Characterisation of Bovine Amniotic Membrane with Hydroxyapatite Bio-Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Materials
2.1.1. BAM Preparation
2.1.2. HAp Preparation
2.1.3. Bio-Composite Fabrication
2.2. Physicochemical Characterization
2.2.1. FTIR Analysis
2.2.2. XRD Analysis
2.2.3. SEM Analysis
2.2.4. Porosity Analysis
2.2.5. Biological Activity Using Fibroblasts
3. Results
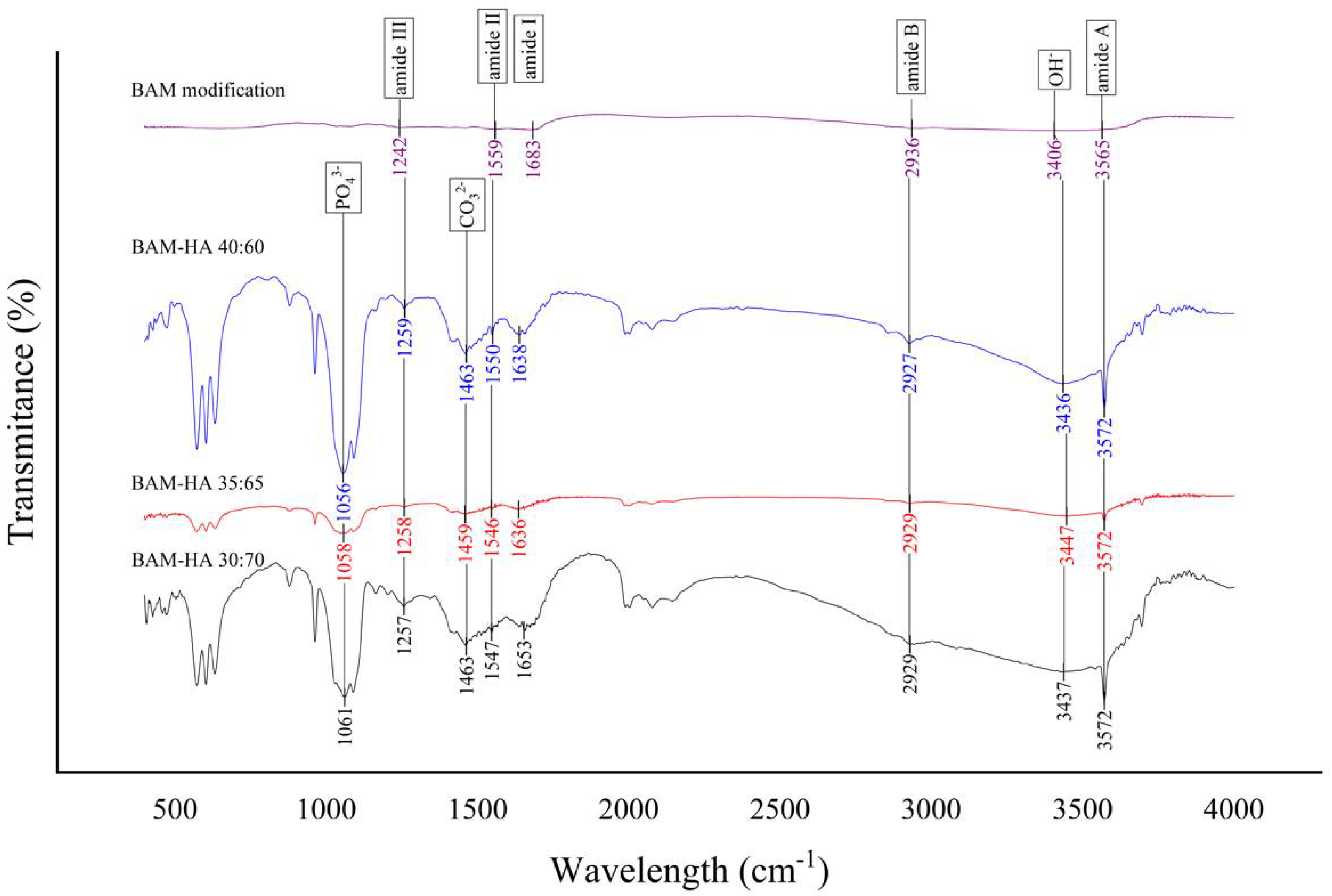
3.1. FTIR Analysis
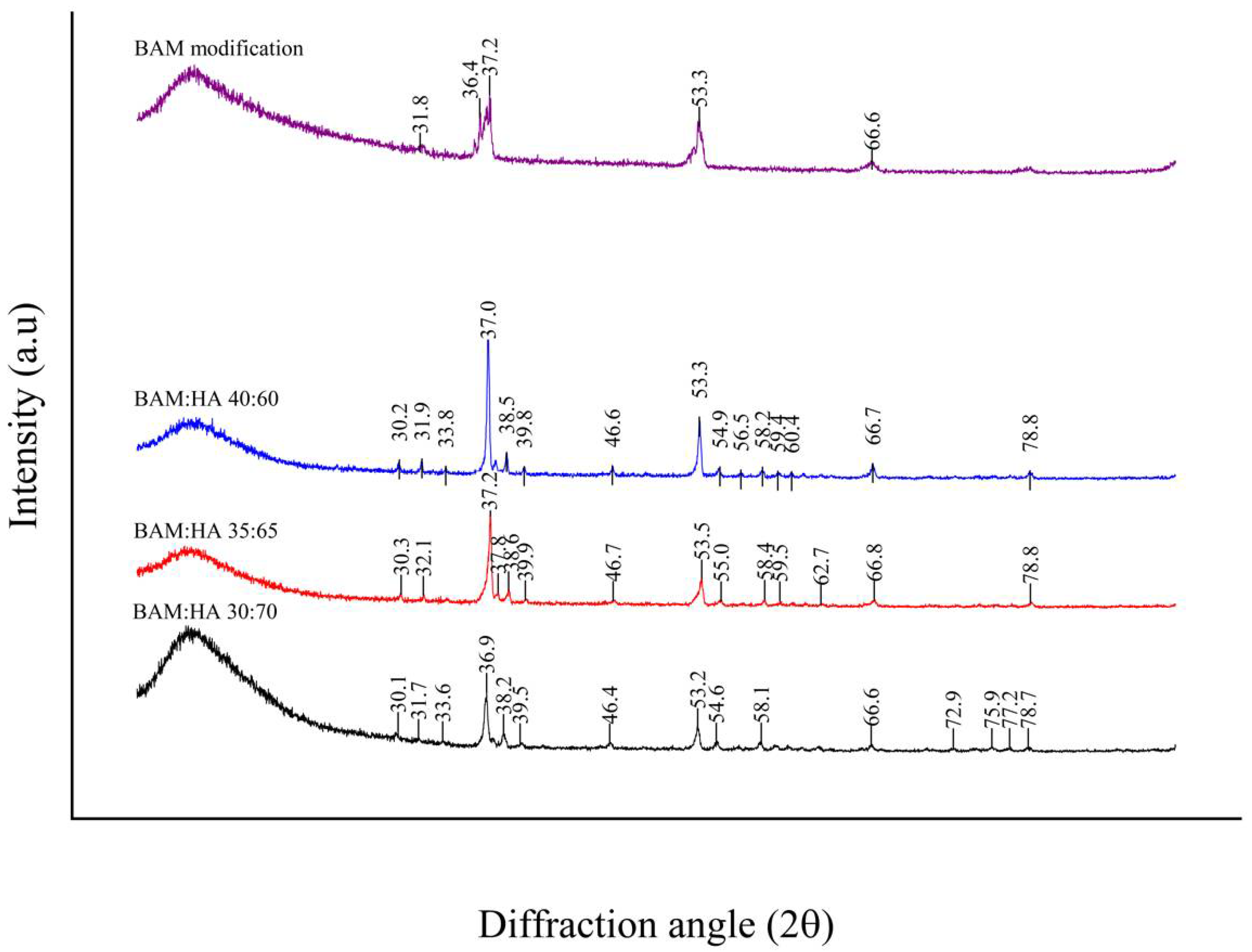
3.2. XRD Analysis
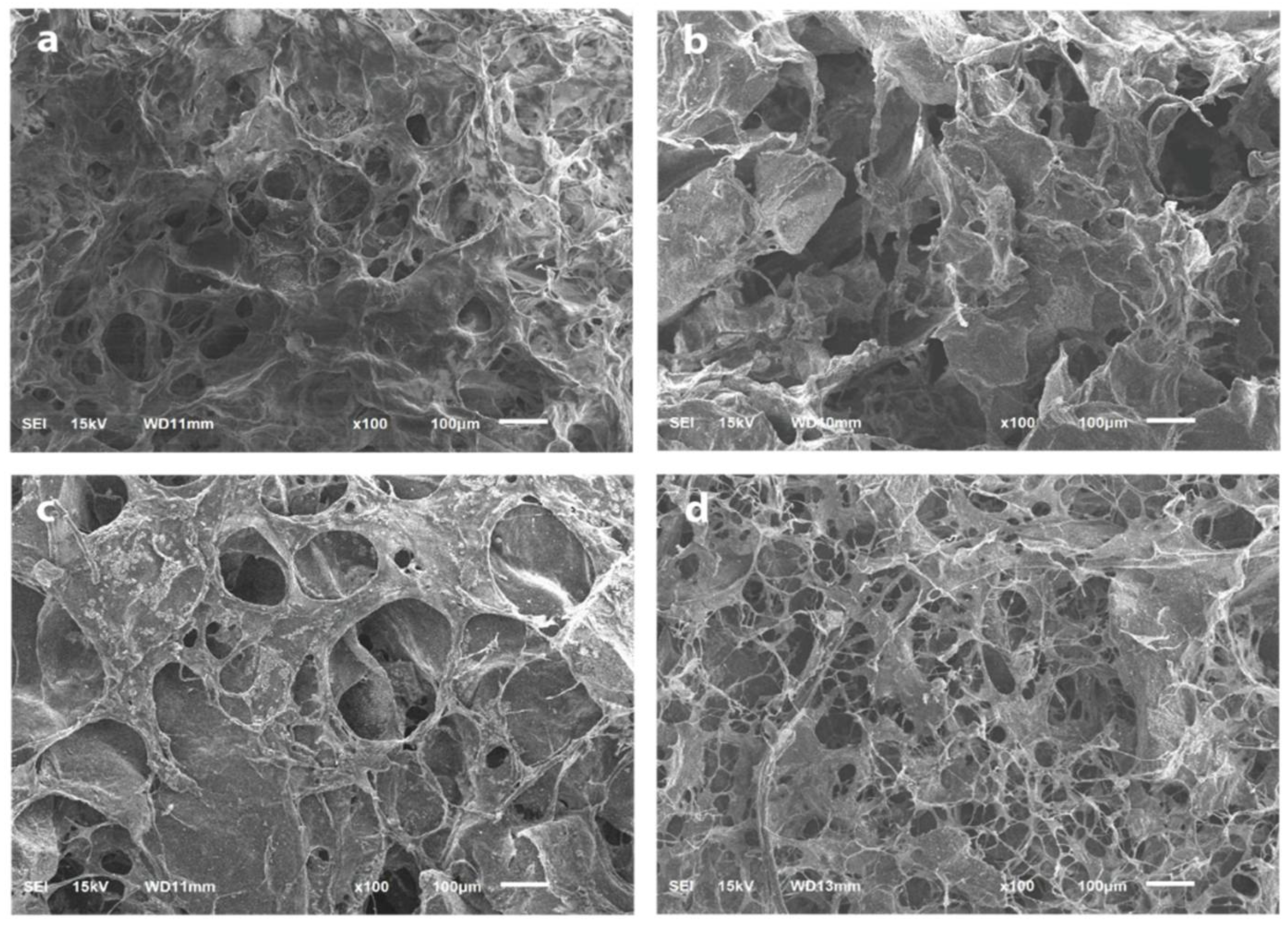
3.3. SEM Analysis
3.4. Porosity
3.5. Viability of Fibroblast Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Madhan, G. Comparison of ability of platelet-rich fibrin vs collaplug in maintaining the buccal bone height of sockets following extractions in 20 patients. J. Health Sci. Res. 2017, 8, 1–6. [Google Scholar]
- Siswanto, R.; Rizkawati, D.M.; Ifada, A.A.; Putra, A.P.; Rachmayani, F.; Widiyanti, P. Bovine freeze dried amniotic membrane (FD-AM) covered sterile gauze for wound dressing. Asian Acad. Soc. Int. Conf. Proc. Ser. 2013, 68–71. [Google Scholar]
- Koizumi, N.; Inatomi, T.; Sotozono, C.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye. Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniontic membrane for potential use in tissue enginering. Eur. Cells Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef]
- Gunasekaran, D.; Thada, R.; Jeyakumar, G.F.S.; Manimegalai, N.P.; Shanmugam, G.; Sivagnanam, U.T. International Journal of Biological Macromolecules Physicochemical characterization and self-assembly of human amniotic membrane and umbilical cord collagen: A comparative study. Int. J. Biol. Macromol. 2020, 165, 2920–2933. [Google Scholar] [CrossRef]
- Tang, K.; Wu, J.; Xiong, Z.; Ji, Y.; Sun, T. Human acellular amniotic membrane: A potential osteoinductive biomaterial for bone regeneration. J. Biomater. Appl. 2017, 32, 754–764. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Castro-ceseña, A.B.; Camacho-villegas, T. Effect of starch on the mechanical and in vitro properties of collagen-hydroxyapatite sponges for applications in dentistry. Carbohydr. Polym. 2016, 148, 78–85. [Google Scholar] [CrossRef]
- Fickl, S.; Zuhr, O.; Wachtel, H.; Stappert, C.F.J.; Stein, J.M.; Hürzeler, M.B. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J. Clin. Periodontol. 2008, 35, 906–913. [Google Scholar] [CrossRef]
- Starecki, M.; Schwartz, J.A.; Grande, D.A. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Adv. Orthop. Surg. 2014, 2014, 72586. [Google Scholar] [CrossRef]
- Dewey, M.J.; Johnson, E.M.; Slater, S.T.; Milner, D.J.; Wheeler, M.B.; Harley, B.A.C. Mineralized collagen scaffolds fabricated with amniotic membrane matrix increase osteogenesis under inflammatory conditions. Regen. Biomater. 2020, 7, 247–258. [Google Scholar] [CrossRef]
- Sabouri, L.; Farzin, A.; Kabiri, A.; Milan, P.B.; Farahbakhsh, M.; Mehdizadehkashi, A.; Kajbafzadeh, A.; Samadikuchaksaraei, A.; Yousefbeyk, F.; Azami, M.; et al. Mineralized human amniotic membrane as a biomimetic scaffold for hard tissue engineering applications. ACS Biomater. Sci. Eng. 2020, 6, 6285–6298. [Google Scholar] [CrossRef]
- Machtei, E.E.; Mayer, Y.; Horwitz, J.; Zigdon-Giladi, H. Prospective randomized controlled clinical trial to compare hard tissue changes following socket preservation using alloplasts, xenografts vs no grafting: Clinical and histological findings. Clin. Implant Dent. Relat. Res. 2019, 21, 14–20. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Gould, M.L.; Shavandi, A.; Mucalo, M.; Dias, G.J. Development and characterization of a xenograft material from New Zealand sourced bovine cancellous bone. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1054–1062. [Google Scholar] [CrossRef]
- Mozartha, M. Hidroksiapatit dan aplikasinya di bidang kedokteran gigi. J. Vis. Lang. Comput. 2015, 11, 287–301. [Google Scholar]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically inspired collagen/apatite composite biomaterials for potential use in bone tissue regeneration—A review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef]
- Shahmoradi, M.; Bertassoni, L.E.; Elfallah, H.M.; Swain, M. Fundamental Structure and Properties of Enamel, Dentin and Cementum; Springer: Berlin/Heidelberg, Germany, 2014; pp. 511–547. [Google Scholar]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In Vitro mineralization of collagen. Adv. Mater. 2021, 33, 2004418. [Google Scholar] [CrossRef]
- Abdullah, M.; Khairurrijal, K. Review: Karakterisasi nanomaterial. J. Nanosains Nanoteknol. 2009, 2, 1–9. [Google Scholar]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) microanalysis: A powerful tool in biomedical research and diagnosis. Eur. J. Histochem. 2018, 62, 2841. [Google Scholar] [CrossRef]
- Choudhary, O.P.; Husbandry, A.; Choudhary, P. Scanning electron microscope: Advantages and disadvantages in imaging scanning electron microscope: Advantages and disadvantages in imaging components. Int. J. Curr. Microbiol. Appl. Sci. 2017, 85, 1–7. [Google Scholar]
- El Milla, L.; Indrani, D.J.; Irawan, B. Sintesis dan uji porositas scaffold hidroksiapatit/alginat. ODONTO Dent. J. 2018, 5, 49. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Mahdani, F.Y.; Ayuningtyas, N.F.; Santosh, A.B.R.; Ernawati, D.S.; Mansur, D.; Arundina, I.; Nagoro, A.A.B.; Rahmadhany, I.P. The cytotoxicity, anti-inflammation, anti-nociceptive and oral ulcer healing properties of coconut shell liquid smoke. J. Herbmed Pharmacol. 2021, 10, 459–467. [Google Scholar] [CrossRef]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Dey, P. Bone mineralization. In Contemporary Topics about Phosphorus in Biology and Materials; Churchill, D.G., Maja Dutour, S., Čolović, B., Füredi Milhofer, H., Eds.; IntechOpen: London, UK, 2020; pp. 1–18. [Google Scholar]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744, 657–661. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Chen, L.; Wu, Z.; Zhou, Y.; Li, L.; Wang, Y.; Wang, Z.; Chen, Y.; Zhang, P. Biomimetic porous collagen/hydroxyapatite scaffold for bone tissue engineering. J. Appl. Polym. Sci. 2017, 134, 45271. [Google Scholar] [CrossRef]
- Sripriya, R.; Kumar, R. Denudation of human amniotic membrane by a novel process and its characterisations for biomedical applications. Prog. Biomater. 2016, 5, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Seckler, M.M.; Danese, M.; Derenzo, S.; Valarelli, J.V.; Giulietti, M.; Rodríguez-Clemente, R. Influence of process conditions on hydroxyapatite crystallinity obtained by direct crystallization. Mater. Res. 1999, 2, 59–62. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, G.; Zheng, L.; Liu, H.; Niu, X.; Fan, Y. Micro-/Nano-sized hydroxyapatite directs differentiation of rat bone marrow derived mesenchymal stem cells towards an osteoblast lineage. Nanoscale 2012, 4, 2484–2490. [Google Scholar] [CrossRef]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emerg. Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Shukla, S. Freeze drying process: A review. Int. J. Pharm. Sci. Res. 2011, 2, 3061–3068. [Google Scholar]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Hydroxyapatite and gelatin composite foams processed via novel freeze-drying and crosslinking for use as temporary hard tissue scaffolds. J. Biomed. Mater. Res. A 2005, 72, 136–145. [Google Scholar] [CrossRef]
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In Vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460. [Google Scholar] [CrossRef]
- Angulo, D.E.L.; do Amaral Sobral, P.J. Characterization of gelatin/chitosan scaffold blended with aloe vera and snail mucus for biomedical purpose. Int. J. Biol. Macromol. 2016, 92, 645–653. [Google Scholar] [CrossRef]
- Danilevicius, P.; Georgiadi, L.; Pateman, C.J.; Claeyssens, F.; Chatzinikolaidou, M.; Farsari, M. The effect of porosity on cell ingrowth into accurately defined, laser-made, polylactide-based 3D scaffolds. Appl. Surf. Sci. 2015, 336, 2–10. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.; Zhang, Y.; Ye, Z.; Tan, W.; Lang, M. Effect of porosity on long-term degradation of poly (ε-caprolactone) scaffolds and their cellular response. Polym. Degrad. Stab. 2013, 98, 209–218. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; Al Mushref, F.R.A.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, S.; Gao, P.; Zhang, M.; Fan, C.; Lu, Q.; Li, C.; Chen, C.; Lin, B.; Jiang, Y. A tough chitosan-alginate porous hydrogel prepared by simple foaming method. J. Solid State Chem. 2021, 294, 121797. [Google Scholar] [CrossRef]
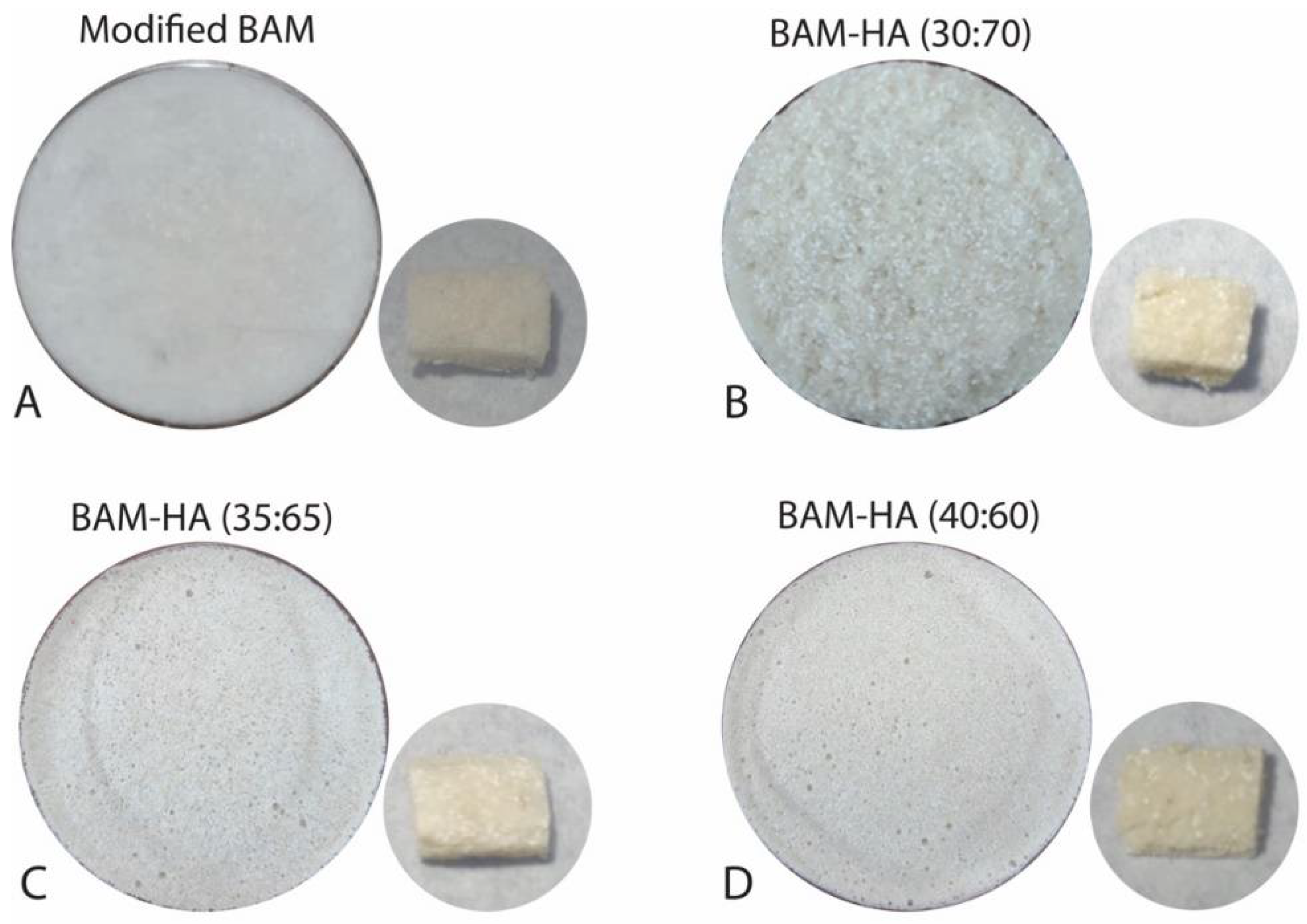

| Group | Pore Size (µm) | Porosity (%) | |||
|---|---|---|---|---|---|
| Mean | Minimum | Maximum | Mean ± SD | p | |
| modified BAM | 89.09 | 208.25 | 14.13 | 105.93 ± 3.81 | 0.0001 |
| bio-composite (30:70) | 153.02 | 420.44 | 37.99 | 76.89 ± 6.15 | |
| bio-composite (35:65) | 155.62 | 316.10 | 43.39 | 89.23 ± 7.51 | |
| bio-composite (40:60) | 72.424 | 279.89 | 20.51 | 103.25 ± 9.50 | |
| Group | Mean ± SD OD | Viability (%) | p-Value |
|---|---|---|---|
| Control media | 0.0670 ± 0.0088 | 0 | 0.860 |
| Control cells | 0.5080 ± 0.0592 | 100 | |
| bio-composite (30:70) | 0.4998 ± 0.0483 | 98.14 | |
| bio-composite (35:65) | 0.4866 ± 0.0507 | 95.14 | |
| bio-composite (40:60) | 0.4943 ± 0.0541 | 96.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Octarina; Munadziroh, E.; Razak, F.A.; Surboyo, M.D.C. Characterisation of Bovine Amniotic Membrane with Hydroxyapatite Bio-Composite. Coatings 2022, 12, 1403. https://doi.org/10.3390/coatings12101403
Octarina, Munadziroh E, Razak FA, Surboyo MDC. Characterisation of Bovine Amniotic Membrane with Hydroxyapatite Bio-Composite. Coatings. 2022; 12(10):1403. https://doi.org/10.3390/coatings12101403
Chicago/Turabian StyleOctarina, Elly Munadziroh, Fathilah Abdul Razak, and Meircurius Dwi Condro Surboyo. 2022. "Characterisation of Bovine Amniotic Membrane with Hydroxyapatite Bio-Composite" Coatings 12, no. 10: 1403. https://doi.org/10.3390/coatings12101403
APA StyleOctarina, Munadziroh, E., Razak, F. A., & Surboyo, M. D. C. (2022). Characterisation of Bovine Amniotic Membrane with Hydroxyapatite Bio-Composite. Coatings, 12(10), 1403. https://doi.org/10.3390/coatings12101403








