Preparation and Tribological Properties of Graphene-Based Coatings on Tungsten Carbide
Abstract
:1. Introduction
2. Materials and Experimental Section
2.1. Materials and TBCs Preparation
2.2. Friction Experiment
3. Results
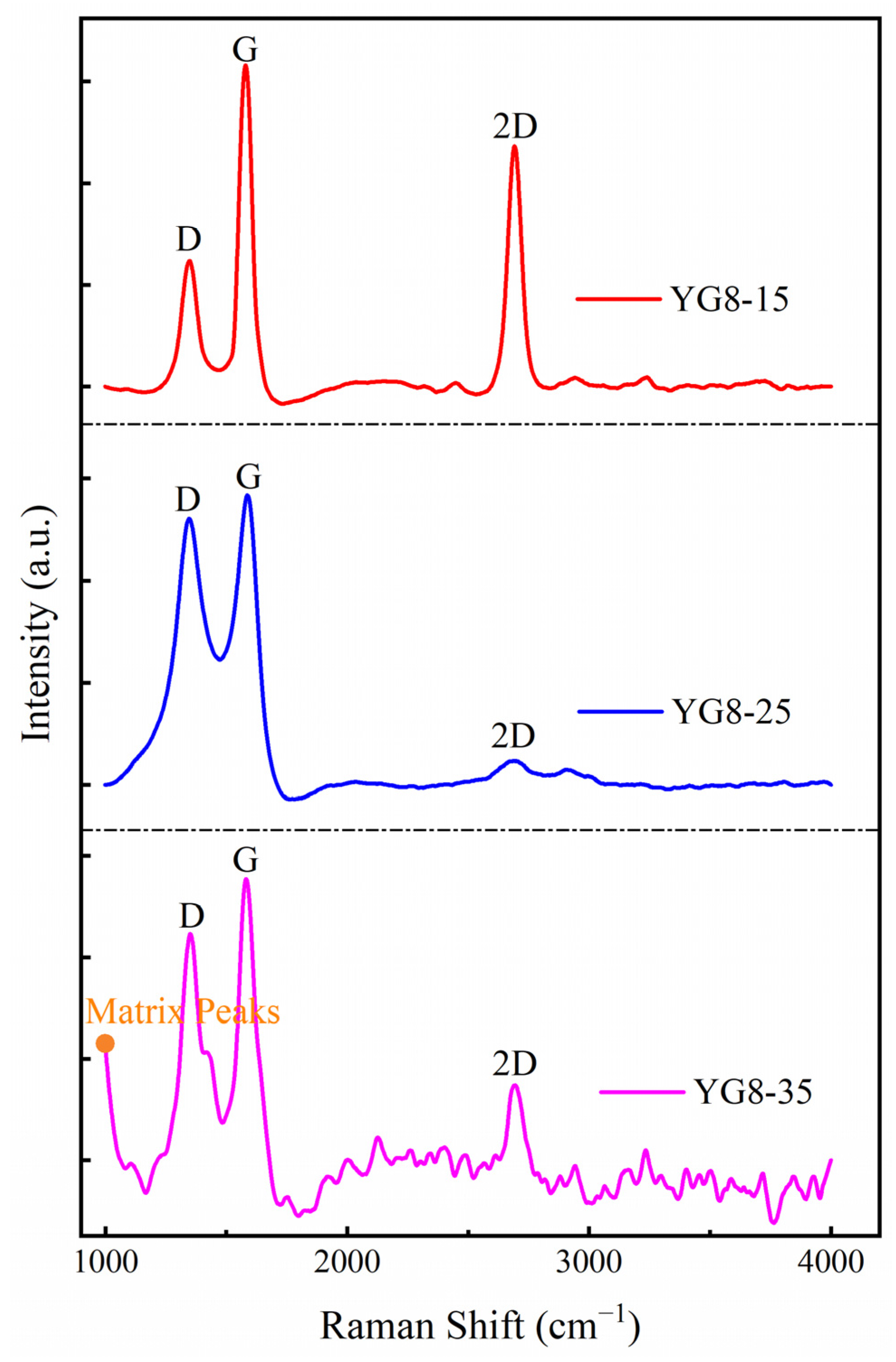
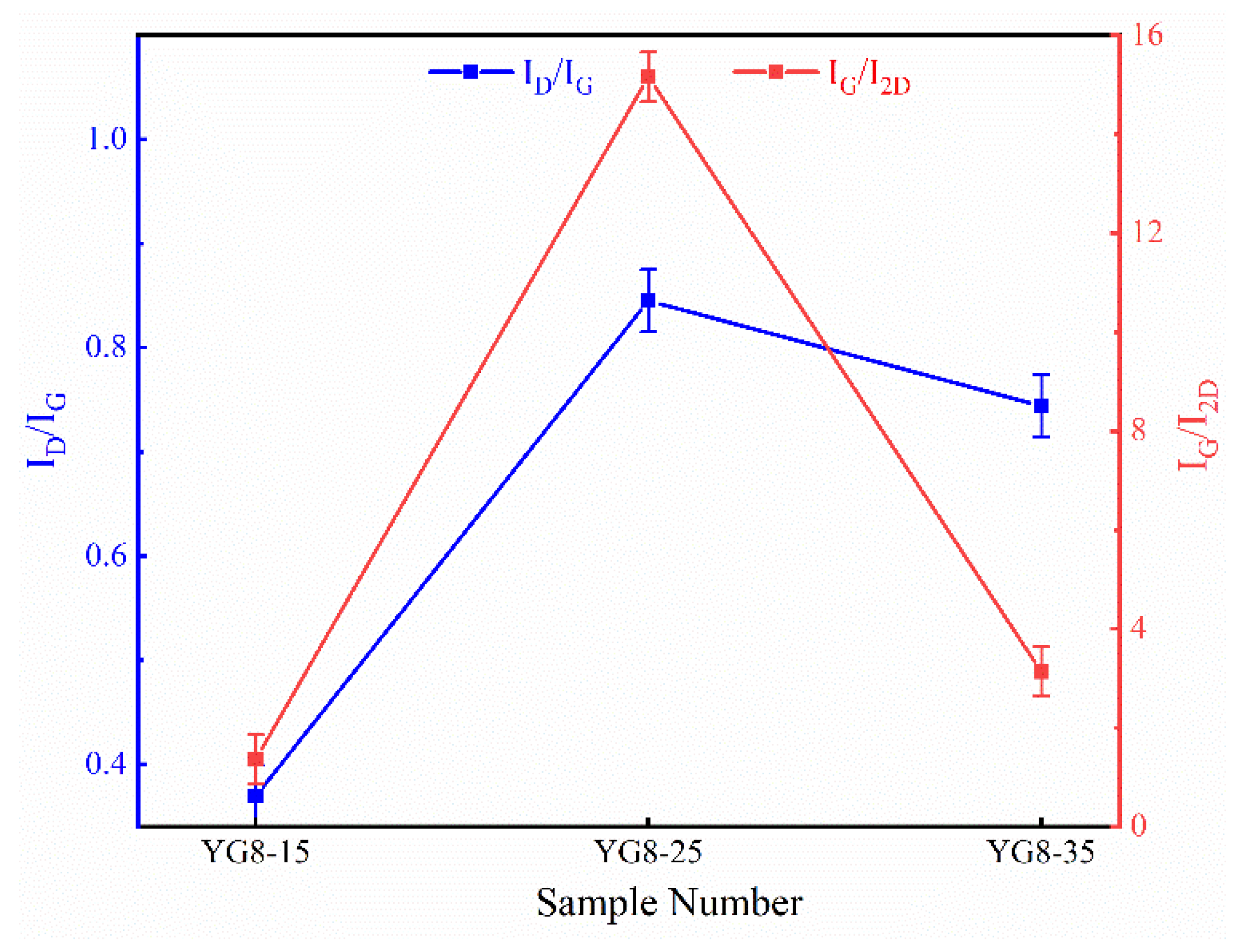
3.1. Effect of Cooling Rate on Deposition of Graphene-Based Coatings
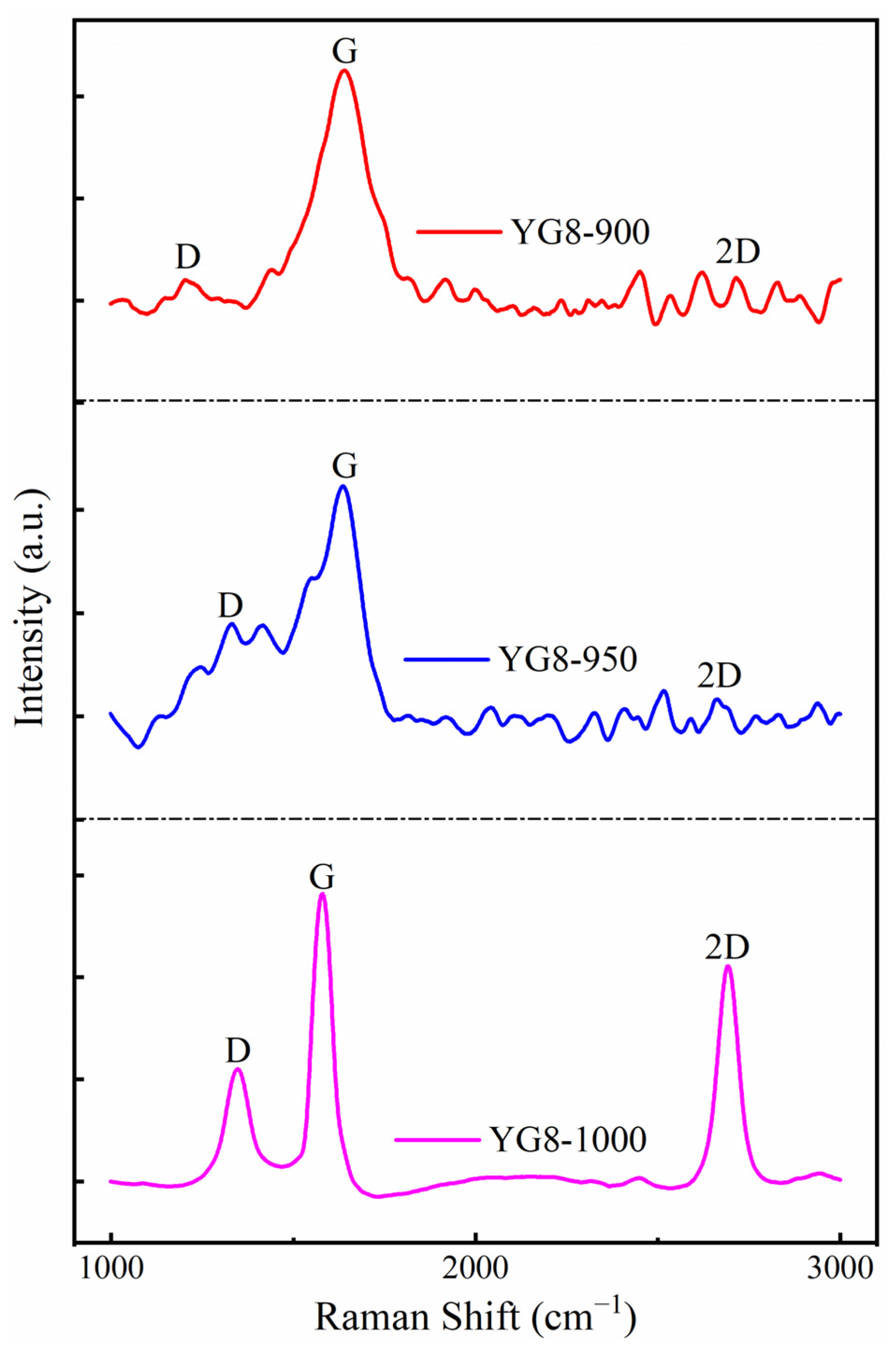
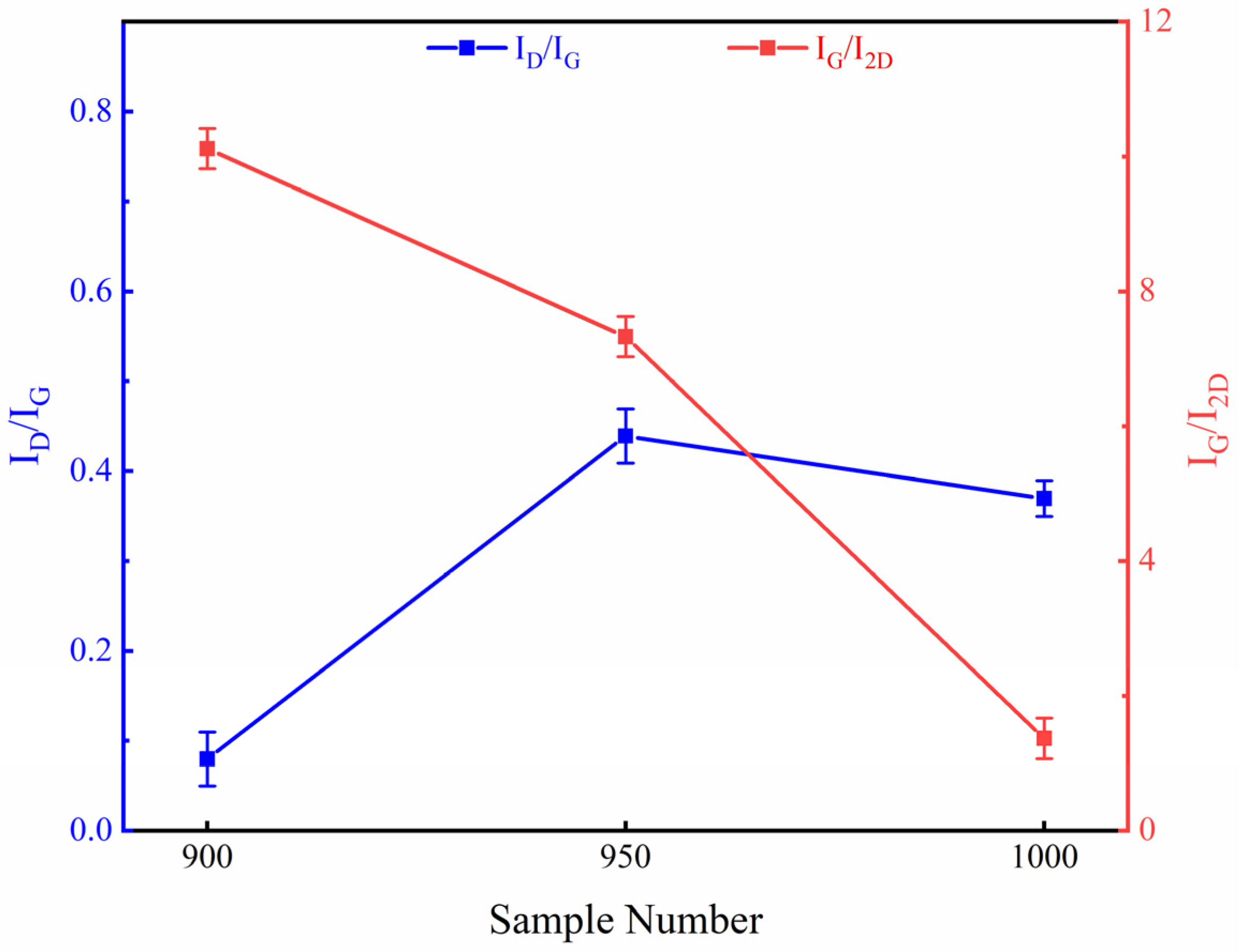
3.2. Effects of Growth Temperature on Deposition of Graphene-Based Coatings
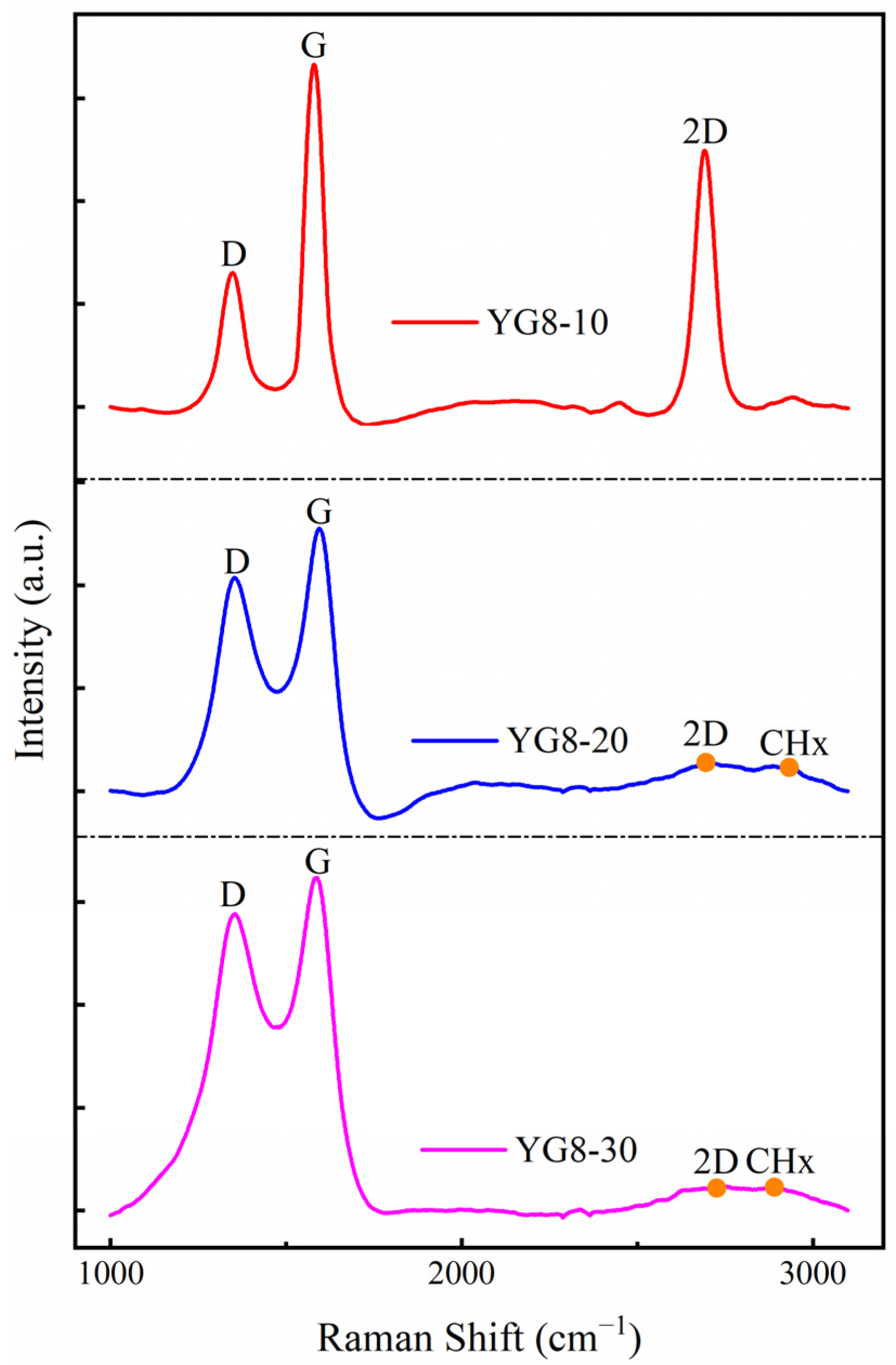
3.3. Effect of Methane Flow on Deposition of Graphene-Based Coatings
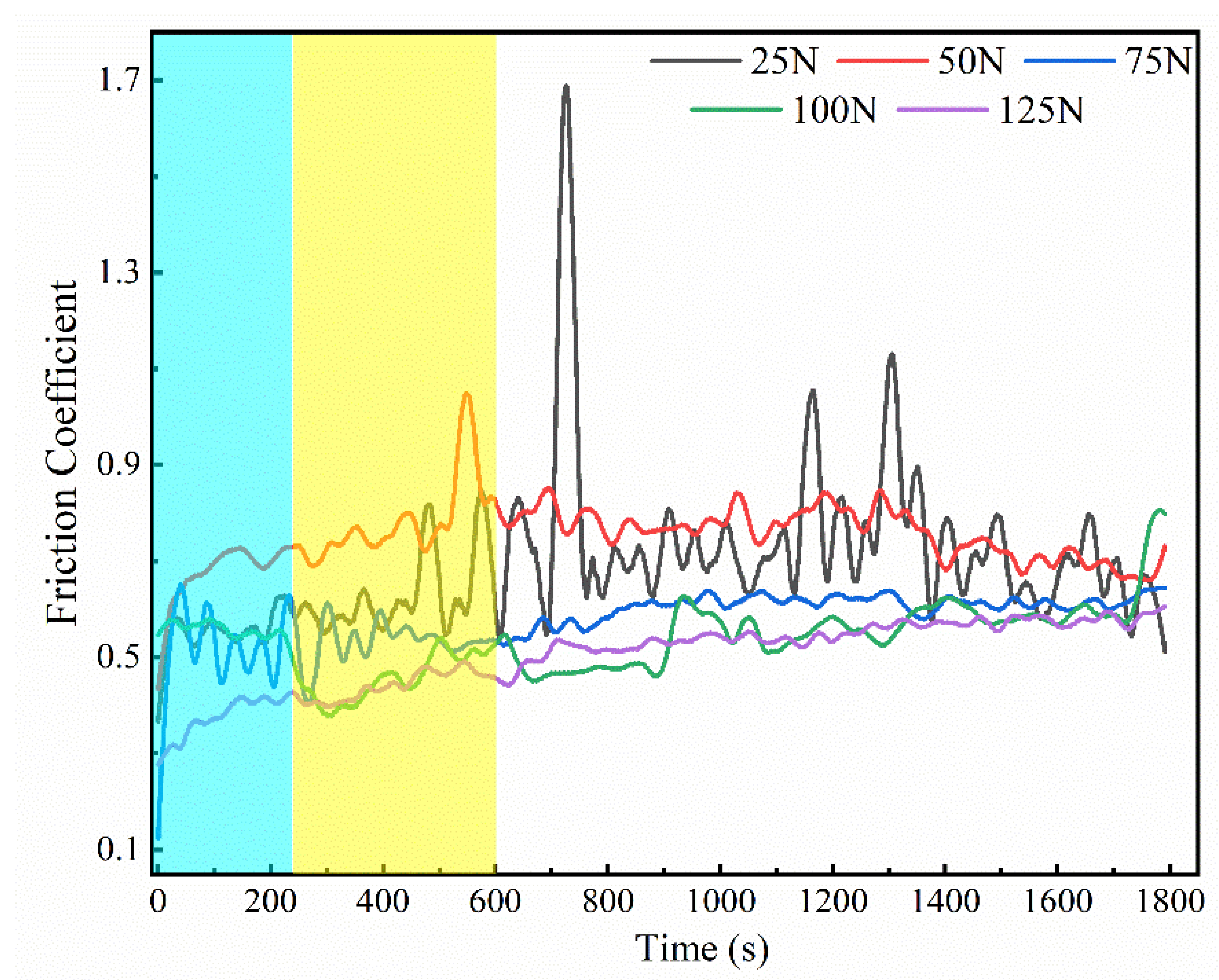
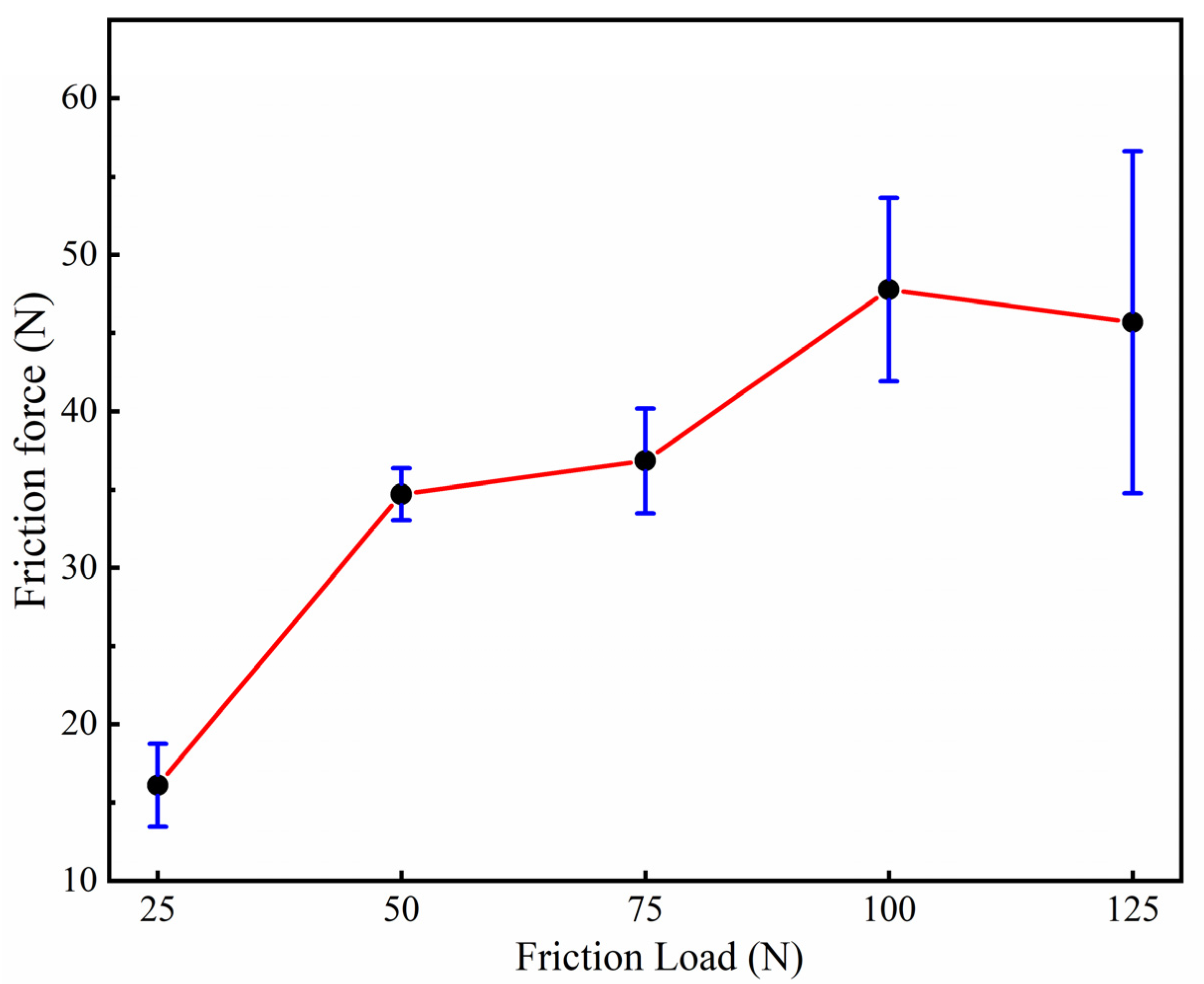
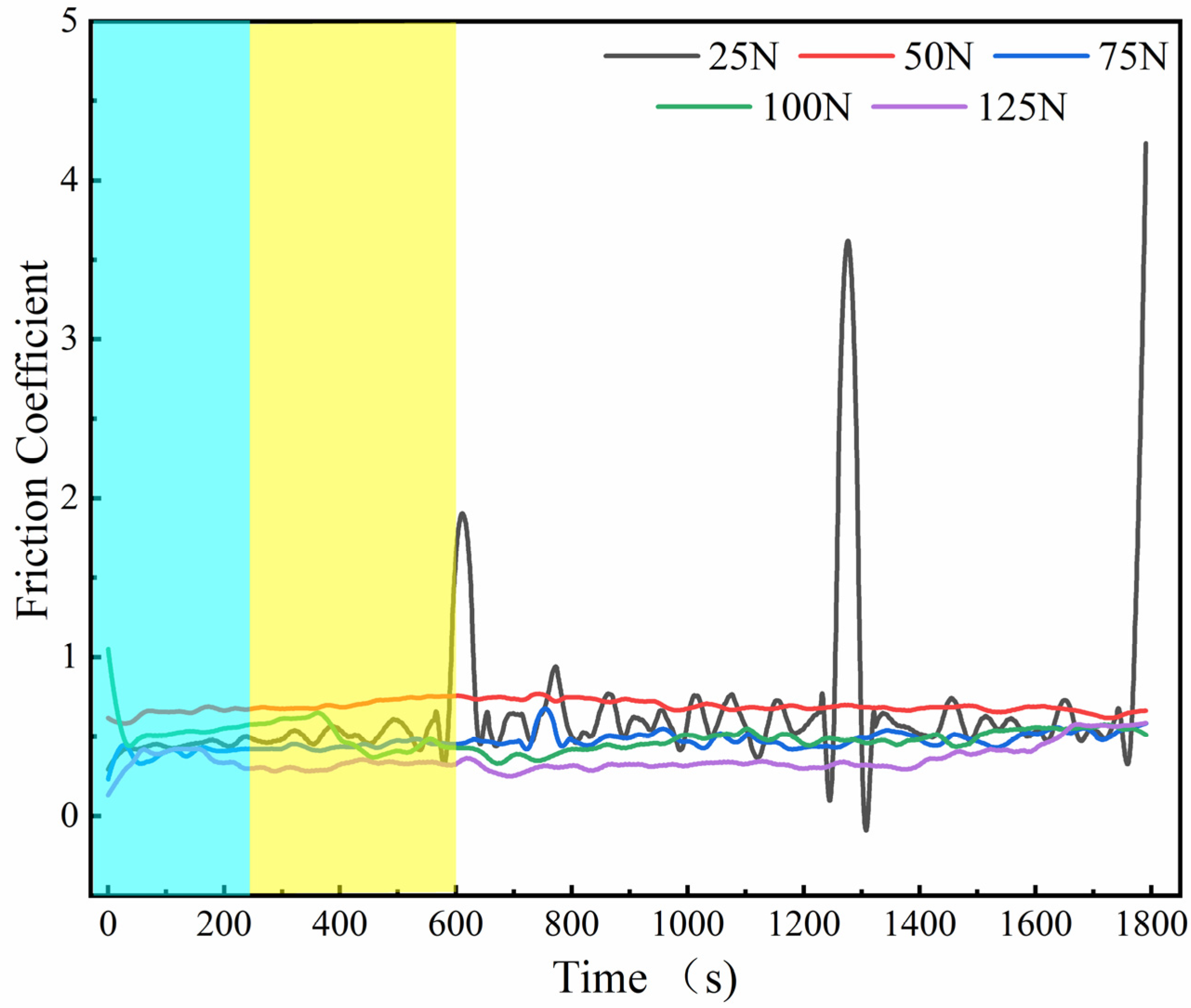
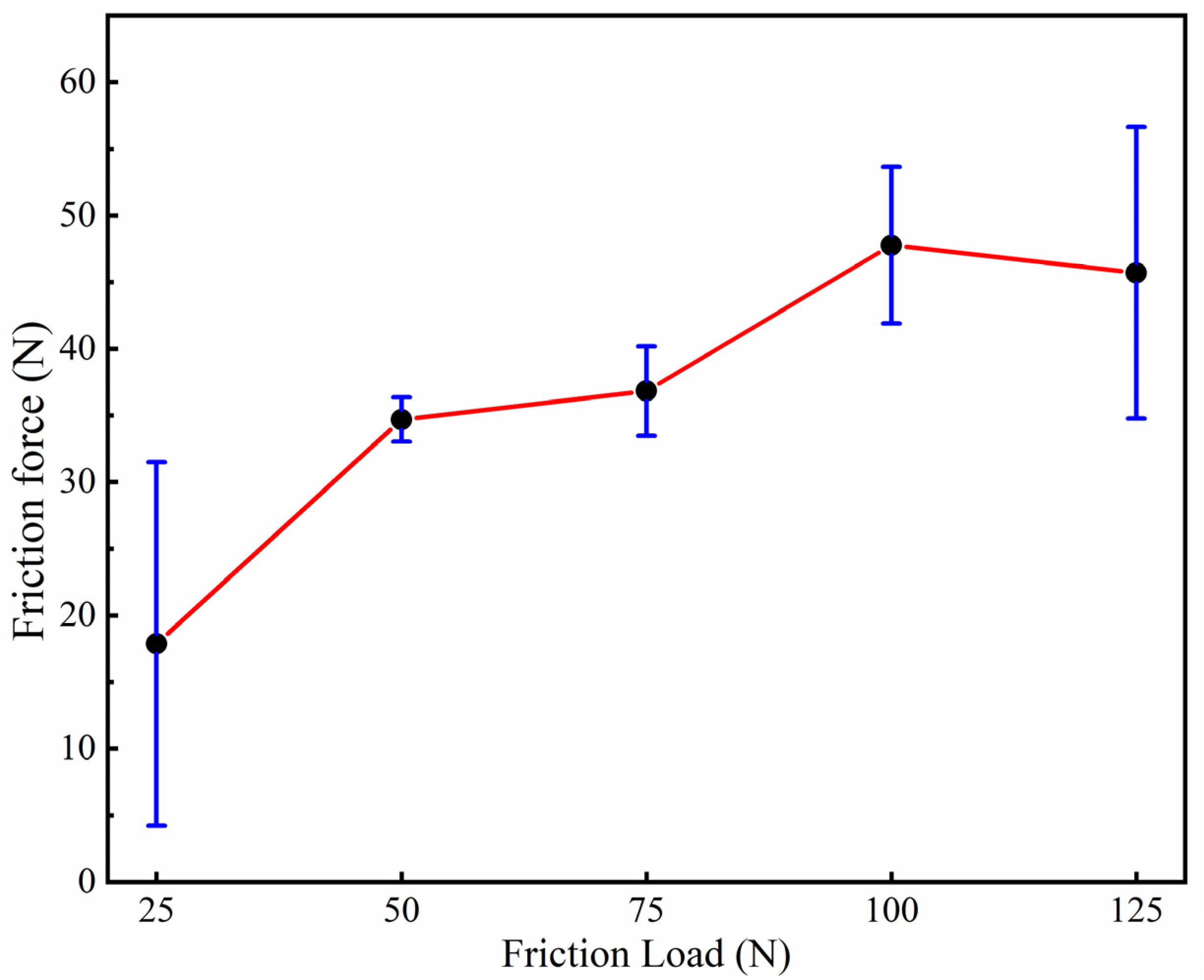
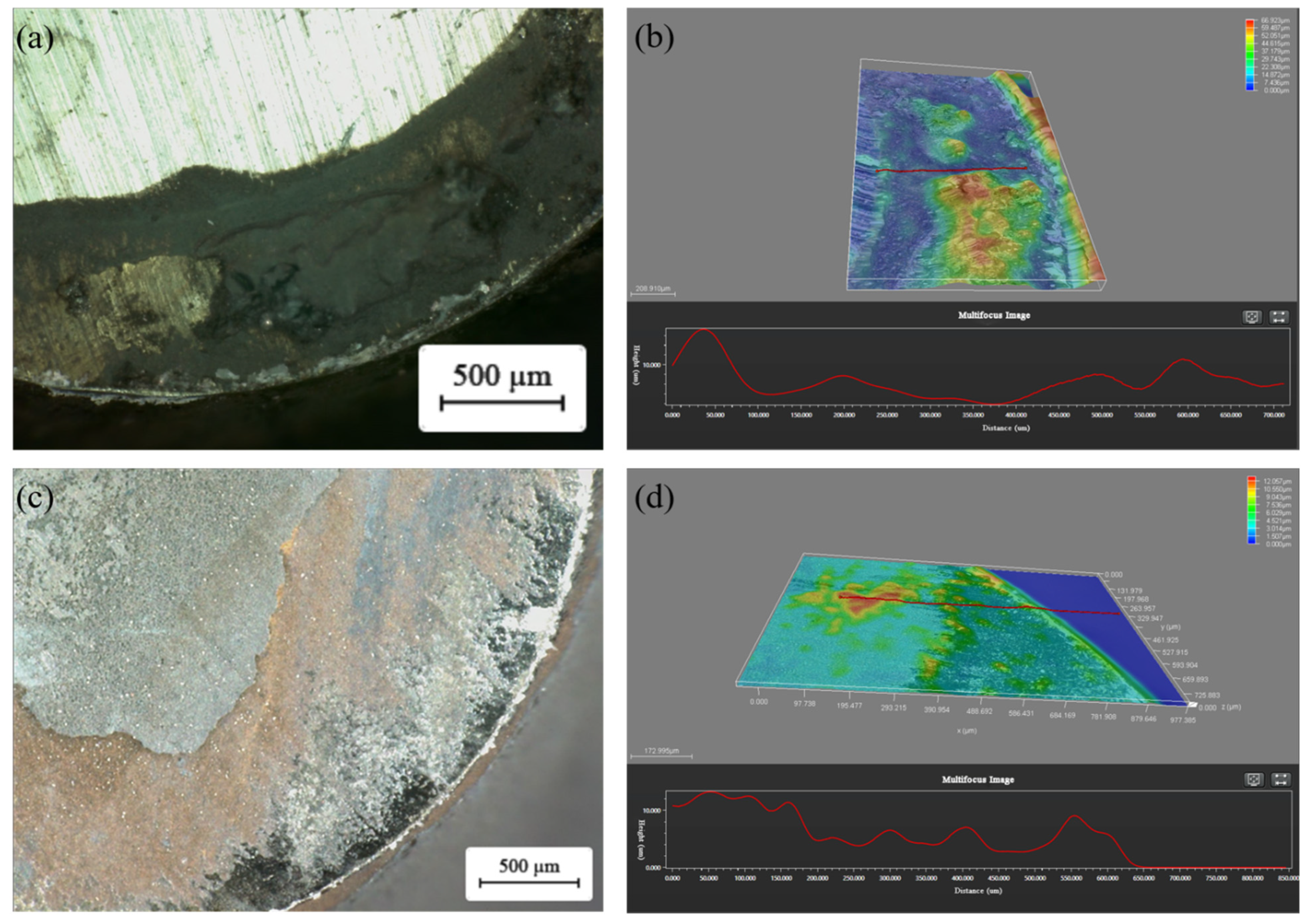
3.4. Tribological Properties of Graphene-Based Coatings
4. Discussion
- Research the effects of growth time, type of carbon source, and growth pressure on the preparation of graphene-based coatings.
- Try to decrease the growth temperature of graphene-based coating to reduce the influence of a high temperature environment on the mechanical properties of the cemented carbide matrix.
- Study the bonding strength between graphene-based coating and the matrix.
- Carry out experimental research on the actual cutting performance of graphene-based coated tools.
5. Conclusions
- The pure graphene coating with fewer layers on a cemented carbide surface is the best growth condition: the growth temperature was 1000 °C, the cooling rate was 15 °C/min, and the methane flow rate was 10 sccm.
- An increase in the flow of methane would lead to an increase in the number of graphene layers, which would generate amorphous carbon.
- The friction coefficient of the graphene coating is inversely proportional to the applied friction load.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, T.Y.; Long, Y.Y.; Mu, C.L.; Zhou, W.; Lian, Y.S. Performance of soft/hard composite dual-effect coated tool in dry cutting of carbon fiber–reinforced polymer. Int. J. Adv. Manuf. Technol. 2020, 109, 2814–2823. [Google Scholar] [CrossRef]
- Kumar, C.S.; Majumder, H.; Khan, A.; Patel, S.K. Applicability of DLC and WC/C low friction coatings on Al2O3/TiCN mixed ceramic cutting tools for dry machining of hardened 52100 steel. Ceram. Int. 2020, 46, 11889–11897. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, W.L.; Li, B.X.; Wang, C.Y.; Kuang, T.C.; Li, Y.Q. Physical vapor deposition technology for coated cutting tools: A review. Ceram. Int. 2020, 46, 18373–18390. [Google Scholar] [CrossRef]
- Boing, D.; Oliveira, A.J.D.; Schroeter, R.B. Evaluation of wear mechanisms of PVD and CVD coatings deposited on cemented carbide substrates applied to hard turning. Int. J. Adv. Manuf. Technol. 2020, 106, 5441–5451. [Google Scholar] [CrossRef]
- Chen, J.; He, G.Y.; Han, Y.T.; Yuan, Z.W.; Li, Z.; Zhang, Z.L.; Han, X.; Yan, S.W. Structural toughness and interfacial effects of multilayer TiN erosion-resistant coatings based on high strain rate repeated impact loads. Ceram. Int. 2021, 19, 27660–27667. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chich, P.C.; Huang, J.H. Effect of Ti interlayer thickness on mechanical properties and wear resistance of TiZrN coatings on AISI D2 steel. Surf. Coat. Technol. 2020, 394, 125690. [Google Scholar] [CrossRef]
- Lian, Y.S.; Long, Y.Y.; Zhao, G.L.; Mu, C.L.; Li, X.M.; Deng, J.X.; Xie, C.P. Performance of CrCN-WS2 hard/soft composite coated tools in dry cutting of titanium alloys. J. Manuf. Process. 2020, 54, 201–209. [Google Scholar] [CrossRef]
- Tyagi, R.; Das, A.K.; Mandal, A. Formation of superhydrophobic surface with enhanced hardness and wear resistance by electrical discharge coating process. Tribol. Int. 2021, 157, 106897. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, G.C.; Chen, Z.Q.; Ji, L.G.; Xu, C.H.; Yi, M.D.; Zhang, J.J.; Zhou, T.T. Mechanical properties, microstructure and crack healing ability of Al2O3/TiC/TiB2/h-BN@-Al2O3 self-lubricating ceramic tool material. Ceram. Int. 2021, 47, 14551–14560. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Li, Y.; Gao, F.; Zhang, G.J. Self-lubricating TiN/MoN and TiAlN/MoN nano-multilayer coatings for drilling of austenitic stainless steel. Ceram. Int. 2019, 45, 24248–24253. [Google Scholar] [CrossRef]
- Ji, L.G.; Chen, Z.Q.; Guo, R.X.; Xu, C.H.; Guo, N.S. Preparation of nano-coating powder CaF2@-Al(OH)3 and its application in Al2O3/Ti(C,N) self-lubricating ceramic tool materials. Ceram. Int. 2020, 46, 15949–15957. [Google Scholar] [CrossRef]
- Liu, J.X.; Wang, X.; Liu, Y.; Liu, X.Y.; Fan, K. Bioinspired three-dimensional and multiple adsorption effects toward high lubricity of solvent-free graphene-based nanofluid. Carbon 2022, 188, 166–176. [Google Scholar] [CrossRef]
- Wu, H.S.; Shen, G.Z.; Li, R.X.; Zhang, L.Y.; Jie, X.H.; Liu, G. Influence of embedded reduced graphene oxide on the corrosion-wear performance of cold sprayed Zn-rGO/Al coating in NaCl solution. Surf. Coat. Technol. 2022, 429, 127856. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.Q.; Ma, W.; Wang, X.Z.; Jiang, H.Y.; Ren, W.C.; Sun, D.M. A silicon-graphene-silicon transistor with an improved current gain. J. Mater. Sci. Technol. 2022, 104, 127–130. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, D.H.; Zhang, Y.M.; Zhao, J.Q.; Zhang, G.J. Bubble Behavior and Its Effect on Surface Integrity in Laser-Induced Plasma Micro-Machining Silicon Wafer. J. Manuf. Sci. Eng. 2022, 144, 091008. [Google Scholar] [CrossRef]
- Low, W.H.; Lim, S.S.; Siong, C.W.; Chia, C.H.; Khiew, P.S. One dimensional MnV2O6 nanobelts on graphene as outstanding electrode material for high energy density symmetric supercapacitor. Ceram. Int. 2021, 47, 9560–9568. [Google Scholar] [CrossRef]
- Ming, W.Y.; Shen, F.; Zhang, G.J.; Liu, G.D.; Du, J.G.; Chen, Z.J. Green machining: A framework for optimization of cutting parameters to minimize energy consumption and exhaust emissions during electrical discharge machining of Al 6061 and SKD 11. J. Clean. Prod. 2021, 285, 124889. [Google Scholar] [CrossRef]
- Mu, J.; Gao, F.J.; Cui, G.; Wang, S.; Tang, S.; Li, Z.L. A comprehensive review of anticorrosive graphene-composite coatings. Prog. Org. Coat. 2021, 157, 106321. [Google Scholar] [CrossRef]
- Gara, D.K.; Raghavendra, G.; Prasad, P.S.; Ojha, S. Enhanced mechanical properties of glass fibre epoxy composites by 2D exfoliated graphene oxide filler. Ceram. Int. 2021, 47, 34860–34868. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, S.; Salman, M.; Baluch, A.H. A comparative study on the effect of carbon-based and ceramic additives on the properties of fiber reinforced polymer matrix composites for high temperature applications. Ceram. Int. 2021, 47, 33956–33971. [Google Scholar] [CrossRef]
- Tseng, S.F.; Yang, Y.H. Superhydrophobic graphene/ceramic templates for the preparation of particulate drugs. Ceram. Int. 2022, 48, 2021–2030. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Ming, W.Y.; Zhang, Y.M.; Cao, C.; Zhang, G.J. A review on magnetic field assisted electrical discharge machining. J. Manuf. Process. 2021, 64, 694–722. [Google Scholar] [CrossRef]
- Ai, Z.S.; Feng, G.X.; Ping, Y.R.; Qin, X.; Ping, X.L.; Xun, W.Z. Self-lubrication and wear-resistance mechanism of graphene-modified coatings. Ceram. Int. 2020, 46, 15915–15924. [Google Scholar] [CrossRef]
- Ollik, K.; Lieder, M. Review of the application of graphene-based coatings as anticorrosion layers. Coatings 2020, 10, 883. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, L.J.; Fu, J.J. Research Status of Graphene Polyurethane Composite Coating. Coatings 2022, 12, 264. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhao, J.; Gan, Y.L.; Tang, X.K.; Gai, S.L.; Sun, X.S. Cutting performance and wear mechanisms of the graphene-reinforced Al2O3-WC-TiC composite ceramic tool in turning hardened 40Cr steel. Ceram. Int. 2022, 48, 13695–13705. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Xu, Q.; Ma, T.B.; Hu, Y.Z.; Luo, J.B. Atomistic insights into friction and wear mechanisms of graphene oxide. Appl. Surf. Sci. 2021, 546, 149130. [Google Scholar] [CrossRef]
- Singh, S.; Chen, X.C.; Zhang, C.H.; Gautam, R.K.; Tyagi, R.; Luo, J.B. Nickel-catalyzed direct growth of graphene on bearing steel (GCr15) by thermal chemical vapor deposition and its tribological behavior. Appl. Surf. Sci. 2020, 502, 144135. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Ogata, Y.; Tachibana, M. Pit formation with graphene growth on copper foils by ethanol chemical vapor deposition. Diam. Relat. Mater. 2020, 101, 107602. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, H.L.; Lee, C.; Lee, S.K.; Jang, H.; Ahn, J.H.; Kim, J.H.; Lee, H.J. Chemical Vapor Deposition-Grown Graphene: The Thinnest Solid Lubricant. ACS Nano. 2011, 5, 5107–5114. [Google Scholar] [CrossRef]
- Jiang, X.F.; Song, J.J.; Su, Y.F.; Zhang, Y.S.; Hu, L.T. Novel Design of Copper-Graphite Self-Lubricating Composites for Reliability Improvement Based on 3D Network Structures of Copper Matrix. Tribol. Lett. 2018, 66, 143. [Google Scholar] [CrossRef]
- Huang, Y.C.; Shi, X.L.; Yang, K.; Liu, X.Y.; Wang, Z.H. Effects of frictional heat on the tribological properties of Ni3Al matrix self-lubricating composite containing graphene nanoplatelets under different loads. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2018, 232, 645–656. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Duong, D.L.; Sang, H.L.; Avila, J.; Lee, Y.H. Layer-controlled single-crystalline graphene film with stacking order via Cu–Si alloy formation. Nat. Nanotechnol. 2020, 15, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ren, E.Z.; Ma, J.; Cao, Y.; Du, J.G.; Ming, W.Y.; Li, X.K.; Li, B. Controllable preparation of graphene-based film deposited on cemented carbides by chemical vapor deposition. J. Mater. Sci. 2020, 55, 4251–4264. [Google Scholar] [CrossRef]
- Liu, W.; Chung, C.H.; Miao, C.Q.; Wang, Y.J.; Li, B.Y.; Ruan, L.Y.; Patel, K.; Park, Y.J.; Woo, J.; Xie, Y.H. Chemical vapor deposition of large area few layer graphene on Si catalyzed with nickel films. Thin Solid Films. 2010, 518, S128–S132. [Google Scholar] [CrossRef]
- Regmi, M.; Chisholm, M.F.; Eres, G. The effect of growth parameters on the intrinsic properties of large-area single layer graphene grown by chemical vapor deposition on Cu. Carbon 2012, 50, 134–141. [Google Scholar] [CrossRef]
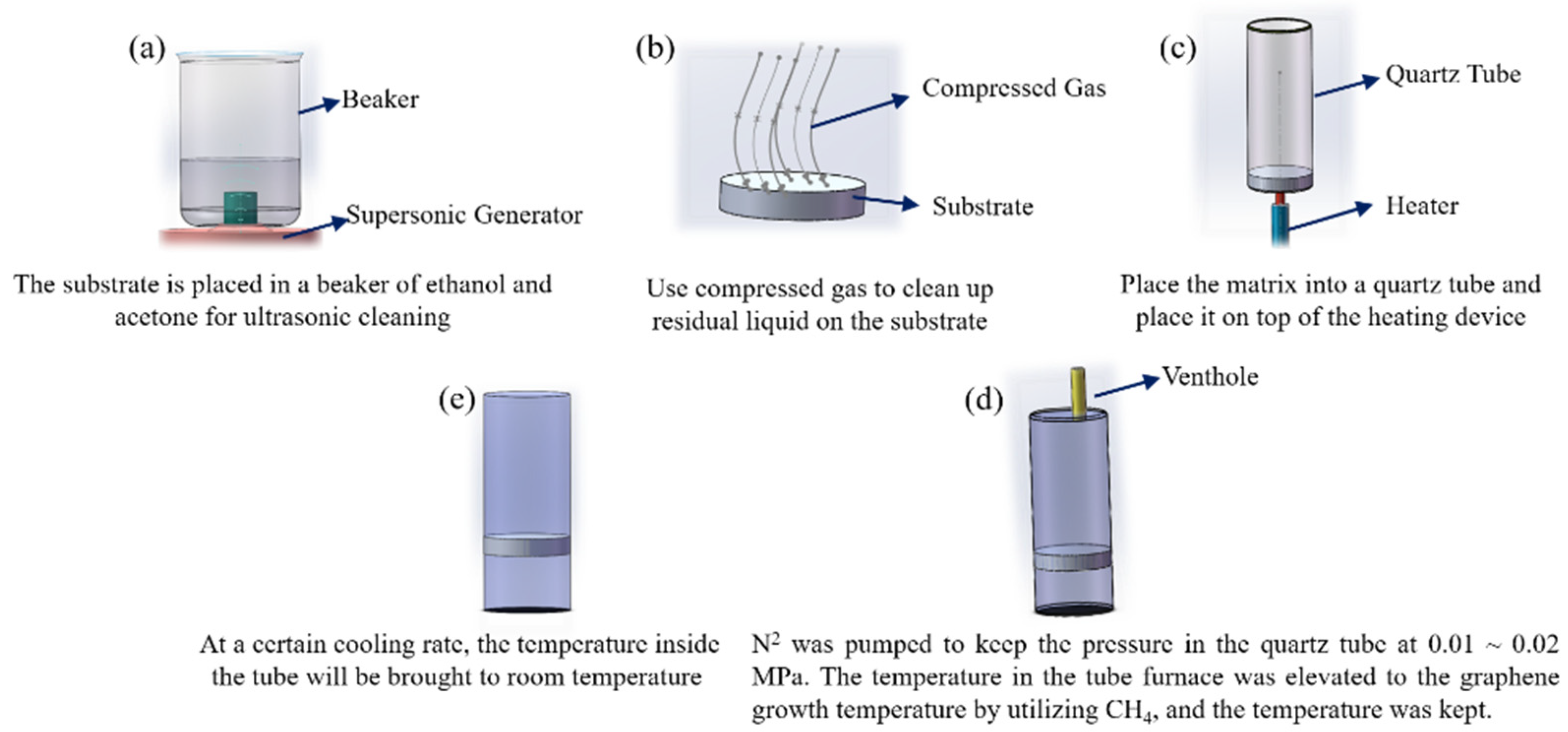




| Sample Number | Matrix Model | Methane Flow (sccm) | Argon Flow (sccm) | Growth Temperature (°C) | Growth Time (min) | Cooling Rate (°C/min) |
|---|---|---|---|---|---|---|
| YG8–15 | YG8 | 10 | 400 | 1000 | 10 | 15 |
| YG8–25 | YG8 | 10 | 400 | 1000 | 10 | 25 |
| YG8–35 | YG8 | 10 | 400 | 1000 | 10 | 35 |
| Sample Number | Matrix Model | Methane Flow (sccm) | Argon Flow (sccm) | Growth Temperature (°C) | Growth Time (min) | Cooling Rate (°C/min) |
|---|---|---|---|---|---|---|
| YG8–900 | YG8 | 10 | 400 | 900 | 10 | 15 |
| YG8–950 | YG8 | 10 | 400 | 950 | 10 | 15 |
| YG8–1000 | YG8 | 10 | 400 | 1000 | 10 | 15 |
| Sample Number | Matrix Model | Methane Flow (sccm) | Argon Flow (sccm) | Growth Temperature (°C) | Growth Time (min) | Cooling Rate (°C/min) |
|---|---|---|---|---|---|---|
| YG8–10 | YG8 | 10 | 400 | 1000 | 10 | 15 |
| YG8–20 | YG8 | 20 | 400 | 1000 | 10 | 15 |
| YG8–30 | YG8 | 30 | 400 | 1000 | 10 | 15 |
| Sample Number | Methane Flow (sccm) | Argon Flow (sccm) | Growth Temperature (°C) | Growth Time (min) | Cooling Rate (°C/min) |
|---|---|---|---|---|---|
| YG8–10 | 10 | 400 | 1000 | 10 | 15 |
| YG8–20 | 20 | 400 | 1000 | 10 | 15 |
| YG8–30 | 30 | 400 | 1000 | 10 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Du, K.-P.; Ren, E.-Z.; Ye, G.-Y.; Wang, X.-S.; Ming, W.-Y.; Ma, J.; He, W.-B. Preparation and Tribological Properties of Graphene-Based Coatings on Tungsten Carbide. Coatings 2022, 12, 1385. https://doi.org/10.3390/coatings12101385
Liu K, Du K-P, Ren E-Z, Ye G-Y, Wang X-S, Ming W-Y, Ma J, He W-B. Preparation and Tribological Properties of Graphene-Based Coatings on Tungsten Carbide. Coatings. 2022; 12(10):1385. https://doi.org/10.3390/coatings12101385
Chicago/Turabian StyleLiu, Kun, Kang-Ping Du, Er-Zhou Ren, Guo-Yong Ye, Xin-Sheng Wang, Wu-Yi Ming, Jun Ma, and Wen-Bin He. 2022. "Preparation and Tribological Properties of Graphene-Based Coatings on Tungsten Carbide" Coatings 12, no. 10: 1385. https://doi.org/10.3390/coatings12101385
APA StyleLiu, K., Du, K.-P., Ren, E.-Z., Ye, G.-Y., Wang, X.-S., Ming, W.-Y., Ma, J., & He, W.-B. (2022). Preparation and Tribological Properties of Graphene-Based Coatings on Tungsten Carbide. Coatings, 12(10), 1385. https://doi.org/10.3390/coatings12101385








