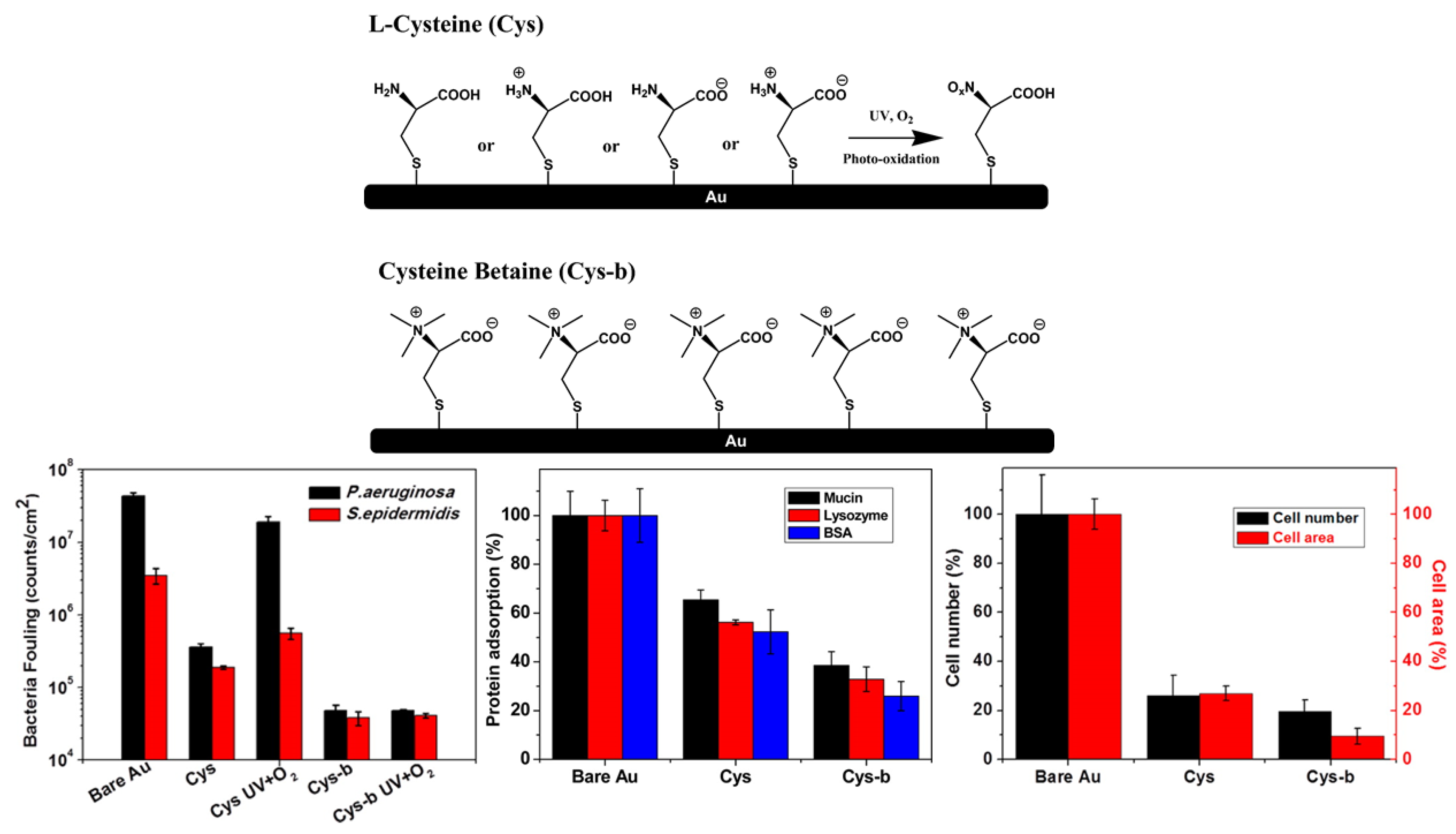
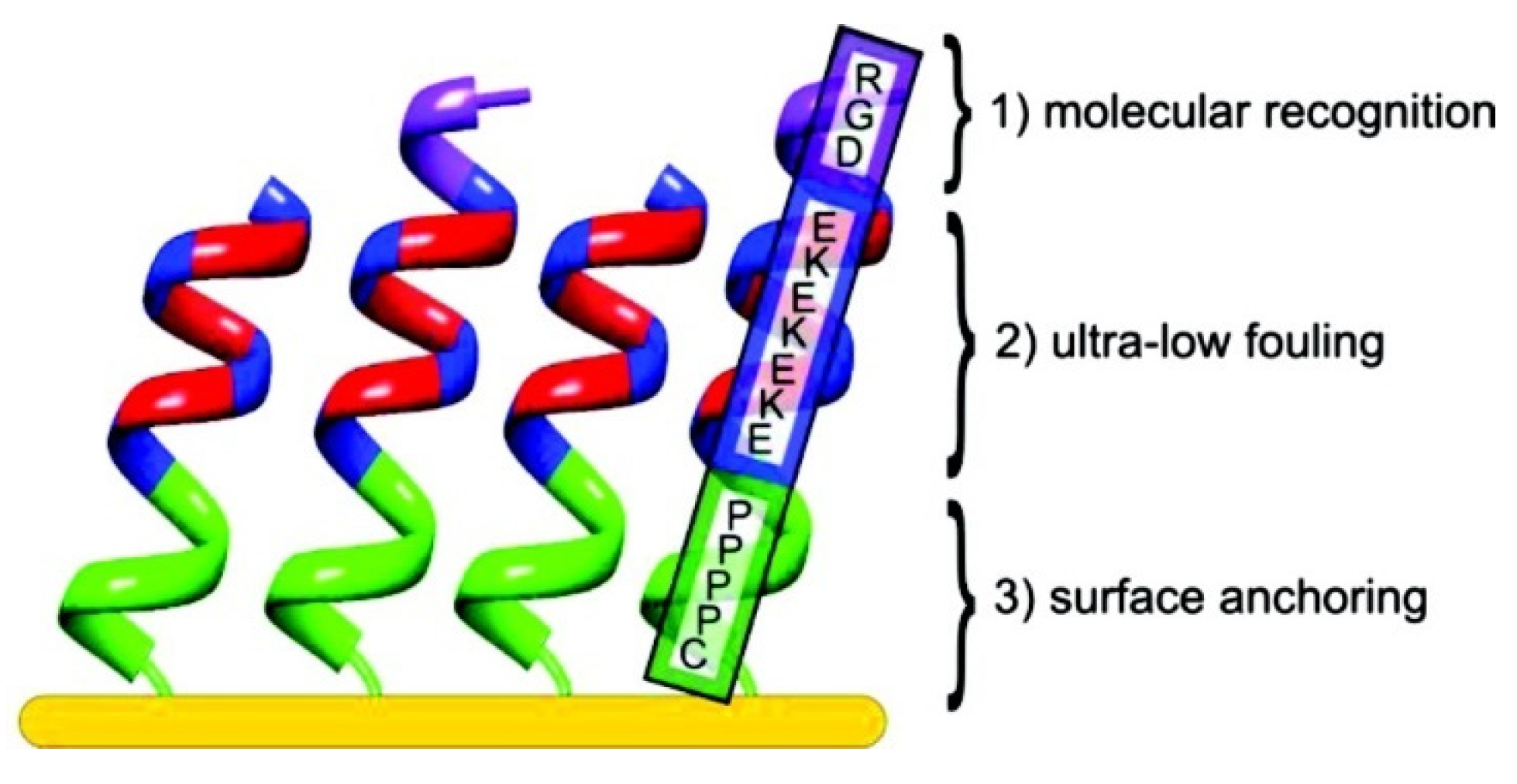
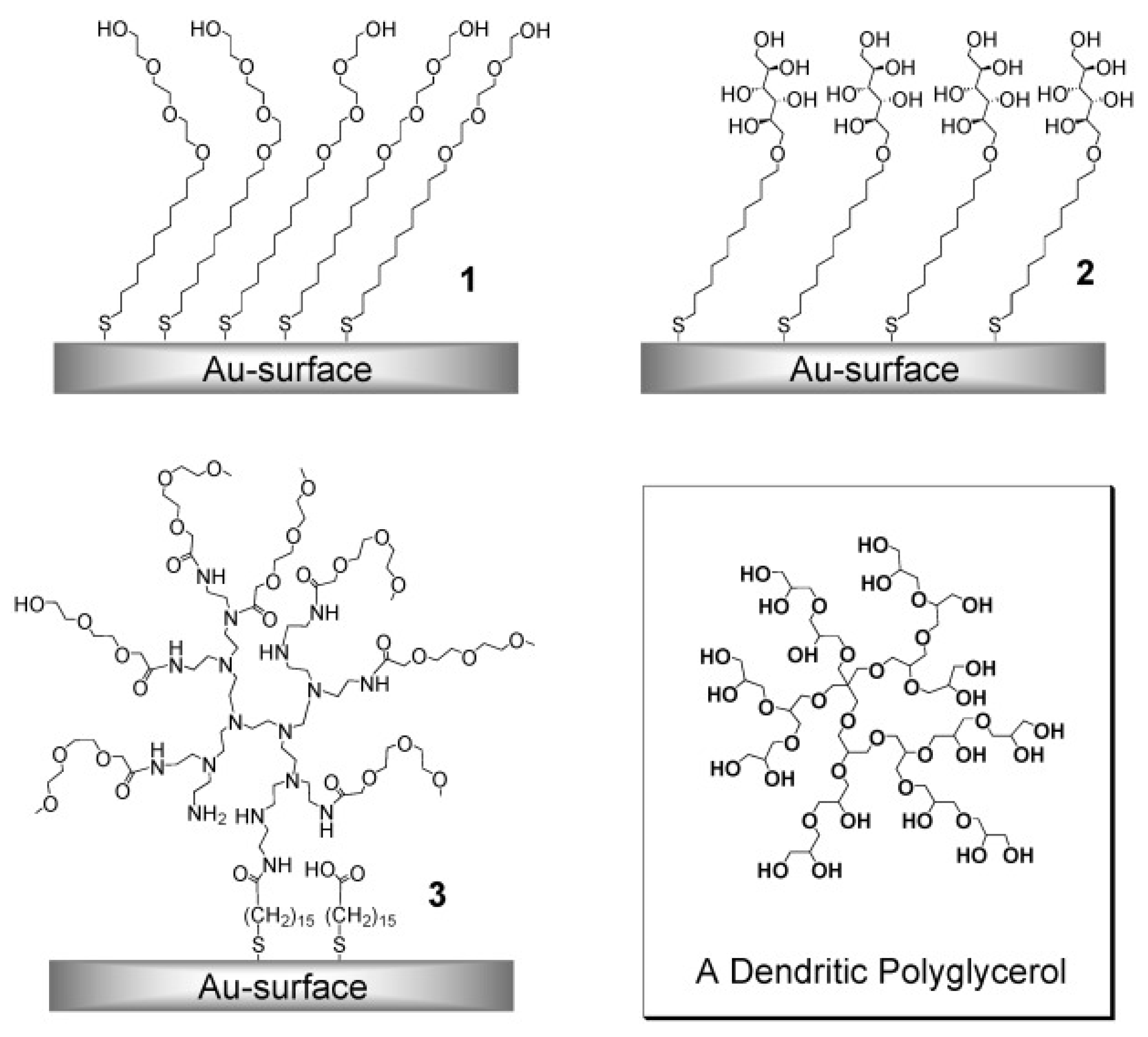
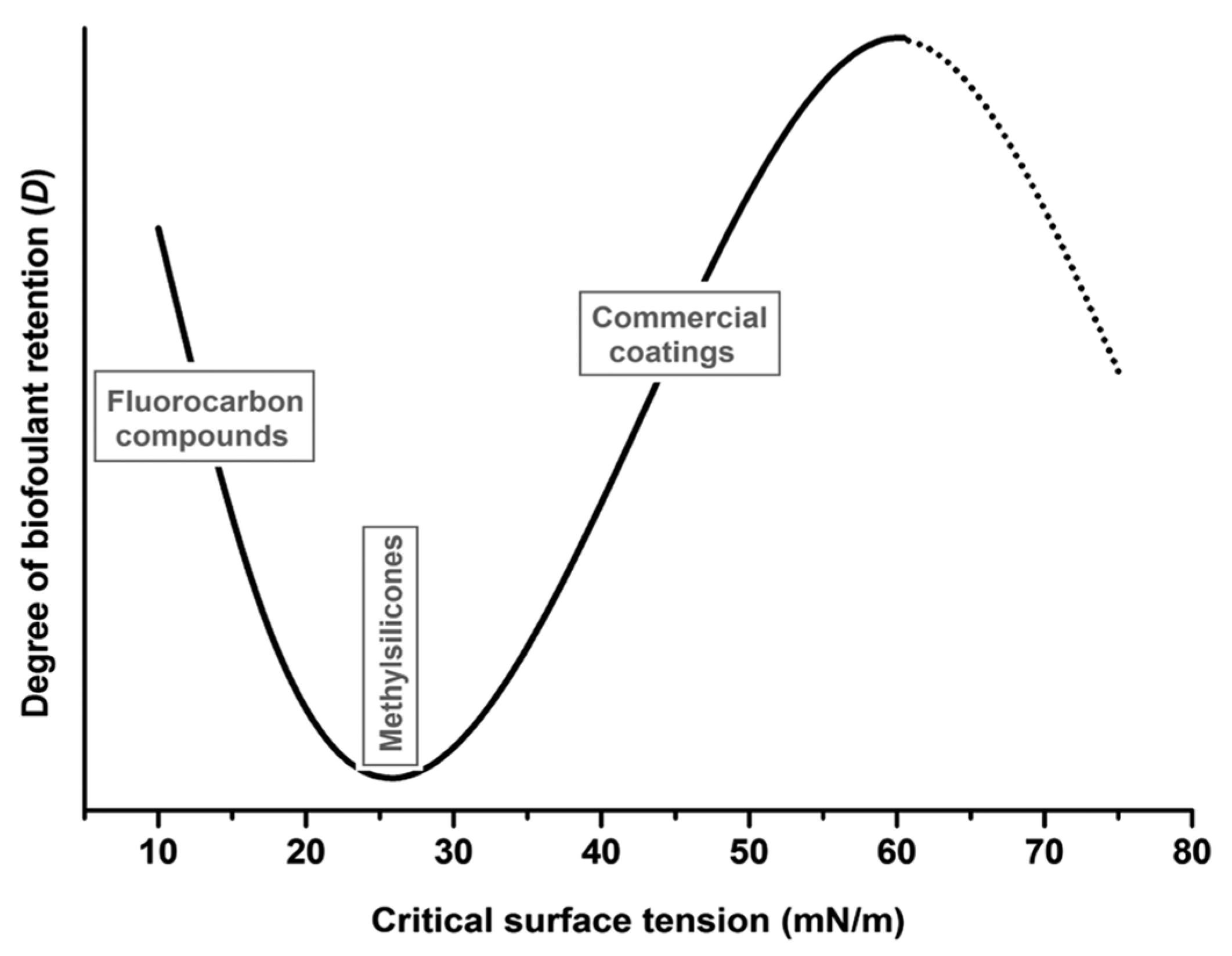
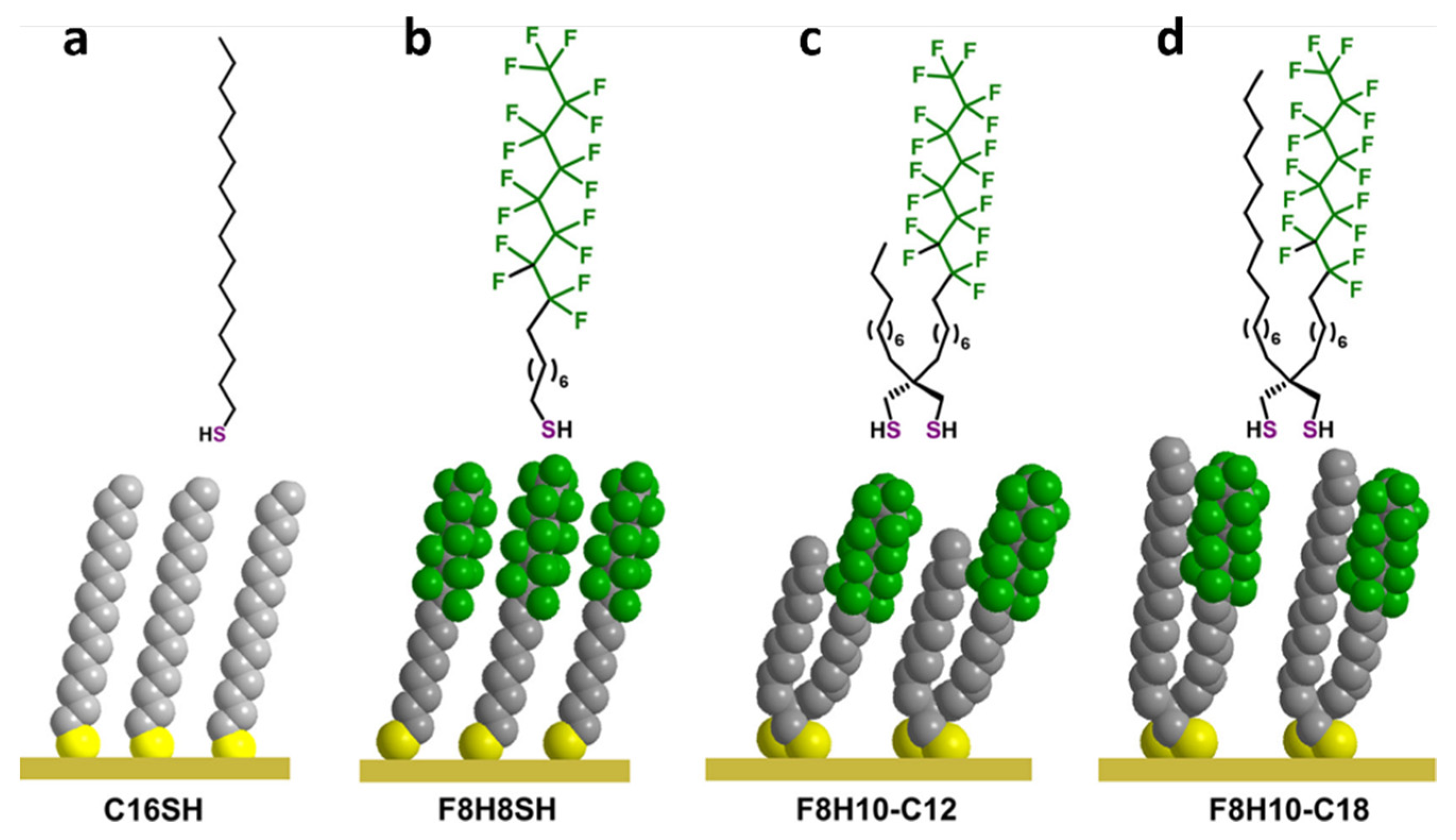
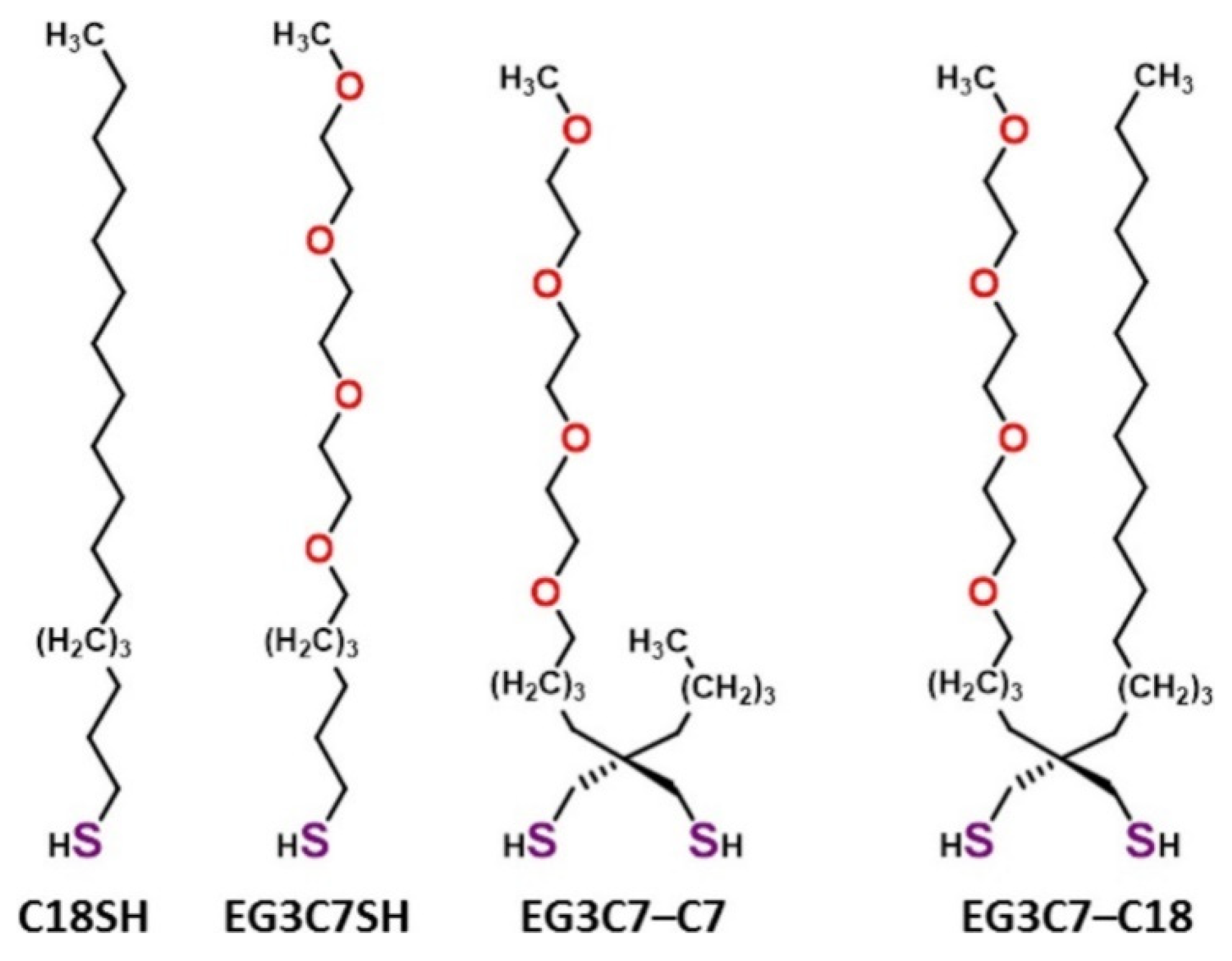
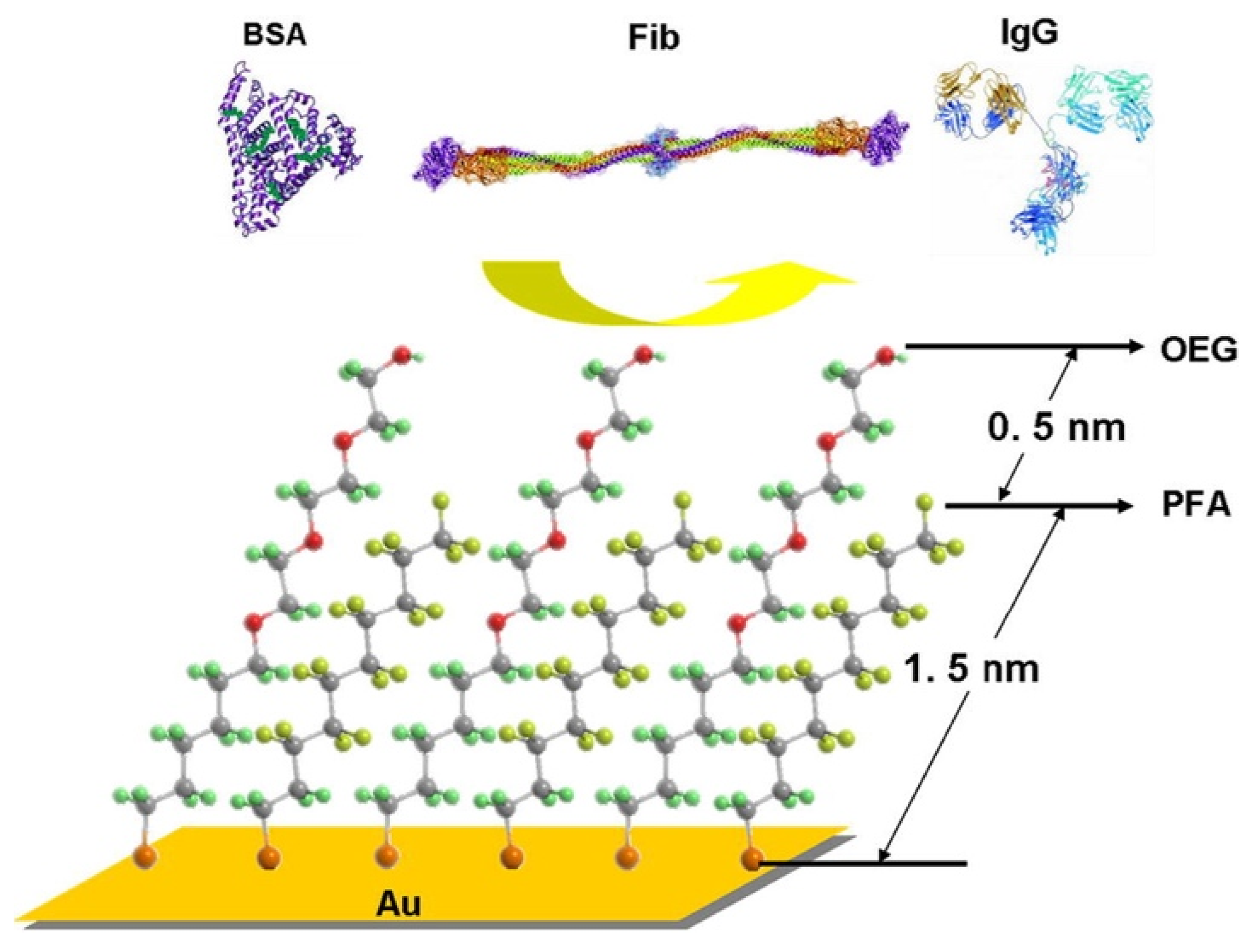

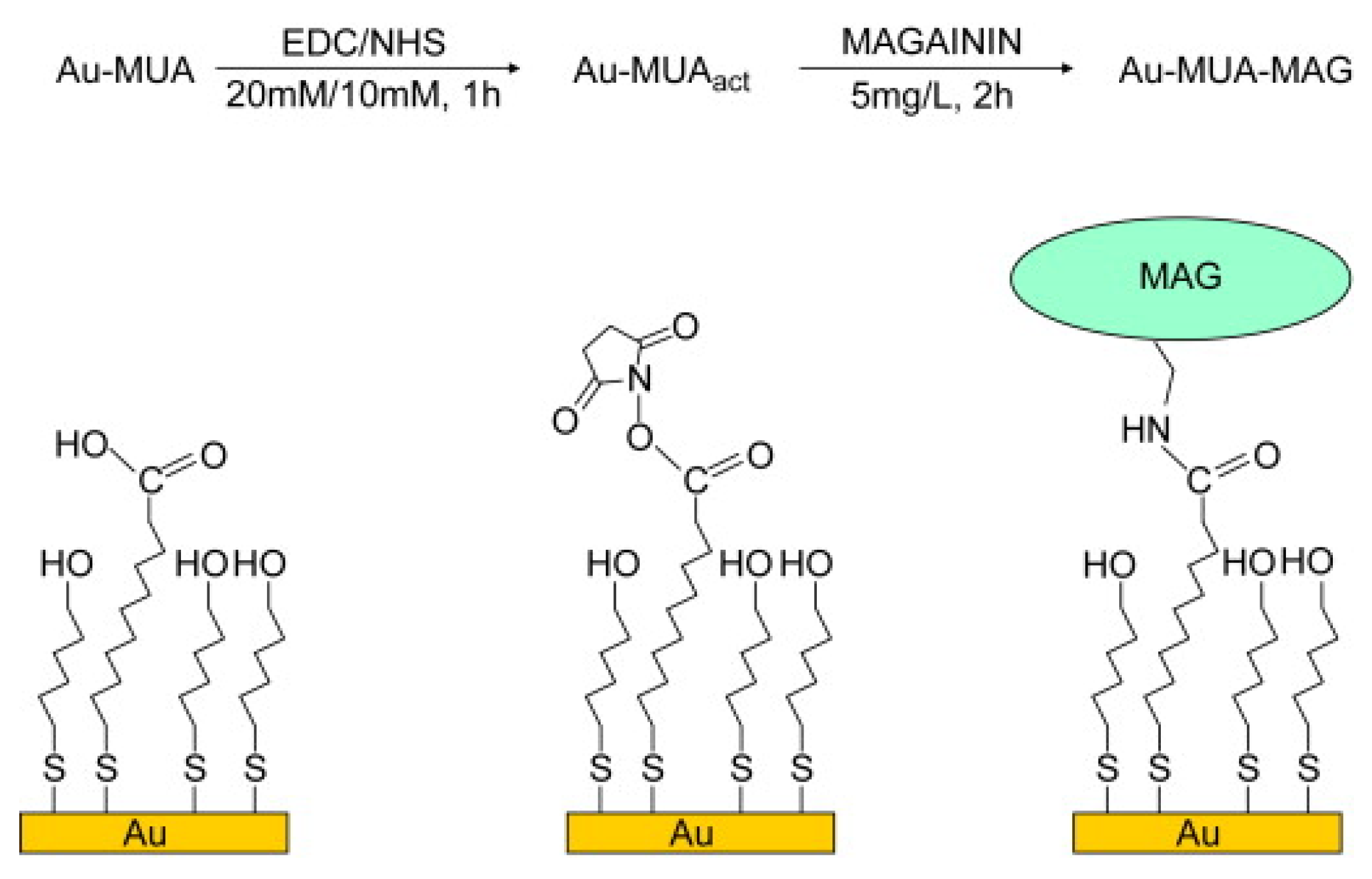
| Antifouling Approach | Material Type | Headgroup/Substrate | Functional Group | Detection Method | Tested Fouling Materials | Ref. |
|---|---|---|---|---|---|---|
| Hydrophilic | OEG | SH/Au | –(OCH2CH2)3OH | SPR, microscope | Fibrinogen, lysozyme, S. aureus, S. epidermidis, BCE cells | [44] |
| SH/Au | –C(O)NH(CH2CH2O)6CH3 | SPR | Fibrinogen, ribonuclease A, lysozyme, carbonic anhydrase | [45] | ||
| SH/Au | –(OCH2CH2)6OH | Ellipsometry | RNase A, pyruvate kinase, fibrinogen | [46] | ||
| SH/Au or Ag | –(OCH2CH2)3OCH3 | FT-IR | Albumin, IgG, fibrinogen | [47] | ||
| SH/Au | –(OCH2CH2)nOH n = 2, 4 and 6 | SPR | Fibrinogen, lysozyme, BSA | [48] | ||
| SH/Au | –(OCH2CH2)6OR R = H or CH3 | Ellipsometry | Fibrinogen, pyruvate kinase, lysozyme, ribonuclease A | [49] | ||
| SH/Au | –(OCH2CH2)nOR n = 1, 2, 3, 6 and R = H, CH3, CH2CH3, CH2CH2CH3 | Ellipsometry | Fibrinogen | [50] | ||
| SH/Au | –(OCH2CH2)3OH | SPR | BSA, β-galactosidase, carbonic anhydrase II, fibrinogen, myoglobin, ribonuclease A, cytochrome c, lysozyme | [51] | ||
| SH/Au | –(OCH2CH2)3OH | Microscope | S. epidermidis, P. aeruginosa, 3T3 fibroblasts | [52] | ||
| SH/Au | –(OCH2CH2)3OH | SPR, microscope | Fibrinogen, pepsin, lysozyme, insulin, trypsin 3T3 fibroblast | [53] | ||
| SH/Au | –C(O)NH(CH2CH2O)nR n = 3, 6 and R = H, CH3 | SPR | Fibrinogen, lysozyme | [54] | ||
| Zwitterion | SH/Au | –OP(O)2−OCH2CH2N+(CH3)3 –N+(CH3)2CH2CH2CH2SO3− | SPR, microscope | Fibrinogen, lysozyme, S. aureus, S. epidermidis, BCE cells | [44] | |
| SH/Au | –OP(O)2−OCH2CH2N+(CH3)3 | QCM | BSA, fibrinogen, FBS | [38] | ||
| SH/Au | 1:1 mixture of –OPO3− and –N+(CH3)3 | SPR | Fibrinogen | [55] | ||
| SH/Au | –SO3− –N+(CH3)3 –N+(CH3)2CH2CH2CH2SO3− –OP(O)2−OCH2CH2N+(CH3)3 | SPR | BSA, β-galactosidase, carbonic anhydrase II, fibrinogen, myoglobin, ribonuclease A, cytochrome c, lysozyme | [51] | ||
| SH/Au | –OP(O)2−OCH2CH2N+(CH3)3 | SPR | Fibrinogen, BSA | [56] | ||
| SH/Au | –CH(N+(CH3)3)COO− | Microscope | S. aureus, S. epidermidis, 3T3 fibroblasts | [52] | ||
| SH/Au | –CH(N+(CH3)3)COO− | ELISA, microscope | BSA, lysozyme, mucin, S. aureus, S. epidermidis, 3T3 fibroblasts | [57] | ||
| Peptide-base | SH/Au | Magainin I | Microscope, AFM, PM-IRRAS | L. ivanovii, E. faecalis, S. aureus | [58] | |
| SH/Au | Glutamic acid/lysine peptide | SPR | Fibrinogen, lysozyme | [59] | ||
| Others | SH/Au | Permethylated sorbitol | SPR, microscope | Fibrinogen, lysozyme, S. aureus, S. epidermidis, BCE cells | [44] | |
| SH/Au | Saccharide | Ellipsometry, microscope | Lysozyme, fibrinogen, BSA, pepsin, Ulva linza, Balanus amphitrite | [60] | ||
| SH/Au | Mannitol | SPR, microscope | Fibrinogen, pepsin, lysozyme, insulin, trypsin 3T3 fibroblast | [53] | ||
| SH/Au | Dendritic polyglycerol | SPR | Fibrinogen | [61] | ||
| SH/Au | Saccharide or sorbitol | SPR | Fibrinogen, lysozyme | [54] | ||
| Hydrophobic | - | Silane/SiO2 | –(CF2)7CF3 | Microscope | Ulva linza | [62] |
| SH/Au | –(CH2)11CH3 | Microscope | N. europaea, N. multiformis, E. coli | [63] | ||
| Silane/SiO2 | –(CF2)7CF3 –(CH2)7CH3 | SDS-PAGE and immunoblotting, bicinchoninic acid assay | Human saliva, serum | [64] | ||
| Silane/SiO2 | –(CF2)5CF3 | Microscope | Fibroblast cells, embryonic stem cells | [65] | ||
| SH/Au | –(CH2)11CH3 | SPR, microscope | C. marina, M. hydrocarbonoclasticus | [66] | ||
| Silane/SiO2 | –(CH2)17CH3 | Microscope | Fibroblasts | [67] | ||
| Silane/SiO2 | –(CH2)17CH3 –(CF2)5CF3 | Microscope | Astrocyte, Choroid plexus, | [68] | ||
| (CH2SH)2/Au | 1:1 mixture of –(CH2)8(CF2)7CF3 and –(CH2)15CH3 | Ellipsometry, QCM, SPR | Protamine, lysozyme, BSA, fibrinogen | [35] | ||
| Amphiphilic | - | (CH2SH)2/Au | 1:1 mixture of –(CH2)5(OCH2CH2)3OCH3 and –(CH2)15CH3 | Ellipsometry, QCM, SPR | Protamine, lysozyme, BSA, fibrinogen | [25,36] |
| SH/Au | 1:1 mixture of –(OCH2CH2)3OH and –(CF2)7CF3 | SPR, microscope | BSA, fibrinogen, immunoglobulin, Hela cell | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Tran, H.-V.; Lee, T.R. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings 2022, 12, 1462. https://doi.org/10.3390/coatings12101462
Choi Y, Tran H-V, Lee TR. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings. 2022; 12(10):1462. https://doi.org/10.3390/coatings12101462
Chicago/Turabian StyleChoi, Yunsoo, Hung-Vu Tran, and T. Randall Lee. 2022. "Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review" Coatings 12, no. 10: 1462. https://doi.org/10.3390/coatings12101462
APA StyleChoi, Y., Tran, H.-V., & Lee, T. R. (2022). Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings, 12(10), 1462. https://doi.org/10.3390/coatings12101462








