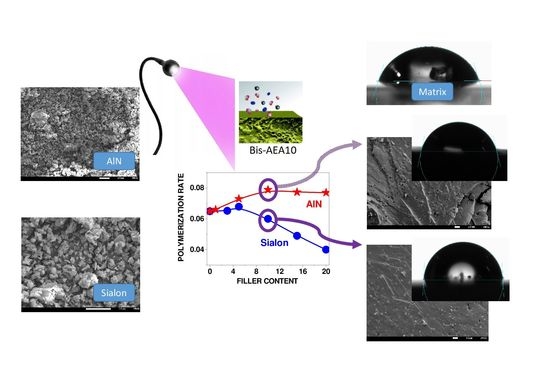
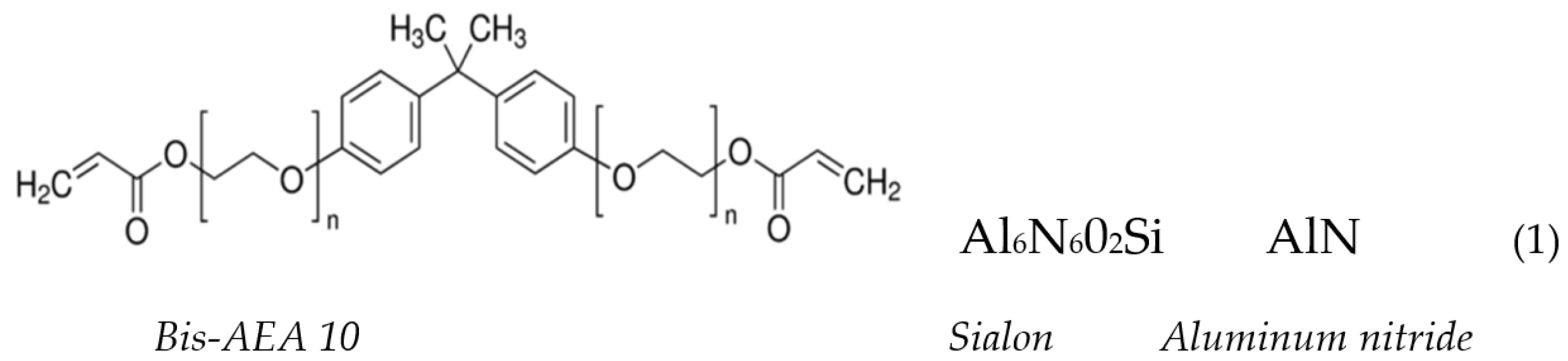
Influence of the Addition of Sialon and Aluminum Nitride Fillers on the Photocuring Process of Polymer Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Viscosity
2.3. Photopolymerization Kinetics
2.4. Thermal Properties
2.5. Mechanical Properties
2.6. FTIR Analysis
2.7. SEM
2.8. Surface Wettability
3. Test Results and Discussion
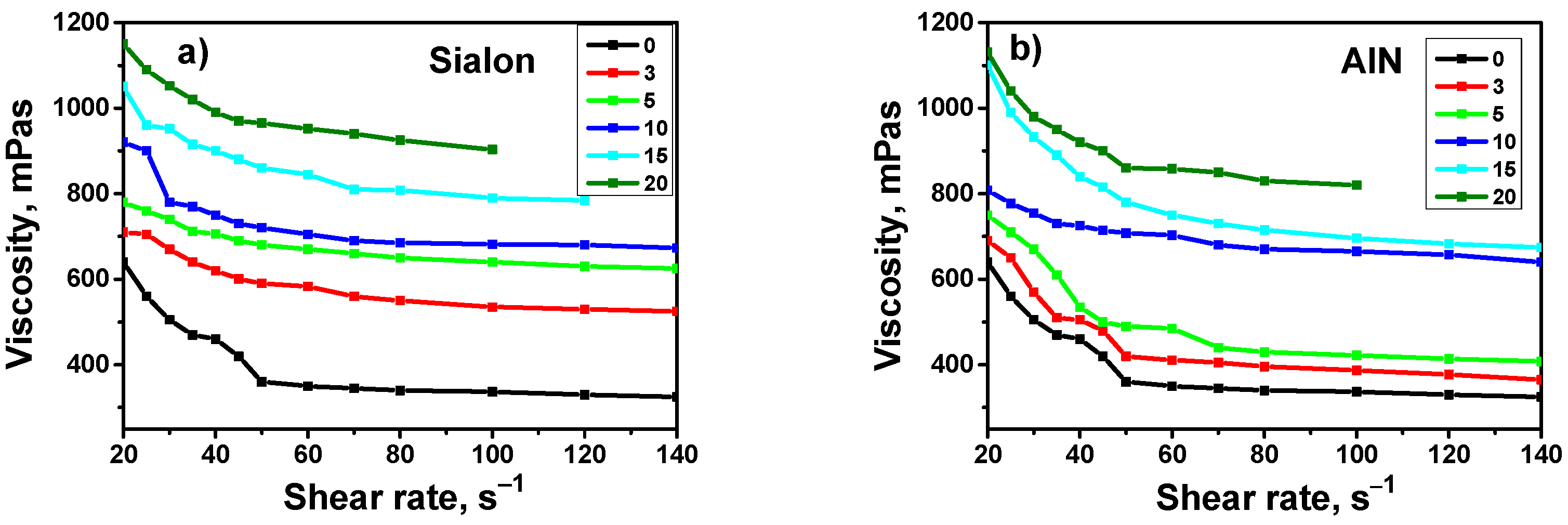
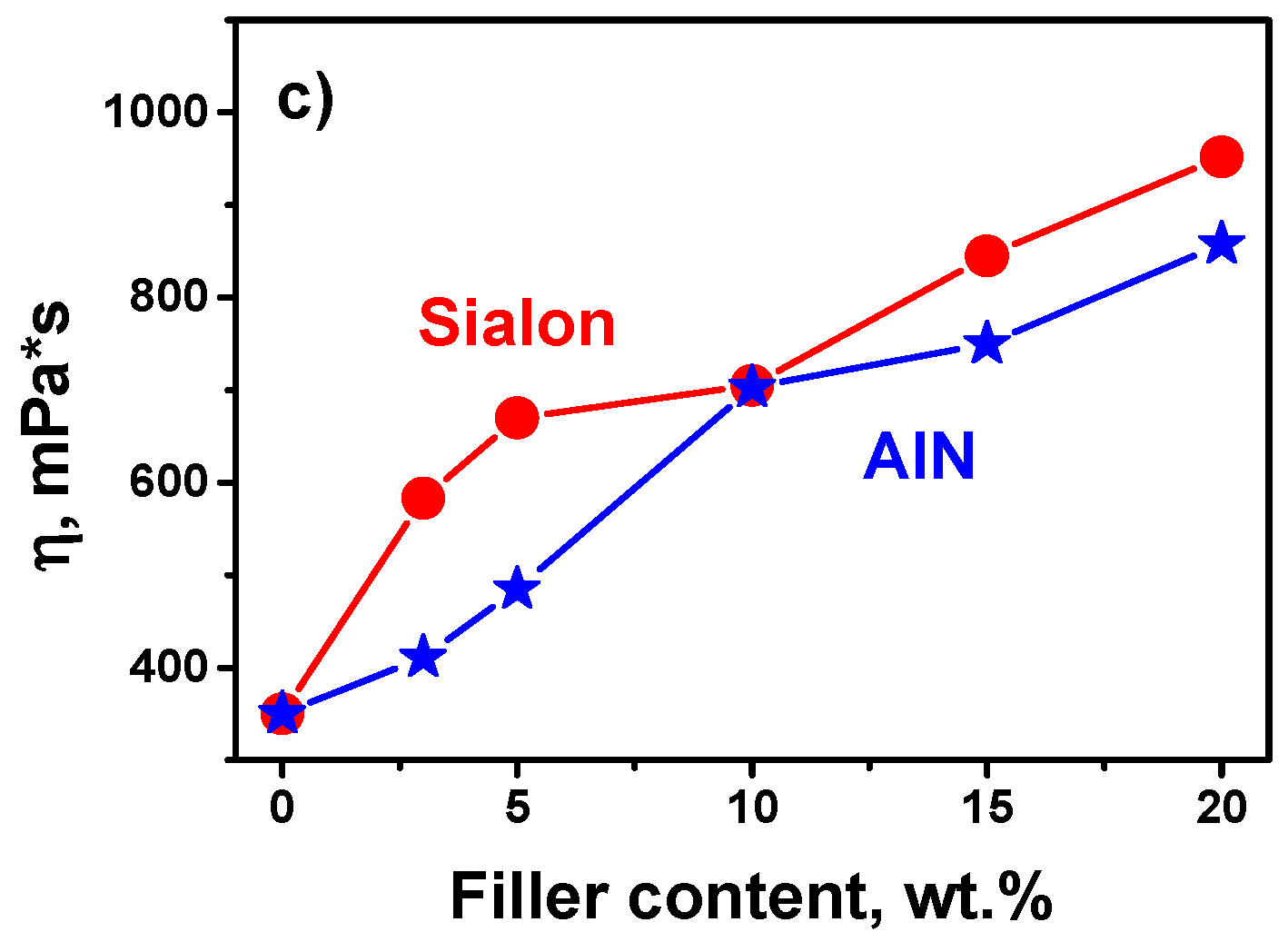
3.1. Viscosity Test
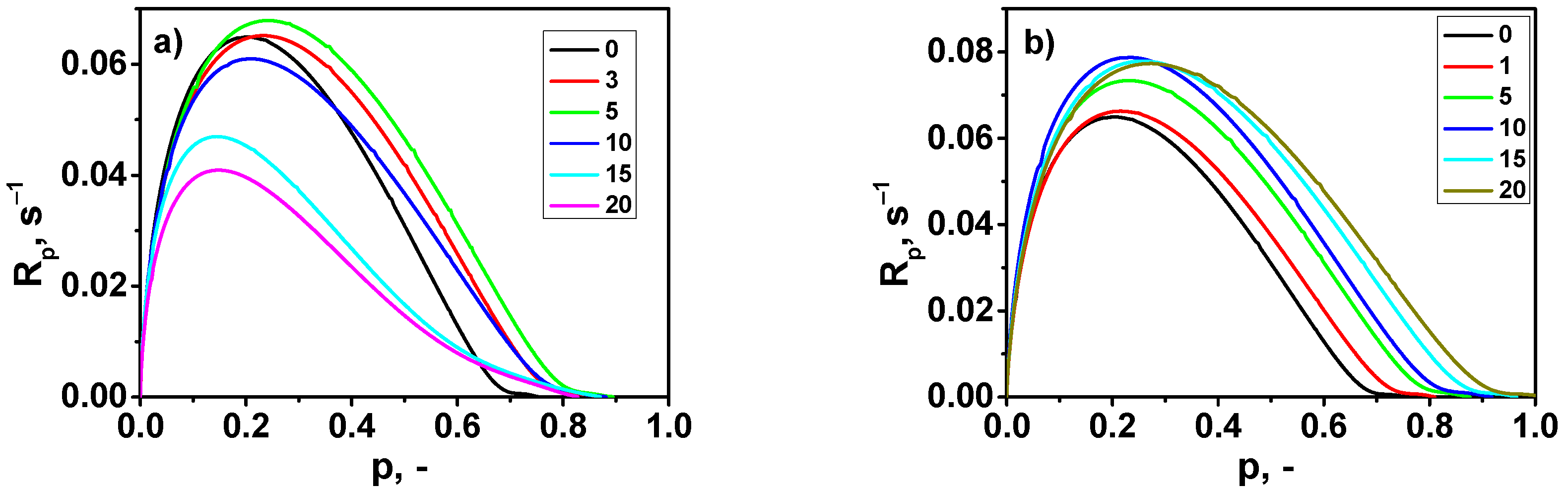
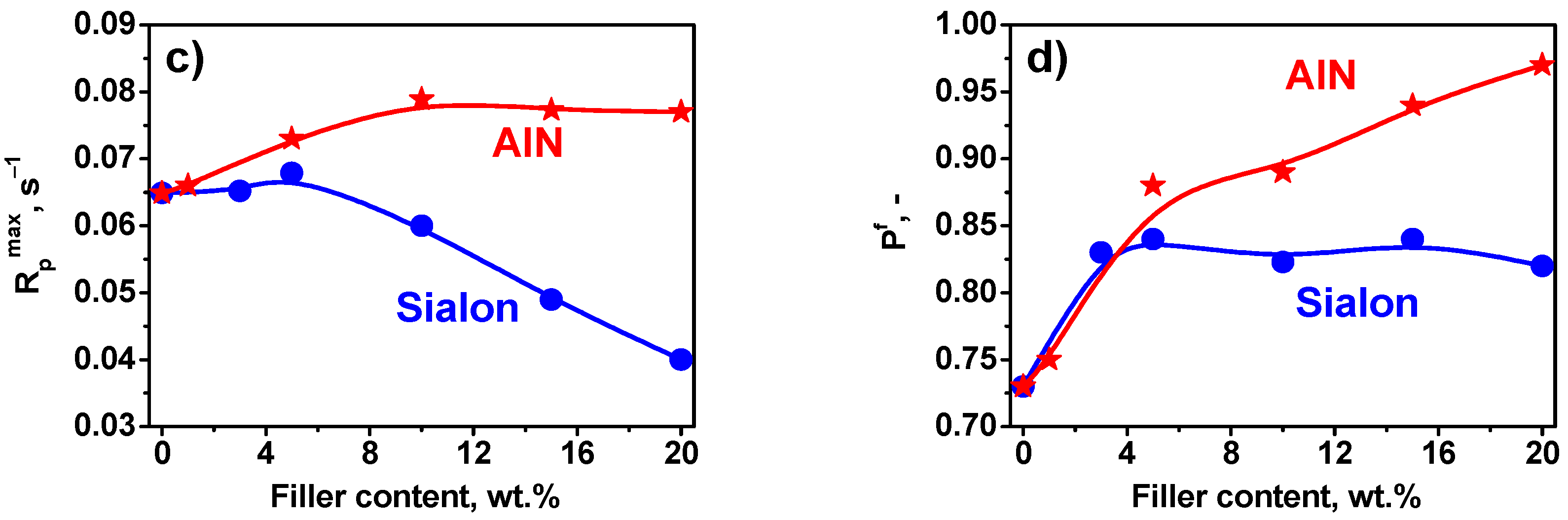
3.2. Photopolymerization Kinetics
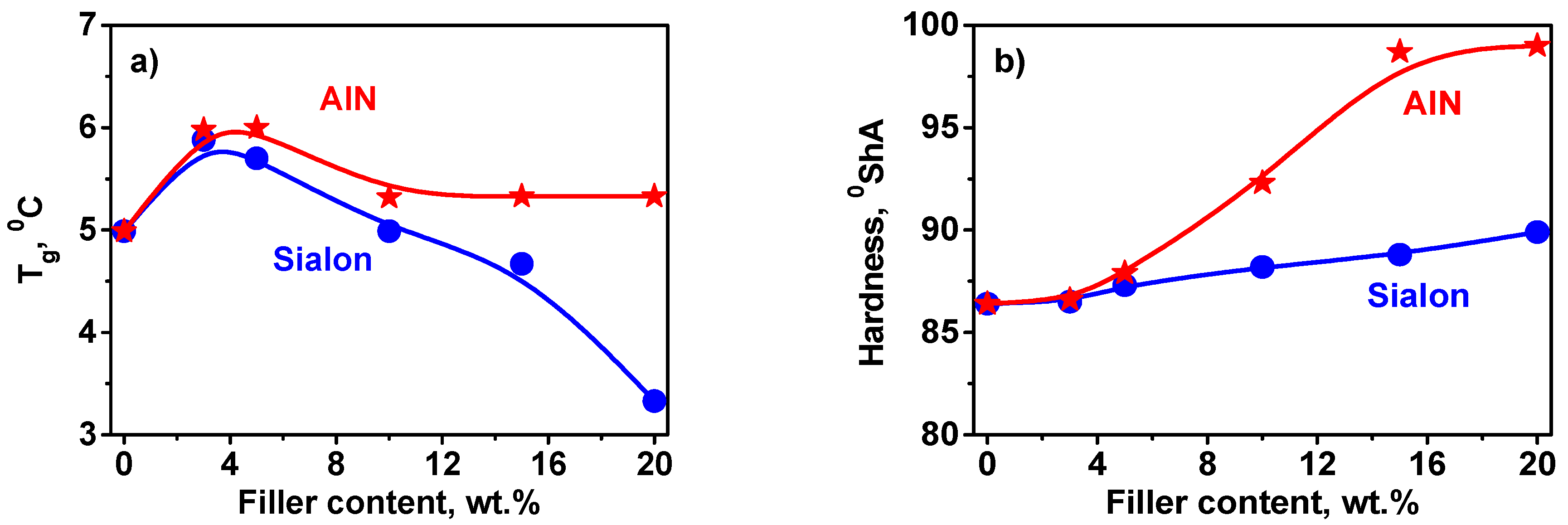
3.3. Thermal and Mechanical Properties
3.4. FTIR-ATR Spectra
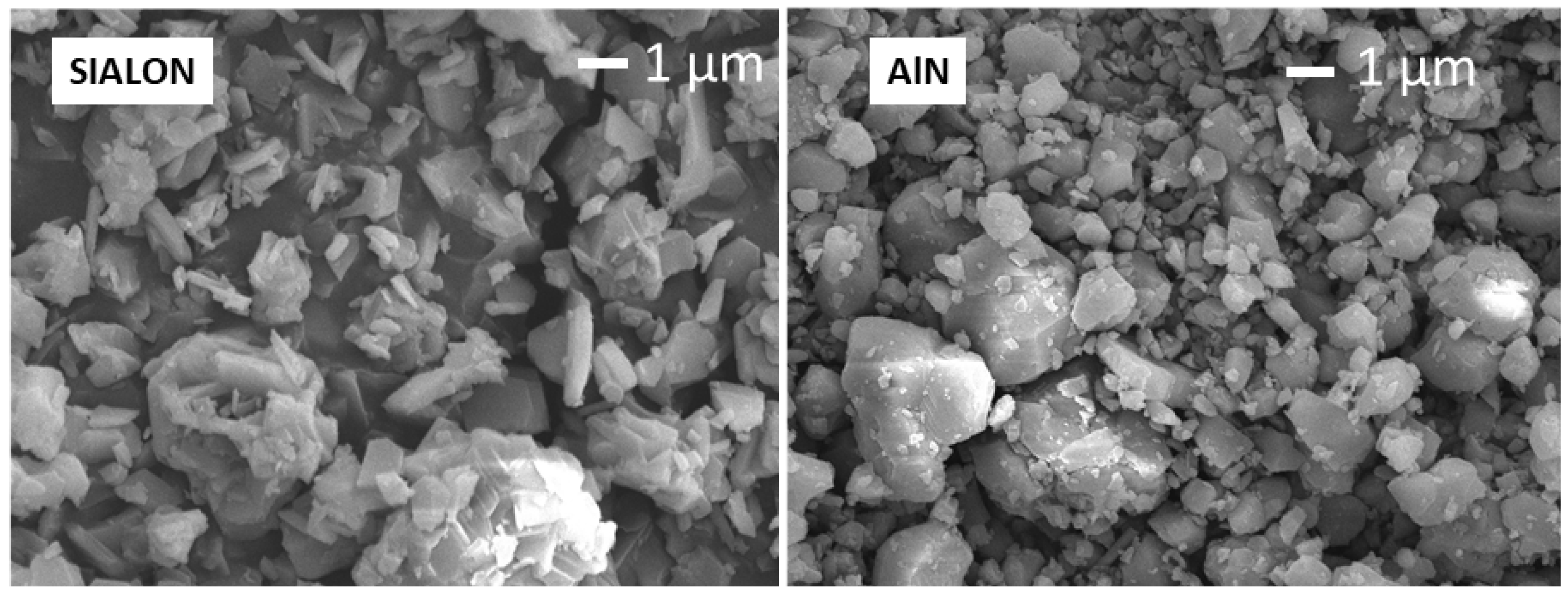
3.5. Cross-Sectional Surface Morphology
3.6. Contact Angle
4. Conclusions
- The addition of both types of fillers to the composition strongly influenced the photopolymerization rate and the viscosity of the photocurable composition.
- The addition of 20 wt.% Sialon and AlN increased the contact angle of the samples by approximately 40% and 31%, respectively, resulting in coatings with hydrophobic surface properties.
- The AlN filler embedded in the matrix reinforced the polymer (hardness) than Sialon, which was associated with better interaction between AlN and the polymer. The presence of fillers led to a slight enhancement of thermal stability of the coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barick, P.; Saha, B.P. Effect of Boron Nitride Addition on Densification. Microstructure. Mechanical. Thermal. and Dielectric Properties of β-SiAlON Ceramic. J. Mater. Eng. Perform 2021, 30, 3603–3611. [Google Scholar] [CrossRef]
- Ahmed, B.A.; Hakeem, A.S.; Laoui, T.; Khan, R.M.A.; Al Malki, M.M.; Ul-Hamid, A.; Khalid, F.A.; Bakhsh, N. Effect of precursor size on the structure and mechanical properties of calcium-stabilized sialon/cubic boron nitride nanocomposites. J. Alloys Compd. 2017, 728, 836–843. [Google Scholar] [CrossRef]
- Falak, M.Z.; Ahmed, B.A.; Saeed, H.A.; Butt, S.U.; Hakeem, A.S.; Akbar, U.A. Spark Plasma Sintering of SiAlON Ceramics Synthesized via Various Cations Charge Stabilizers and Their Effect on Thermal and Mechanical Characteristics. Crystals 2021, 11, 1378. [Google Scholar] [CrossRef]
- Hou, X.; Yue, C.; Guo, M.; Wang, X.; Zhang, M.; Chou, K.C. Synthesis, phase transformation and mechanical property of SiAlON ceramics from coal gangue. J. Min. Met. A 2014, 50, 47–55. [Google Scholar] [CrossRef]
- Mohamedkhair, A.K.; Hakeem, A.S.; Drmosh, Q.A.; Mohammed, A.S.; Baig, M.M.A.; Ul-Hamid, A.; Gondal, M.A.; Yamani, Z.H. Fabrication and Characterization of Transparent and Scratch-Proof Yttrium/Sialon Thin Films. Nanomater. 2020, 10, 2283. [Google Scholar] [CrossRef]
- Garrett, J.C.; Sigalas, I.; Wolfrum, A.K.; Herrmann, M. Effect of cubic boron nitride grain size in the reinforcing of α-Sialon ceramics sintered via SPS. J. Eur. Ceram. Soc. 2015, 35, 451–462. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Ali, S.; Jonson, B. Preparation and properties of mixed La–Pr silicate oxynitride glasses. J. Non-Cryst. Solids 2013, 368, 93–97. [Google Scholar] [CrossRef]
- Ayas, E.; Kara, A. Novel electrically conductive α–β SiAlON/TiCN composites. J. Eur. Ceram. Soc. 2011, 31, 903–911. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lee, Y.-S.; Chung, D.; Kim, B.-J. Studies on Preparation and Characterization of Aluminum Nitride-Coated Carbon Fibers and Thermal Conductivity of Epoxy Matrix Composites. Coatings 2017, 7, 121. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, W.; Ge, Y.; Chen, K.; Xie, Z. Carbothermal synthesis of spherical AlN granules: Effects of synthesis parameters and Y2O3 additive. Ceram. Int. 2015, 41, 6715–6721. [Google Scholar] [CrossRef]
- Gao, Z.; Wan, Y.; Xiong, G.; Guo, R.; Luo, H. Synthesis of aluminum nitride nanoparticles by a facile urea glass route and influence of urea/metal molar ratio. Appl. Surf. Sci. 2013, 280, 42–49. [Google Scholar] [CrossRef]
- Robakowska, M.; Gierz, Ł.; Gojzewski, H. Sialon and Alumina Modified UV-Curable Coatings with Improved Mechanical Properties and Hydrophobicity. Coatings 2021, 11, 1424. [Google Scholar] [CrossRef]
- Sadej, M.; Andrzejewska, E. Silica/aluminum oxide hybrid as a filler for photocurable composites. Prog. Org. Coat. 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Sadej, M.; Gojzewski, H.; Andrzejewska, E. Photocurable polymethacrylate-silica nanocomposites: Correlation between dispersion stability. curing kinetics. morphology and properties. J. Polym. Res. 2016, 23, 116. [Google Scholar] [CrossRef]
- Sadej-Bajerlain, M.; Gojzewski, H.; Andrzejewska, E. Monomer/modified nanosilica systems: Photopolymerization kinetics and composite characterization. Polymer 2011, 52, 1495–1503. [Google Scholar] [CrossRef]
- Sadej, M.; Gojzewski, H.; Gajewski, P.; Vancso, G.J.; Andrzejewska, E. Photocurable acrylate-based composites with enhanced thermal conductivity containing boron and silicon nitrides. Express Polym. Lett. 2018, 12, 790–807. [Google Scholar] [CrossRef]
- Sadej, M.; Andrzejewska, E.; Kurc, B.; Gojzewski, H.; Jesionowski, T. Surface-dependent effect of functional silica fillers on photocuring kinetics of hydrogel materials. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3472–3487. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Y.; Chen, C.-Y.; Moon, K.; Wang, H.; Wong, C.-P. The use of polyimide-modified aluminum nitride fillers in AlN@PI/epoxy composites with enhanced thermal conductivity for electronic encapsulation. Sci. Rep. 2014, 4, 4779. [Google Scholar] [CrossRef]
- Gojzewski, H.; Sadej, M.; Andrzejewska, E.; Kokowska, M. Nanoscale Young’s modulus and surface morphology in photocurable polyacrylate/nanosilica composites. Eur. Polym. J. 2017, 88, 205–220. [Google Scholar] [CrossRef]
- Chen, X.; Gonsalves, K.E. Synthesis and properties of an aluminum nitride/polyimide nanocomposite prepared by a nonaqueous suspension process. J. Mater. Res. 1997, 12, 1274–1286. [Google Scholar] [CrossRef]
- Amorim, A.A.P.O.; Oliveira, M.G.; Mancini, M.C.; Sirqueira, A.S. Rheological, EMI and corrosion properties of epoxy coating with nanoparticle and conductive carbon black. SN Appl. Sci. 2021, 3, 236. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Huang, Y.-L.; Ma, C.-C.M.; Yuen, S.-M.; Teng, C.-C.; Yang, S.-Y. Mechanical, thermal and electrical properties of aluminum nitride/polyetherimide composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1573–1583. [Google Scholar] [CrossRef]
- Gilev, V.G. IR Spectra and Structure of Si–Al–O–N Phases Prepared by Carbothermal Reduction of Kaolin in Nitriding Atmosphere. Inorg. Mater. 2001, 37, 1041–1045. [Google Scholar] [CrossRef]
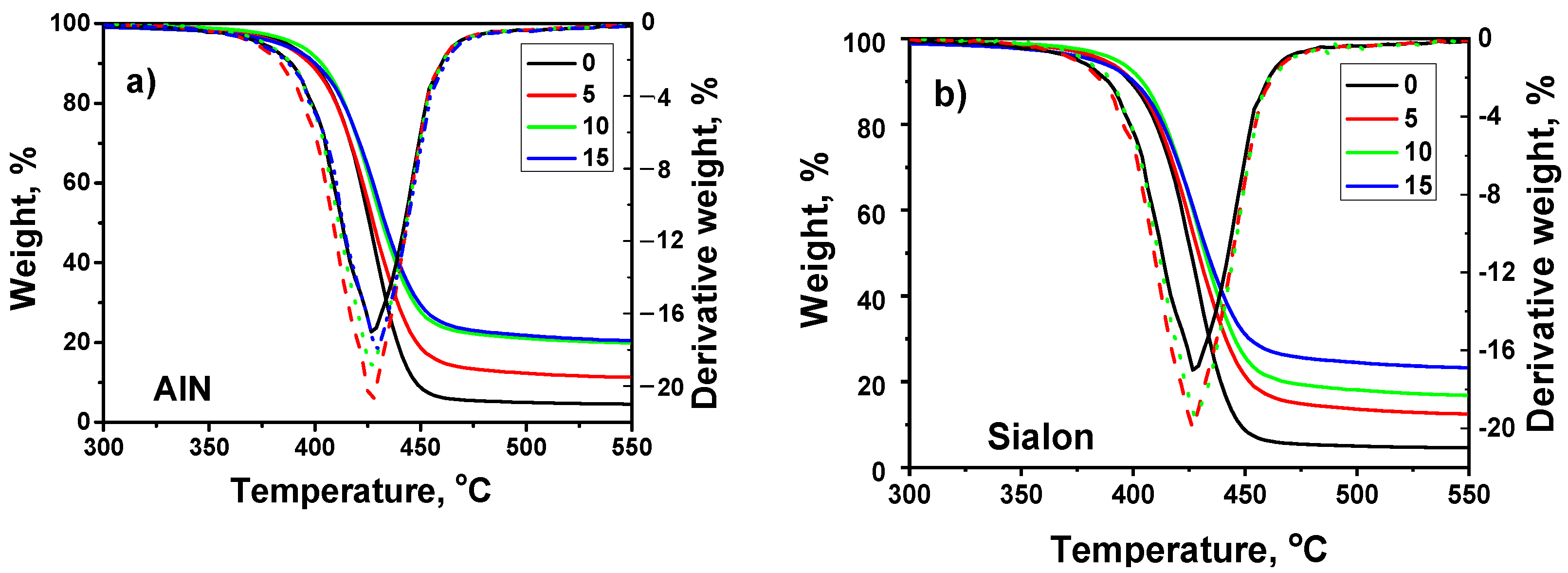
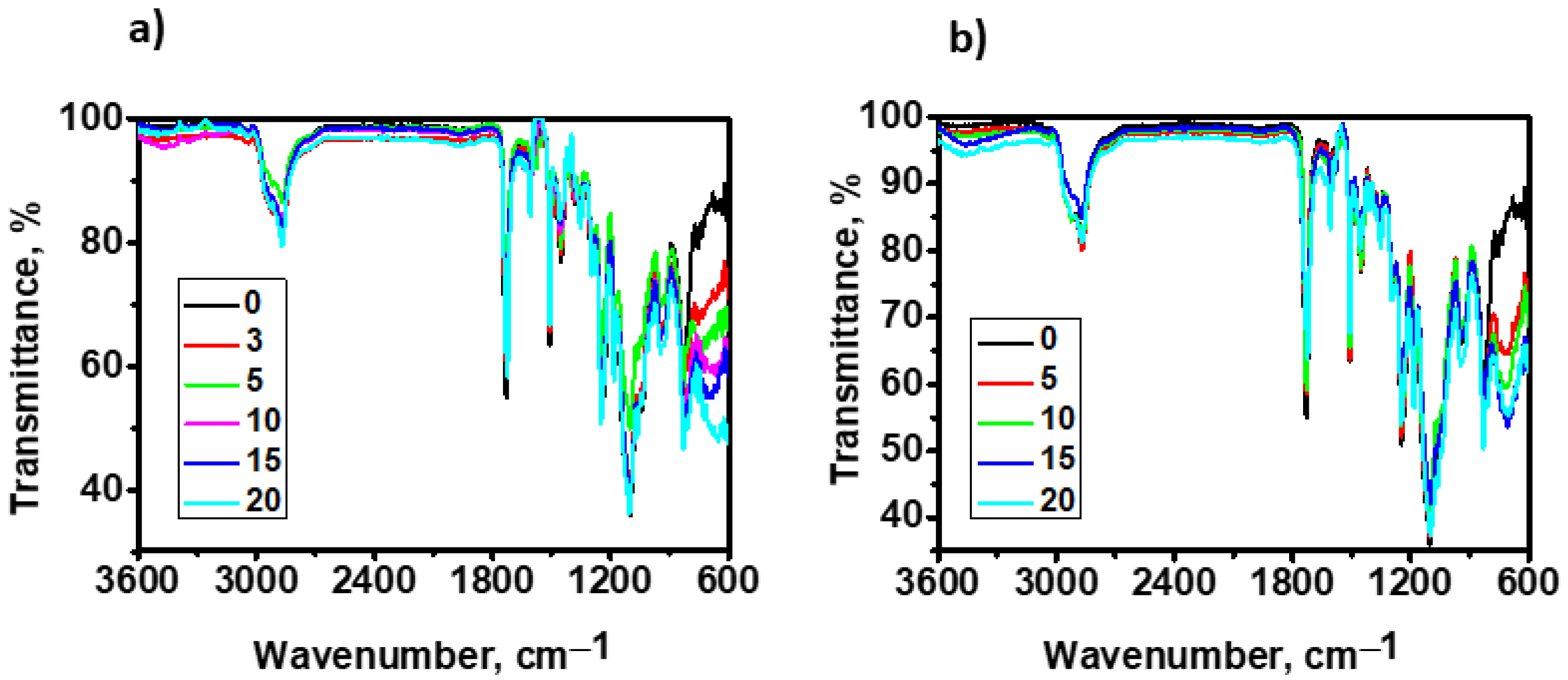

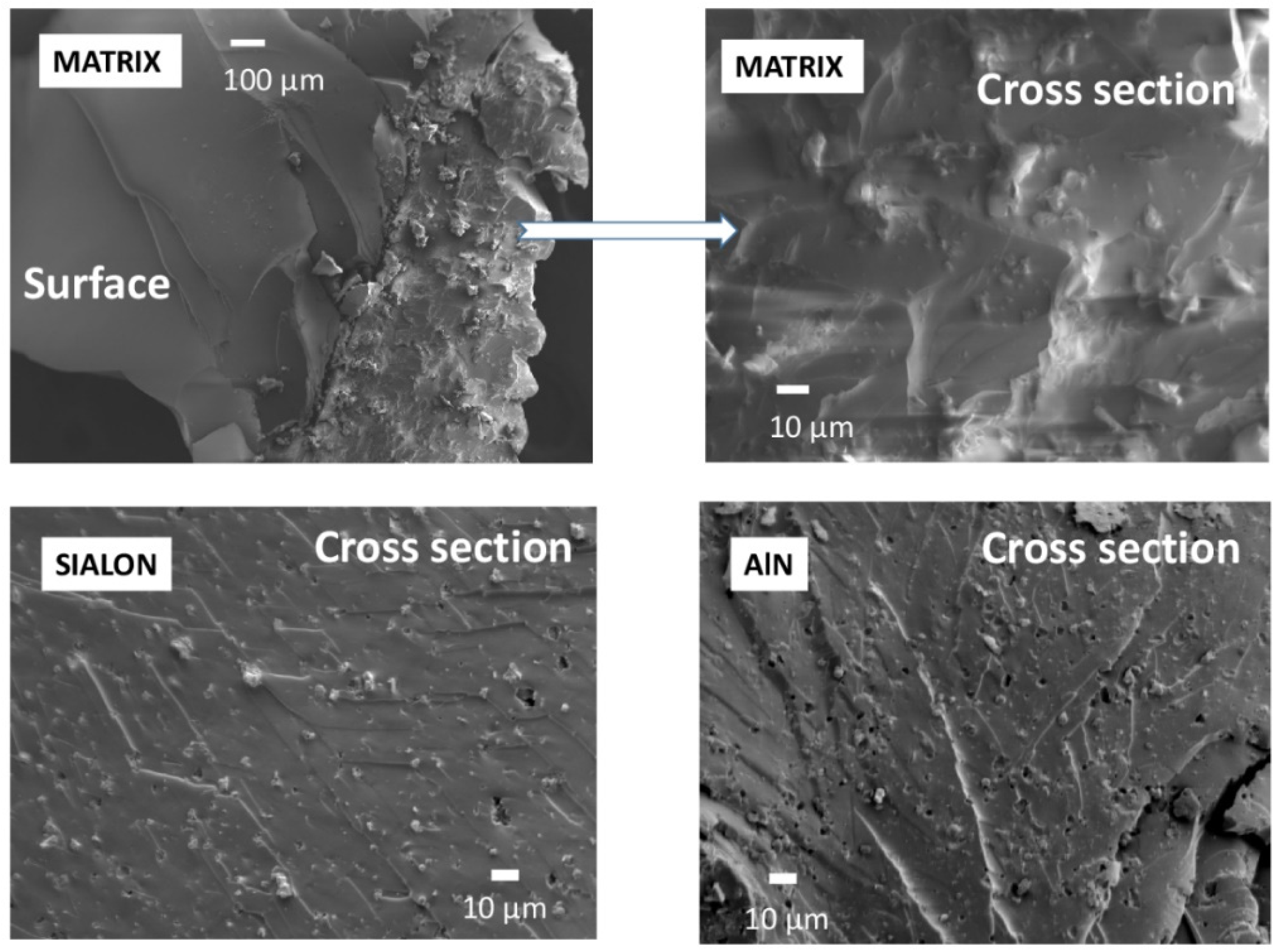
| Sample | Fillers Content [wt.%] | Residue [%] | Weight Loss Temperature [°C] | Tmax [°C] | |
|---|---|---|---|---|---|
| T10% | T50% | ||||
| - | 0 | 4.51 | 398.7 | 425.8 | 423.0 |
| Sialon | 5 | 12.30 | 400.1 | 429.0 | 479.0 |
| 10 | 16.63 | 404.1 | 432.0 | 427.7 | |
| 15 | 23.02 | 400.1 | 433.2 | 427.1 | |
| 20 | 24.89 | 403.1 | 433.9 | 426.9 | |
| AlN | 5 | 11.14 | 397.7 | 427.7 | 426.7 |
| 10 | 19.74 | 403.2 | 432.0 | 477.8 | |
| 15 | 20.26 | 400.0 | 433.2 | 479.0 | |
| Fillers Content [wt.%] | - | Contact Angle [°] θW | ||
|---|---|---|---|---|
| 0 | - | θW = 69° 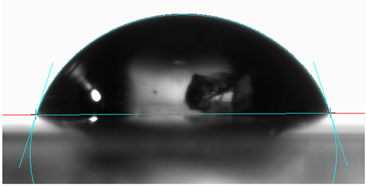 | ||
| - | θW [°] | Sialon | θW [°] | AlN |
| 5 | 82 |  | 87 | 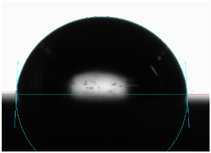 |
| 10 | 87 |  | 78 |  |
| 15 | 92 |  | 88 |  |
| 20 | 97 |  | 91 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robakowska, M.; Gierz, Ł.; Mayer, P.; Szcześniak, K.; Marcinkowska, A.; Lewandowska, A.; Gajewski, P. Influence of the Addition of Sialon and Aluminum Nitride Fillers on the Photocuring Process of Polymer Coatings. Coatings 2022, 12, 1389. https://doi.org/10.3390/coatings12101389
Robakowska M, Gierz Ł, Mayer P, Szcześniak K, Marcinkowska A, Lewandowska A, Gajewski P. Influence of the Addition of Sialon and Aluminum Nitride Fillers on the Photocuring Process of Polymer Coatings. Coatings. 2022; 12(10):1389. https://doi.org/10.3390/coatings12101389
Chicago/Turabian StyleRobakowska, Mariola, Łukasz Gierz, Paulina Mayer, Katarzyna Szcześniak, Agnieszka Marcinkowska, Aneta Lewandowska, and Piotr Gajewski. 2022. "Influence of the Addition of Sialon and Aluminum Nitride Fillers on the Photocuring Process of Polymer Coatings" Coatings 12, no. 10: 1389. https://doi.org/10.3390/coatings12101389
APA StyleRobakowska, M., Gierz, Ł., Mayer, P., Szcześniak, K., Marcinkowska, A., Lewandowska, A., & Gajewski, P. (2022). Influence of the Addition of Sialon and Aluminum Nitride Fillers on the Photocuring Process of Polymer Coatings. Coatings, 12(10), 1389. https://doi.org/10.3390/coatings12101389