Enhanced the Efficiency of Photocatalytic Degradation of Methylene Blue by Construction of Z-Scheme g-C3N4/BiVO4 Heterojunction
Abstract
:1. Introduction
2. Experimental
2.1. Materials
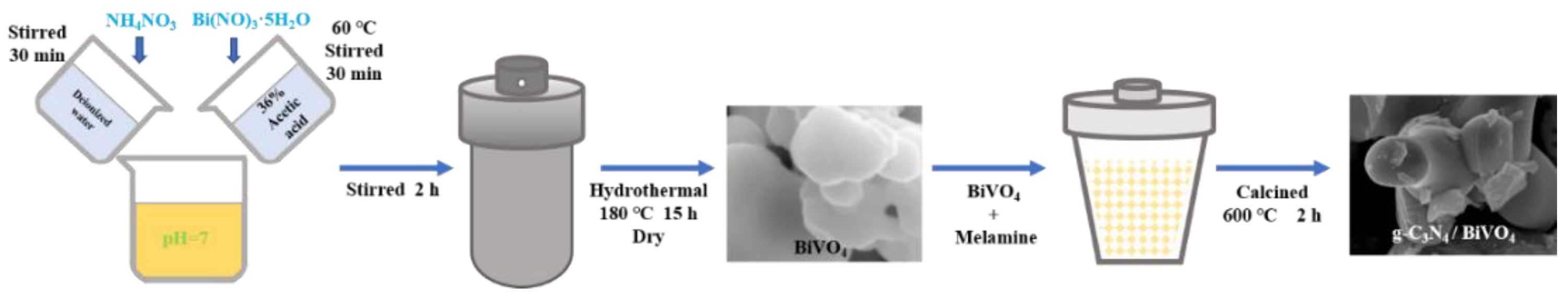
2.2. Synthesis of BiVO4 and g-C3N4/BiVO4 Composites
2.3. Characterization
2.4. Photocatalytic Degradation Experiments
3. Results and Discussion
3.1. XRD Analysis
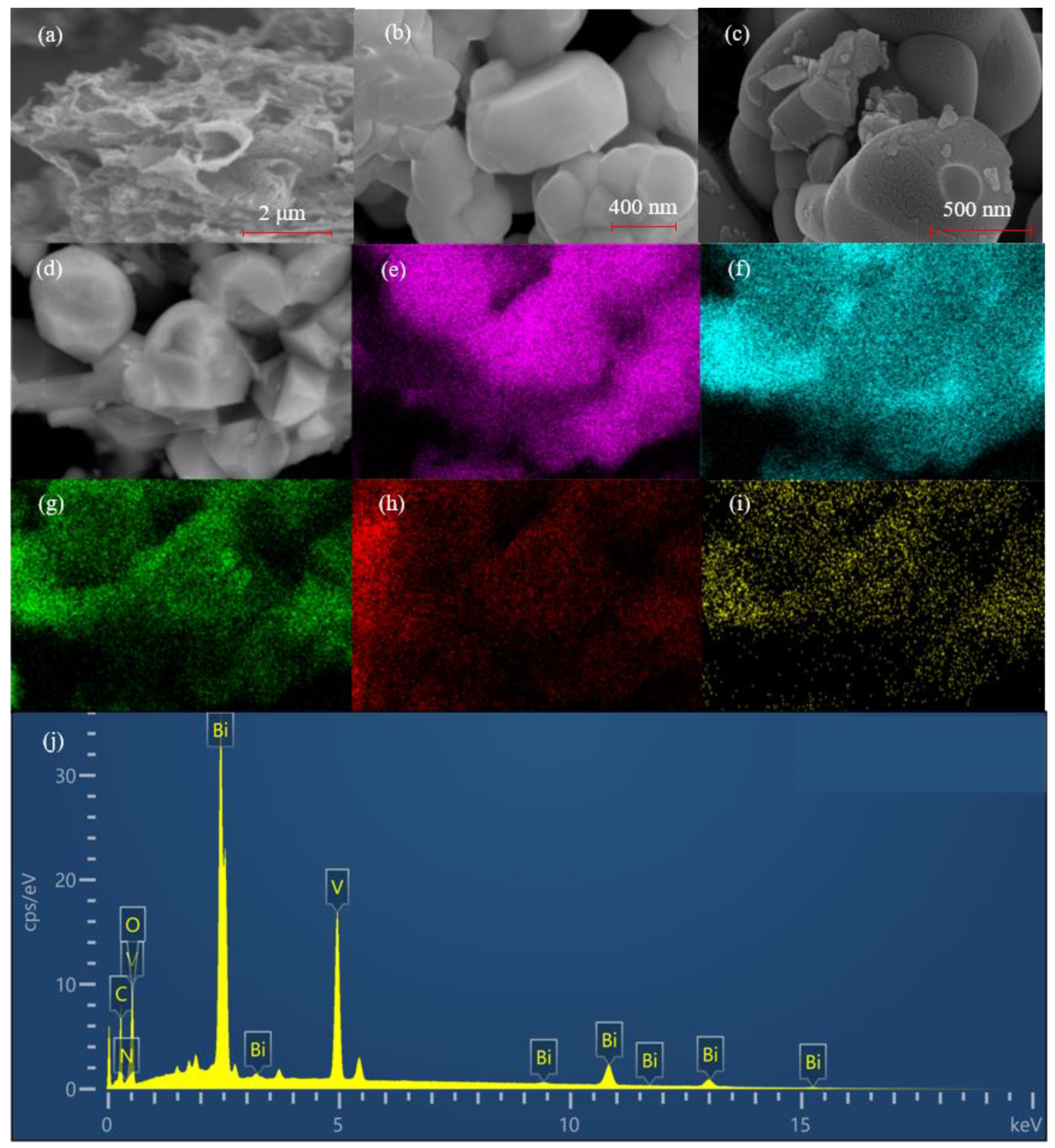
3.2. Morphology of Catalyst
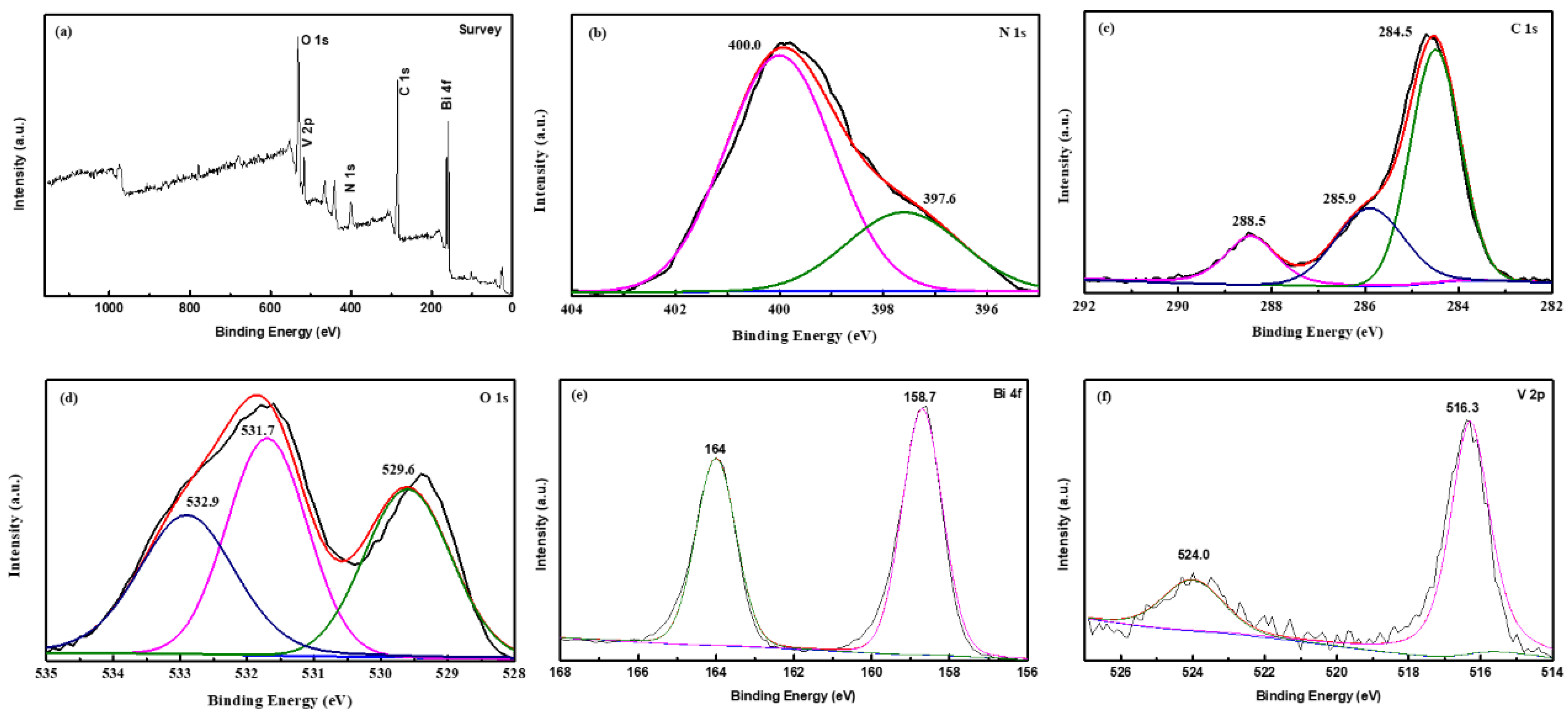
3.3. XPS Analysis
3.4. UV–Vis DRS and Band Structure Analysis
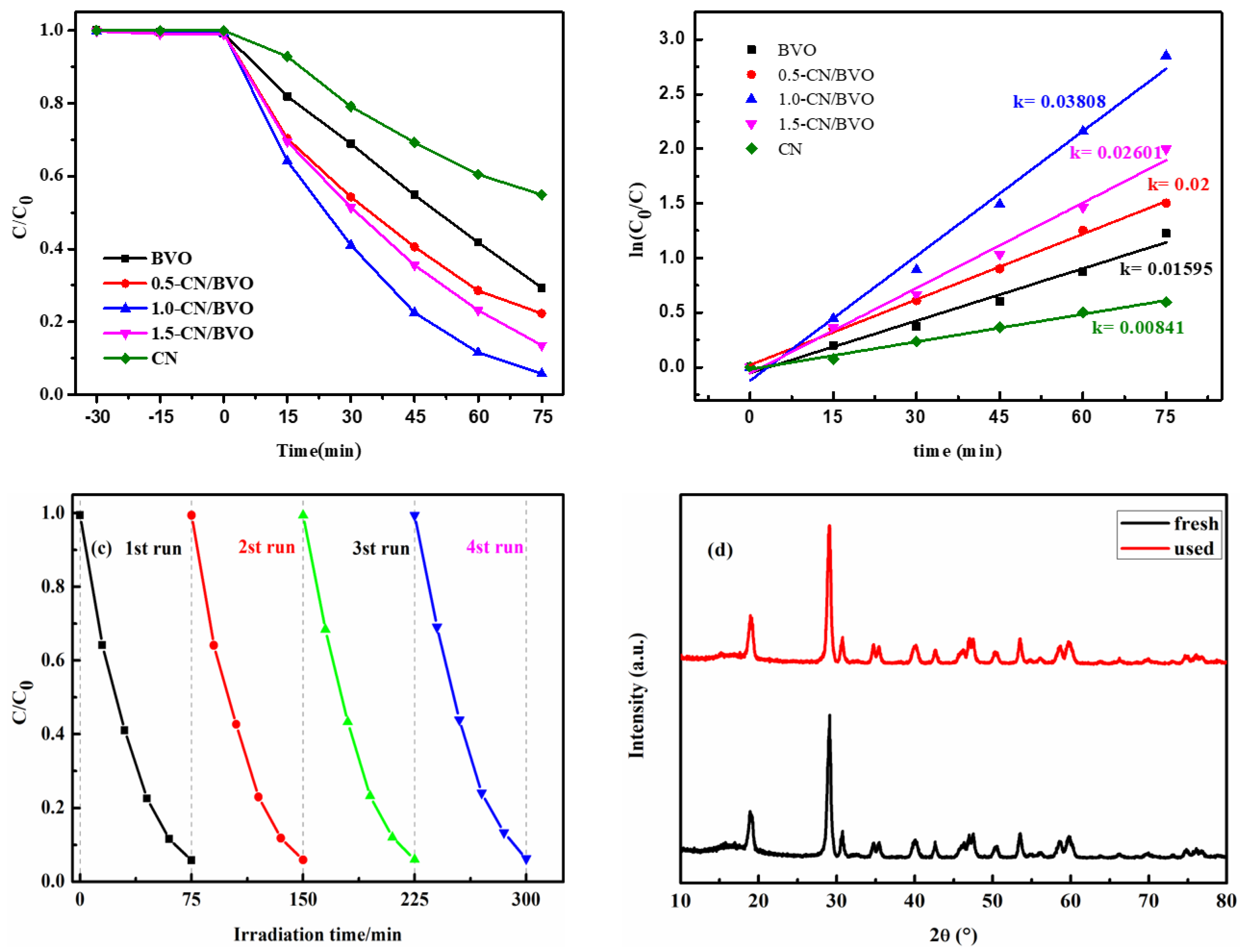
3.5. Photocatalytic Degradation Performance
3.6. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Declaration of competing interest
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.W.; Wang, D.; Wang, D. Nanomaterials for energy conversion and storage. ChemNanoMat 2016, 2, 560–561. [Google Scholar] [CrossRef]
- Xu, M.; Han, L.; Dong, S. Facile fabrication of highly efficient g-C3N4/Ag2O heterostructured photocatalysts with enhanced visible-light photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 12533–12540. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B 2015, 162, 494–500. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Yin, W.; Wang, W.; Zhou, L.; Sun, S.; Zhang, L. CTAB-assisted synthesis of monoclinic BiVO4 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation. J. Hazard. Mater. 2010, 173, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, D.; Wang, L.; Li, H.; Wang, P.; Jiang, T.; Xie, T. Hydrothermal synthesis and photoelectric properties of BiVO4 with different morphologies: An efficient visible-light photocatalyst. Appl. Surf. Sci. 2011, 257, 7758–7762. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, G.; Dong, G.; Ren, H.; Xia, A. Effects of structure, morphology, and up-conversion on Nd-doped BiVO4 system with high photocatalytic activity. Ceram. Int. 2015, 41, 3259–3268. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Nanostructured bismuth vanadate-based materials for solar-energy-driven water oxidation: A review on recent progress. Nanoscale 2014, 6, 14044–14063. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Cui, W.; Sui, H. Synthesis and characterization of a core-shell BiVO4@g-C3N4 photo-catalyst with enhanced photocatalytic activity under visible light irradiation. RSC Adv. 2017, 7, 8167–8177. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Bai, Y.; Zhang, W.F. Difference in valence band top of BiVO4 with different crystal structure. Mater. Chem. Phys. 2012, 136, 930–934. [Google Scholar] [CrossRef]
- Usai, S.; Obregón, S.; Becerro, A.I.; Colón, G. Monoclinic–tetragonal heterostructured BiVO4 by yttrium doping with improved photocatalytic activity. J. Phys. Chem. C 2013, 117, 24479–24484. [Google Scholar] [CrossRef]
- Tan, G.; Zhang, L.; Ren, H.; Wei, S.; Huang, J.; Xia, A. Effects of pH on the hierarchical structures and photocatalytic performance of BiVO4 powders prepared via the microwave hydrothermal method. ACS Appl Mater. Interfaces 2013, 5, 5186–5193. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kweon, K.E.; Ye, H.; Paek, E.; Hwang, G.S.; Bard, A.J. Factors in the metal doping of BiVO4 for improved photoelectrocatalytic activity as studied by scanning electrochemical microscopy and first-principles density-functional calculation. J. Phys. Chem. C 2011, 115, 17870–17879. [Google Scholar] [CrossRef]
- Ansari, S.A.; Ansari, S.G.; Foaud, H.; Cho, M.H. Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J. Chem. 2017, 41, 9314–9320. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.; Yang, W.; Sun, J.; Hou, H. Efficient visible-light driven photocatalysts: Coupling TiO2(AB) nanotubes with g-C3N4 nanoflakes. J. Mater. Sci.-Mater. Electron. 2017, 28, 1271–1280. [Google Scholar] [CrossRef]
- Zhang, Z.; Xui, R.; Wang, Z.; Dong, M.; Cui, B.; Chen, M. Visible-light neural stimulation on graphitic-carbon nitride/graphene photocatalytic fibers. ACS Appl. Mater. Interfaces 2017, 9, 34736–34743. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1–31. [Google Scholar] [CrossRef]
- Ji, Y.; Cao, J.; Jiang, L.; Zhang, Y.; Yi, Z. g-C3N4/BiVO4 composites with enhanced and stable visible light photocatalytic activity. J. Alloys Compd. 2014, 590, 9–14. [Google Scholar] [CrossRef]
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Mohamad Noh, M.F.; Soh, M.F.; Tahir, A.A.; Ahmad Ludin, N.; Ibrahim, M.A.; Wan Isahak, W.N.R.; Mat Teridi, M.A. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl. Catal. B 2018, 234, 296–310. [Google Scholar] [CrossRef]
- Li, Z.; Jin, C.; Wang, M.; Kang, J.; Wu, Z.; Yang, D.; Zhu, T. Novel rugby-like g-C3N4/BiVO4 core/shell Z-scheme composites prepared via low-temperature hydrothermal method for enhanced photocatalytic performance. Sep. Purif. Technol. 2020, 232, 1383–5866. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; He, Y.; Guo, Y.; Zhang, T.; Zhang, Y. Mediator-free direct Z-scheme photocatalytic system: BiVO4/g-C3N4 organic-inorganic hybrid photocatalyst with highly efficient visible-light-induced photocatalytic activity. Dalton Trans. 2015, 44, 4297–4307. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Jin, X.; Zheng, X.; Wang, Y.; Wei, D.; Zhang, Q.; Feng, Y.; Xie, Z.; Chen, P.; et al. Highly active metal-free carbon dots/g-C3N4 hollow porous nanospheres for solar-light-driven PPCPs remediation: Mechanism insights, kinetics and effects of natural water matrices. Water Res. 2020, 172, 115492. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Lee, G.J.; Zhou, R.; Wu, J.J. Synthesis of g-C3N4/BiVO4 heterojunction composites for photocatalytic degradation of nonylphenol ethoxylate. Sep. Purif. Technol. 2020, 250, 117202. [Google Scholar] [CrossRef]
- Zhong, K.; Feng, J.; Gao, H.; Zhang, Y.; Lai, K. Fabrication of BiVO4@g-C3N4(100) heterojunction with enhanced photocatalytic visible-light-driven activity. J. Solid State Chem. 2019, 274, 142–151. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Wang, Z.; Zhang, X.; Qin, X.; Dai, Y.; Liu, Y.; Wang, P.; Li, Y.; Huang, B. Doping strategy to promote the charge separation in BiVO4 photoanodes. Appl. Catal. B-Environ. 2017, 211, 258–265. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Q.; Yan, X.; Mo, Q.; Chen, Y.; Liu, B.; Teng, L.; Xiao, W.; Ge, L.; Wang, Q. Enhanced photocatalytic activity of the carbon quantum dot-modified bioi microsphere. Nanoscale Res. Lett. 2016, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Yan, X.; Mo, Q.; Liu, B.; Wang, J.; Yang, X.; Li, L. Facile synthesis of g-C3N4/BiVO4 heterojunctions with enhanced visible light photocatalytic performance. Ceram. Int. 2017, 43, 301–307. [Google Scholar] [CrossRef]
- Li, L.; Mao, M.; She, X.; Yi, J.; He, M.; Pan, L.; Chen, Z.; Xu, H.; Li, H. Direct Z-scheme photocatalyst for efficient water pollutant degradation: A case study of 2D g-C3N4/BiVO4. Mater. Chem. Phys. 2020, 241, 122308. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, J.; Zhang, J.; Dai, K.; Liang, C. Facile synthesis of Z-scheme BiVO4 /porous graphite carbon nitride heterojunction for enhanced visible-light-driven photocatalyst. Appl. Surf. Sci. 2018, 430, 595–602. [Google Scholar] [CrossRef]
- Li, C.; Che, H.; Liu, C.; Che, G.; Charpentier, P.A.; Xu, W.Z.; Wang, X.; Liu, L. Facile fabrication of g-C3N4 QDs/BiVO4 Z-scheme heterojunction towards enhancing photodegradation activity under visible light. J. Taiwan Inst. Chem. Eng. 2019, 95, 669–681. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Liu, S.; Zhang, L.; Xu, H.; Zhu, W. A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst. J. Mol. Catal. A-Chem. 2006, 252, 120–124. [Google Scholar] [CrossRef]
- Wang, Y.; Di, Y.; Antonietti, M.; Li, H.; Chen, X.; Wang, X. Excellent visible-light photocatalysis of fluorinated polymeric carbon nitride solids. Chem. Mater. 2010, 22, 5119–5121. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Huang, W.; Kong, Q.; Fan, X.; Wang, M.; Shi, J. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H-2 evolution under visible light. Carbon 2016, 99, 111–117. [Google Scholar] [CrossRef]
- Wang, M.; Guo, P.; Zhang, Y.; Liu, T.; Li, S.; Xie, Y.; Wang, Y.; Zhu, T. Eu doped g-C3N4 nanosheet coated on flower-like BiVO4 powders with enhanced visible light photocatalytic for tetracycline degradation. Appl. Surf. Sci. 2018, 453, 11–22. [Google Scholar] [CrossRef]
- Rathi, V.; Panneerselvam, A.; Sathiyapriya, R. A novel hydrothermal induced BiVO4/g-C3N4 heterojunctions visible-light photocatalyst for effective elimination of aqueous organic pollutants. Vacuum 2020, 180, 109458. [Google Scholar] [CrossRef]
- Norsten, T.B.; Kantchev, E.A.B.; Kantchev, E.A.B. Thiophene-containing pechmann dye derivatives. OL 2010, 20, 4816–4819. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-scheme alpha-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Wang, Y.; Zhao, J.; Wang, D.; Li, X.; Guo, Z.; Wang, H.; Deng, Y.; Niu, C.; et al. Novel ternary heterojunction photcocatalyst of Ag nanoparticles and g-C3N4 nanosheets co-modified BiVO4 for wider spectrum visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B-Environ. 2017, 205, 133–147. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, M.; Liu, C.; Zhang, Z.; Zhang, F.; Liu, Q. Enhanced the Efficiency of Photocatalytic Degradation of Methylene Blue by Construction of Z-Scheme g-C3N4/BiVO4 Heterojunction. Coatings 2021, 11, 1027. https://doi.org/10.3390/coatings11091027
Zhang X, Li M, Liu C, Zhang Z, Zhang F, Liu Q. Enhanced the Efficiency of Photocatalytic Degradation of Methylene Blue by Construction of Z-Scheme g-C3N4/BiVO4 Heterojunction. Coatings. 2021; 11(9):1027. https://doi.org/10.3390/coatings11091027
Chicago/Turabian StyleZhang, Xiong, Minjin Li, Cheng Liu, Zhiyong Zhang, Fuchun Zhang, and Qiaoping Liu. 2021. "Enhanced the Efficiency of Photocatalytic Degradation of Methylene Blue by Construction of Z-Scheme g-C3N4/BiVO4 Heterojunction" Coatings 11, no. 9: 1027. https://doi.org/10.3390/coatings11091027
APA StyleZhang, X., Li, M., Liu, C., Zhang, Z., Zhang, F., & Liu, Q. (2021). Enhanced the Efficiency of Photocatalytic Degradation of Methylene Blue by Construction of Z-Scheme g-C3N4/BiVO4 Heterojunction. Coatings, 11(9), 1027. https://doi.org/10.3390/coatings11091027




