The Protection, Challenge, and Prospect of Anti-Oxidation Coating on the Surface of Niobium Alloy
Abstract
1. Introduction
2. Anti-Oxidation Coating on Niobium Alloys
2.1. Slurry Sintering Method
2.2. Thermal Spraying Method
| Substrate | Material Composition (wt.%) | Process Conditions | Coating Composition and Thickness (μm) | Oxidation System | Oxidation Products | Quality Change (mg/cm2) | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Spray Distance (mm) | Spray Rate (g/min) | Outer Layer | Inner Layer | ||||||
| Nb521 | 10mullite-90MoSi2 | 100 | 20 | h-MoSi2 t-MoSi2 t-WSi2 (150) | Mo5Si3 W5Si3 NbSi2 (50) | 1500 °C 500 h | SiO2 | 4.41 | [42] |
| Nb521 | MoSi2 | 100 | 20 | t-MoSi2 h-MoSi2 (155) | Mo5Si3 Mo (45) | 1500 °C 140 h | SiO2 | 21.38 | [43] |
| 10mullite-90MoSi2 | Mullite SiO2 | −2.77 | |||||||
| 30mullite-70MoSi2 | −4.06 | ||||||||
| Nb521 | MoSi2 | 100 | 25 | b-MoSi2 (98) | Mo5Si3 (23) | 1200 °C 94 h | MoSi2 | − | [44] |
| Nb-W | MoSi2 | 90 | 20 | h-MoSi2 t-MoSi2 SiO2 (170) | Mo5Si3 (34) | 1500 °C 43 h | SiO2 | 5.31 | [45] |
| Nb-Si-Ti | Mo-45Si-45Al | 90 | 25 | Mo(Si,Al)2 (100) | Mo5(Si,Al)3 (25) | 1250 °C 100 h | SiO2 Al2O3 | 8.24 | [46] |
| Nb-Si-Ti | 2NaF-34Si-B-63Al2O3 | 100 | 25 | MoSi2 (72) | Mo Mo5Si3 (55) | 1250 °C 100 h | SiO2 Borosilicate glass cover | 1.28 | [47] |
2.3. Embedding Method
2.4. Other Methods
3. Oxidation Mechanism and Failure Behavior of Coating
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subramanian, P.; Mendiratta, M.; Dimiduk, D. The development of Nb-based advanced intermetallic alloys for structural applications. JOM 1996, 48, 33–38. [Google Scholar] [CrossRef]
- Wadsworth, J.; Froes, F. Developments in metallic materials for aerospace applications. JOM 1989, 41, 12–19. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, W.; Xu, R.; Jiang, P. Review on active thermal protection and its heat transfer for airbreathing hypersonic vehicles. Chin. J. Aeronaut. 2018, 31, 1929–1953. [Google Scholar] [CrossRef]
- Ji, T.; Zhang, R.; Sunden, B.; Xie, G. Investigation on thermal performance of high temperature multilayer insulations for hypersonic vehicles under aerodynamic heating condition. Appl. Therm. Eng. 2014, 70, 957–965. [Google Scholar] [CrossRef]
- Jian, D.; Qiuru, Z. Key technologies for thermodynamic cycle of precooled engines: A review. Acta Astronaut. 2020, 177, 299–312. [Google Scholar] [CrossRef]
- Yuan, B.; Harvey, C.M.; Thomson, R.C.; Critchlow, G.W.; Wang, S. A new spallation mechanism of thermal barrier coatings on aero-engine turbine blades. Theor. Appl. Mech. Lett. 2018, 8, 7–11. [Google Scholar] [CrossRef]
- Prasad, V.S.; Baligidad, R.; Gokhale, A.A. Niobium and other high temperature refractory metals for aerospace applications. In Aerospace Materials and Material Technologies; Springer: Cham, Switzerland, 2017; pp. 267–288. [Google Scholar]
- Inouye, H. Niobium in high temperature applications. In Proceedings of the Niobium-Proceedings of the International Symposium; Metallurgical Society of AIME: Warrendale, PA, USA, 1984. [Google Scholar]
- Eckert, J. Niobium compounds and alloys. Int. J. Refract. Met. Hard Mater. 1993, 12, 335–340. [Google Scholar] [CrossRef]
- Wojcik, C.C. Processing, properties and applications of high-temperature niobium alloys. Mrs Online Proc. Libr. (OPL) 1993, 322, 519. [Google Scholar] [CrossRef]
- Wadsworth, J.; Nieh, T.; Stephens, J. Recent advances in aerospace refractory metal alloys. Int. Mater. Rev. 1988, 33, 131–150. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Zuo, J.; Qin, J.; Cheng, K.; Feng, Y.; Bao, W. Research progress on active thermal protection for hypersonic vehicles. Prog. Aerosp. Sci. 2020, 119, 100646. [Google Scholar] [CrossRef]
- Wojcik, C.C.; Chang, W. Thermomechanical processing and properties of niobium alloys. In Proceedings of the International Symposium on Niobium 2001, Orlando, FL, USA, 2–5 December 2001; pp. 163–173. [Google Scholar]
- Packer, C.M.; Perkins, R.A. Development of a fused slurry silicide coating for the protection of tantalum alloys. J. Less Common Met. 1974, 37, 361–378. [Google Scholar] [CrossRef]
- Streiff, R. Protection of materials by advanced high temperature coatings. J. Phys. IV 1993, 3, C9–C17. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Alam, M.Z.; Kumar, A.; Gopinath, K.; Saha, S.; Singh, V.; Srinivas, V.; Das, D.K. Tensile behavior of a slurry Fe-Cr-Si coated Nb-alloy evaluated by Gleeble testing. Surf. Coat. Technol. 2018, 349, 695–706. [Google Scholar] [CrossRef]
- Alam, M.Z.; Sarin, S.; Kumawat, M.K.; Das, D.K. Microstructure and oxidation behaviour of Fe–Cr–silicide coating on a niobium alloy. Mater. Sci. Technol. 2016, 32, 1826–1837. [Google Scholar] [CrossRef]
- Novak, M.D.; Levi, C.G. Oxidation and Volatilization of Silicide Coatings for Refractory Niobium Alloys. In Proceedings of the ASME 2007 International Mechanical Engineering Congress and Exposition, Seattle, WA, USA, 11–15 November 2007; pp. 261–267. [Google Scholar]
- Pan, Y.; Guan, W.M. The hydrogenation mechanism of PtAl and IrAl thermal barrier coatings from first-principles investigations. Int. J. Hydrog. Energy 2020, 45, 20032–20041. [Google Scholar] [CrossRef]
- Geethasree, K.; Satya Prasad, V.V.; Brahma Raju, G.; Alam, M.Z. Cyclic oxidation behavior of Fe-Cr modified slurry silicide coated Nb-18.7Si alloyed with Ti and Zr. Corros. Sci. 2019, 148, 293–306. [Google Scholar] [CrossRef]
- Geethasree, K.; Alam, M.Z.; Raju, G.B.; Prasad, V.V.S. Microstructure and mechanical properties of uncoated Nb-18.7Si and Nb-18.7Si-5Ti alloys and their improved oxidation resistance after application of silicide coating. Mater. Today Proc. 2019, 15, 36–43. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X.; Hu, Y.; Gao, X.; Yu, J.; Qian, M.; Dong, H.; Huang, W. Microstructure and isothermal oxidation behavior of Nb-Ti-Si-based alloy additively manufactured by powder-feeding laser directed energy deposition. Corros. Sci. 2020, 173, 108757. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D.L.; Yu, E.D. Structural, electronic, mechanical and thermodynamic properties of Cr–Si binary silicides from first-principles investigations. Vacuum 2021, 185, 110024. [Google Scholar] [CrossRef]
- Han, J.; Su, B.; Meng, J.; Zhang, A.; Wu, Y. Microstructure and composition evolution of a fused slurry silicide coating on MoNbTaTiW refractory high-entropy alloy in high-temperature oxidation environment. Materials 2020, 13, 3592. [Google Scholar] [CrossRef]
- Segura-Cedillo, I. Fused Metallic Slurry Coatings for Improving the Oxidation Resistance of Wrought Alloys. Ph.D. Thesis, The University of Manchester, Manchester, UK, 1 August 2011. [Google Scholar]
- Sankar, M.; Satya Prasad, V.V.; Baligidad, R.G.; Alam, M.Z.; Das, D.K.; Gokhale, A.A. Microstructure, oxidation resistance and tensile properties of silicide coated Nb-alloy C-103. Mater. Sci. Eng. A 2015, 645, 339–346. [Google Scholar] [CrossRef]
- Ji, P.F.; Li, B.; Chen, B.H.; Wang, F.; Ma, W.; Zhang, X.Y.; Ma, M.Z.; Liu, R.P. Effect of Nb addition on the stability and biological corrosion resistance of Ti-Zr alloy passivation films. Corros. Sci. 2020, 170, 108696. [Google Scholar] [CrossRef]
- Herman, H. Plasma-sprayed coatings. Sci. Am. 1988, 259, 112–117. [Google Scholar] [CrossRef]
- Fauchais, P. Understanding plasma spraying. J. Phys. D Appl. Phys. 2004, 37, R86. [Google Scholar] [CrossRef]
- Stöver, D.; Pracht, G.; Lehmann, H.; Dietrich, M.; Döring, J.-E.; Vaßen, R. New material concepts for the next generation of plasma-sprayed thermal barrier coatings. J. Therm. Spray Technol. 2004, 13, 76–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Hussain, S.; Cui, K.; Fu, T.; Wang, J.; Javed, M.S.; Lv, Y.; Aslam, B. Microstructure and mechanical properties of MoSi2 coating deposited on Mo substrate by hot dipping processes. J. Nanoelectron. Optoelectron. 2019, 14, 1680–1685. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, E. First-principles investigation of structural stability, mechanical and thermodynamic properties of Pt3Zr5 compounds. Phys. B Condens. Matter 2021, 611, 412936. [Google Scholar] [CrossRef]
- Odhiambo, J.G.; Li, W.; Zhao, Y.; Li, C. Porosity and its significance in plasma-sprayed coatings. Coatings 2019, 9, 460. [Google Scholar] [CrossRef]
- Hu, D.; Fu, Q.; Zhou, L.; Liu, B.; Sun, J. Crack development behavior in thermally sprayed anti-oxidation coating under repeated thermal-oxygen coupling environment. Ceram. Int. 2021, 47, 15328–15336. [Google Scholar] [CrossRef]
- Sun, J.; Li, T.; Zhang, G.-P. Effect of thermodynamically metastable components on mechanical and oxidation properties of the thermal-sprayed MoSi2 based composite coating. Corros. Sci. 2019, 155, 146–154. [Google Scholar] [CrossRef]
- Hou, Q.; Shao, W.; Li, M.; Zhou, C. Interdiffusion behavior of Mo-Si-B/Al2O3 composite coating on Nb-Si based alloy. Surf. Coat. Technol. 2020, 401, 126243. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Y.; Fu, T.; Wang, J.; Zhang, X. Toughening mechanism of mullite matrix composites: A Review. Coatings 2020, 10, 672. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, Y.; Wang, R.; Wang, Y.; Cheng, F.; Ma, Y.; Hei, H.; Gao, J.; Zhou, B.; Wang, Y. Microstructure, oxidation behavior and adhesion of a CoNiCrAlTaY coating deposited on a high Nb-TiAl alloy by plasma surface metallizing technique. Vacuum 2020, 179, 109494. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Bai, C. Microstructure and oxidation behavior of Si–MoSi2 functionally graded coating on Mo substrate. Ceram. Int. 2017, 43, 6250–6256. [Google Scholar] [CrossRef]
- Hou, Q.; Li, M.; Shao, W.; Zhou, C. Oxidation and interdiffusion behavior of Mo-Si-B coating on Nb-Si based alloy prepared by spark plasma sintering. Corros. Sci. 2020, 169, 108638. [Google Scholar] [CrossRef]
- Hu, L.; Li, S.; Li, C.; Fu, G.; He, J.; Dong, Y.; Yang, Y.; Zhao, H.; Qin, Y.; Yin, F. Deposition and properties of plasma sprayed NiCrCoMo–TiC composite coatings. Mater. Chem. Phys. 2020, 254, 123502. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Fu, Q. Microstructure and oxidation behavior of plasma sprayed WSi2-mullite-MoSi2 coating on niobium alloy at 1500 °C. Surf. Coat. Technol. 2020, 400, 126210. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Fu, Q. Effect of mullite on the microstructure and oxidation behavior of thermal-sprayed MoSi2 coating at 1500 °C. Ceram. Int. 2020, 46, 10058–10066. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Q.-G.; Huo, C.-X.; Li, T.; Wang, C.; Cheng, C.-Y.; Yang, G.-J.; Sun, J.-C. Oxidation response determined by multiphase-dependent melting degree of plasma sprayed MoSi2 on Nb-based alloy. J. Alloys Compd. 2018, 762, 922–932. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, E.; Wang, D.; Deng, H. Sulfur vacancy enhances the electronic and optical properties of FeS2 as the high performance electrode material. J. Alloys Compd. 2021, 858, 157662. [Google Scholar] [CrossRef]
- Yao, D.; Gong, W.; Zhou, C. Development and oxidation resistance of air plasma sprayed Mo–Si–Al coating on an Nbss/Nb5Si3 in situ composite. Corros. Sci. 2010, 52, 2603–2611. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Zhou, C. Microstructure and oxidation resistance of Mo–Si–B coating on Nb based in situ composites. Corros. Sci. 2014, 87, 421–426. [Google Scholar] [CrossRef]
- Bianco, R.; Rapp, R.A. Pack cementation diffusion coatings. In Metallurgical and Ceramic Protective Coatings; Stern, K.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 236–260. [Google Scholar]
- Mevrel, R.; Duret, C.; Pichoir, R. Pack cementation processes. Mater. Sci. Technol. 1986, 2, 201–206. [Google Scholar] [CrossRef]
- Goward, G.; Cannon, L. Pack cementation coatings for superalloys: A review of history, theory, and practice. J. Eng. Gas Turbines Power 1988, 110, 150–154. [Google Scholar] [CrossRef]
- Sun, J.; Li, T.; Zhang, G.-P.; Fu, Q.-G. Different oxidation protection mechanisms of HAPC silicide coating on niobium alloy over a large temperature range. J. Alloys Compd. 2019, 790, 1014–1022. [Google Scholar] [CrossRef]
- Alam, M.Z.; Rao, A.S.; Das, D.K. Microstructure and high temperature oxidation performance of silicide coating on Nb-based alloy C-103. Oxid. Met. 2010, 73, 513–530. [Google Scholar] [CrossRef]
- Zheng, H.; Xiong, L.; Luo, Q.; Lu, S. Development of multilayer oxidation resistant coatings on Cr–50Nb alloy. Appl. Surf. Sci. 2015, 359, 515–520. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, M.; Guo, X. Development of silicide coatings over Nb–NbCr2 alloy and their oxidation behavior at 1250 °C. Surf. Coat. Technol. 2014, 258, 921–930. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, X.; Sha, J. Oxidation behaviours of Nb–22Ti–15Si–2Al–2Hf–2V–(2, 14) Cr alloys with Al and Y modified silicide coatings prepared by pack cementation. Prog. Nat. Sci. Mater. Int. 2015, 25, 486–495. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Zhou, C. Characterization of microstructure and oxidation resistance of Y and Ge modified silicide coating on Nb-Si based alloy. Corros. Sci. 2016, 110, 114–122. [Google Scholar] [CrossRef]
- Chaia, N.; Cury, P.; Rodrigues, G.; Coelho, G.; Nunes, C. Aluminide and silicide diffusion coatings by pack cementation for Nb-Ti-Al alloy. Surf. Coat. Technol. 2020, 389, 125675. [Google Scholar] [CrossRef]
- Yoon, J.K.; Kim, G.H.; Hong, K.T.; Doh, J.M.; Lee, J.K.; Lee, K.H.; Son, K.H. NbSi-2 Base Nanocomposite Coating and Manufacturing Method Thereof. U.S. Patent 20060029830A1, 29 December 2004. [Google Scholar]
- Xiao, L.-r.; Cai, Z.-g.; YI, D.-q.; Lei, Y.; Liu, H.-q.; Huang, D.-y. Morphology, structure and formation mechanism of silicide coating by pack cementation process. Trans. Nonferrous Met. Soc. China 2006, 16, s239–s244. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Vourlias, G.; Pavlidou, E.; Skolianos, S.; Chrissafis, K.; Stergioudis, G. Comparative examination of the microstructure and high temperature oxidation performance of NiCrBSi flame sprayed and pack cementation coatings. Appl. Surf. Sci. 2009, 255, 3605–3612. [Google Scholar] [CrossRef]
- Majumdar, S.; Arya, A.; Sharma, I.; Suri, A.; Banerjee, S. Deposition of aluminide and silicide based protective coatings on niobium. Appl. Surf. Sci. 2010, 257, 635–640. [Google Scholar] [CrossRef]
- Qiao, Y.; Guo, X. Formation of Cr-modified silicide coatings on a Ti–Nb–Si based ultrahigh-temperature alloy by pack cementation process. Appl. Surf. Sci. 2010, 256, 7462–7471. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, Y.; Fu, T.; Hussain, S.; Saad AlGarni, T.; Wang, J.; Zhang, X.; Ali, S. Effects of Cr2O3 content on microstructure and mechanical properties of Al2O3 matrix composites. Coatings 2021, 11, 234. [Google Scholar] [CrossRef]
- Sun, Z.; Tian, X.; Guo, X.; Yin, M.; Zhang, F.; Zhang, X. Oxidation resistance and mechanical characterization of silicide coatings on the Nb-18Ti-14Si-9Al alloy. Int. J. Refract. Met. Hard Mater. 2017, 69, 18–26. [Google Scholar] [CrossRef]
- Pu, R.; Sun, Y.; Xu, J.; Zhou, X.; Li, S.; Zhang, B.; Cai, Z.; Liu, S.; Zhao, X.; Xiao, L. Microstructure and properties of Mo-based double-layer MoSi2 thick coating by a new two-step method. Surf. Coat. Technol. 2020, 394, 125840. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Q.-G.; Guo, L.-P.; Wang, L. Silicide coating fabricated by HAPC/SAPS combination to protect niobium alloy from oxidation. Acs Appl. Mater. Interfaces 2016, 8, 15838–15847. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Lei, H.; Li, H.; Gong, J.; Sun, C. Influence of different coating structures on the oxidation resistance of MoSi2 coatings. Ceram. Int. 2020, 46, 5993–5997. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, W.; Wang, C.; Zhou, C. Microstructure and oxidation behavior of Mo-Si-Al coating on Nb-based alloy. J. Alloy. Compd. 2018, 735, 2247–2255. [Google Scholar] [CrossRef]
- Majumdar, S.; Mishra, S.; Paul, B.; Kishor, J.; Mishra, P.; Chakravartty, J. Development of MoSi2 coating on Nb-1Zr-0.1 C alloy. Mater. Today Proc. 2016, 3, 3172–3177. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Fu, T.; Wang, J.; Shen, F.; Zhang, X.; Yu, L. Formation of MoSi2 and Si/MoSi2 coatings on TZM (Mo–0.5 Ti–0.1 Zr–0.02 C) alloy by hot dip silicon-plating method. Ceram. Int. 2021, in press. [Google Scholar]
- Zhang, Y.; Qie, J.; Cui, K.; Fu, T.; Fan, X.; Wang, J.; Zhang, X. Effect of hot dip silicon-plating temperature on microstructure characteristics of silicide coating on tungsten substrate. Ceram. Int. 2020, 46, 5223–5228. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Li, J.; Lei, J.; Cheng, X. Effect of hot-dip siliconizing time on phase composition and microstructure of Mo–MoSi2 high temperature structural materials. Ceram. Int. 2019, 45, 5588–5593. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, T.; Cui, K.; Shen, F.; Wang, J.; Yu, L.; Mao, H. Evolution of surface morphology, roughness and texture of tungsten disilicide coatings on tungsten substrate. Vacuum 2021, 191, 110297. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Fu, T.; Wang, J.; Qie, J. Synthesis WSi2 coating on W substrate by HDS method with various deposition times. Appl. Surf. Sci. 2020, 511, 145551. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, K.; Gao, Q.; Hussain, S.; Lv, Y. Investigation of morphology and texture properties of WSi2 coatings on W substrate based on contact-mode AFM and EBSD. Surf. Coat. Technol. 2020, 396, 125966. [Google Scholar] [CrossRef]
- Lu–Steffes, O.J.; Su, L.; Jackson, D.M.; Perepezko, J.H. Mo–Si–B coating for improved oxidation resistance of niobium. Adv. Eng. Mater. 2015, 17, 1068–1075. [Google Scholar] [CrossRef]
- Bacos, M.-P.; Landais, S.; Morel, A.; Rimpot, E.; Rio, C.; Sanchez, C.; Hannoyer, B.; Lefez, B.; Jouen, S. Characterization of a multiphase coating formed by a vapor pack cementation process to protect Nb-base alloys against oxidation. Surf. Coat. Technol. 2016, 291, 94–102. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, X.; Wang, Y.; Pu, R.; Zhao, G.; Shen, Z.; Huang, Y.; Liu, S.; Cai, Z.; Zhao, X. Formation and oxidation behavior of Ce-modified MoSi2-NbSi2 coating on niobium alloy. Corros. Sci. 2020, 173, 108751. [Google Scholar] [CrossRef]
- Su, L.; Lu-Steffes, O.; Zhang, H.; Perepezko, J.H. An ultra-high temperature Mo–Si–B based coating for oxidation protection of NbSS/Nb5Si3 composites. Appl. Surf. Sci. 2015, 337, 38–44. [Google Scholar] [CrossRef]
- He, J.; Guo, X.; Qiao, Y.; Luo, F. A novel Zr-Y modified silicide coating on Nb-Si based alloys as protection against oxidation and hot corrosion. Corros. Sci. 2020, 177, 108948. [Google Scholar] [CrossRef]
- Liu, L.; Lei, H.; Gong, J.; Sun, C. Deposition and oxidation behaviour of molybdenum disilicide coating on Nb based alloys substrate by combined AIP/HAPC processes. Ceram. Int. 2019, 45, 10525–10529. [Google Scholar] [CrossRef]
- Pang, J.; Wang, W.; Zhou, C. Microstructure evolution and oxidation behavior of B modified MoSi2 coating on Nb–Si based alloys. Corros. Sci. 2016, 105, 1–7. [Google Scholar] [CrossRef]
- Li, G.-R.; Yang, G.-J.; Li, C.-X.; Li, C.-J. Force transmission and its effect on structural changes in plasma-sprayed lamellar ceramic coatings. J. Eur. Ceram. Soc. 2017, 37, 2877–2888. [Google Scholar] [CrossRef]
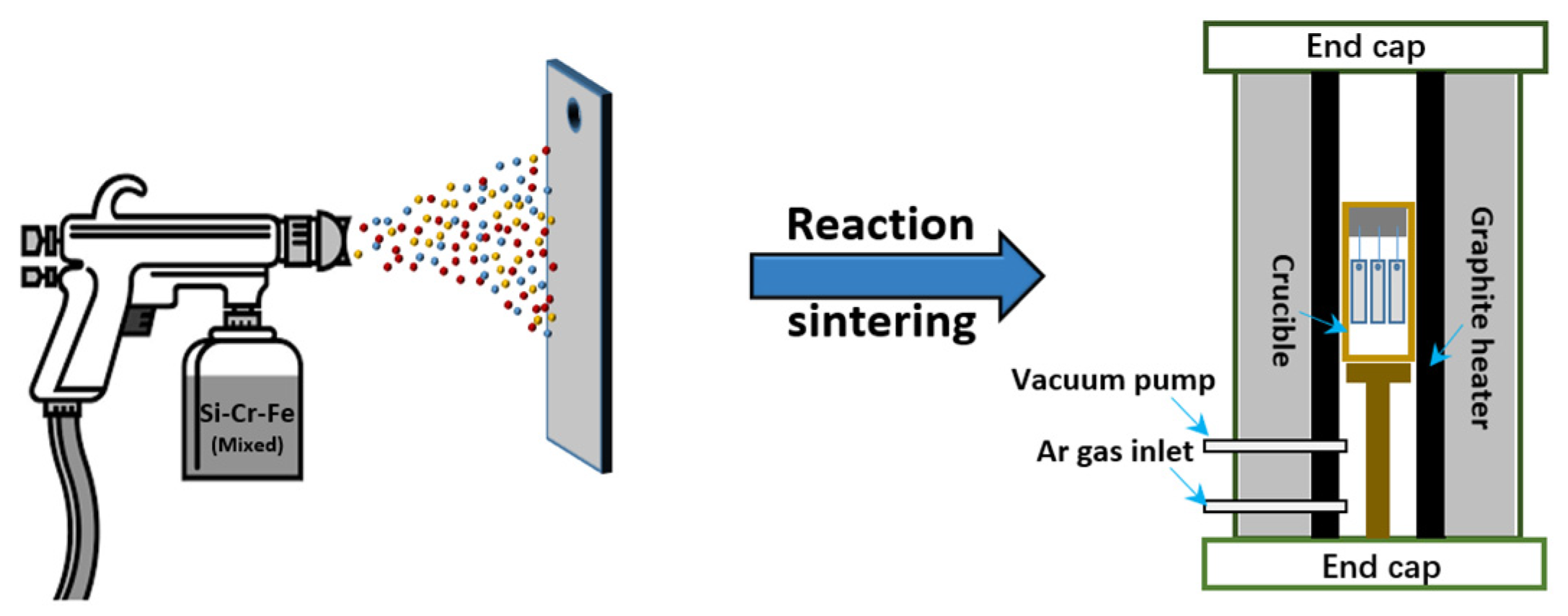
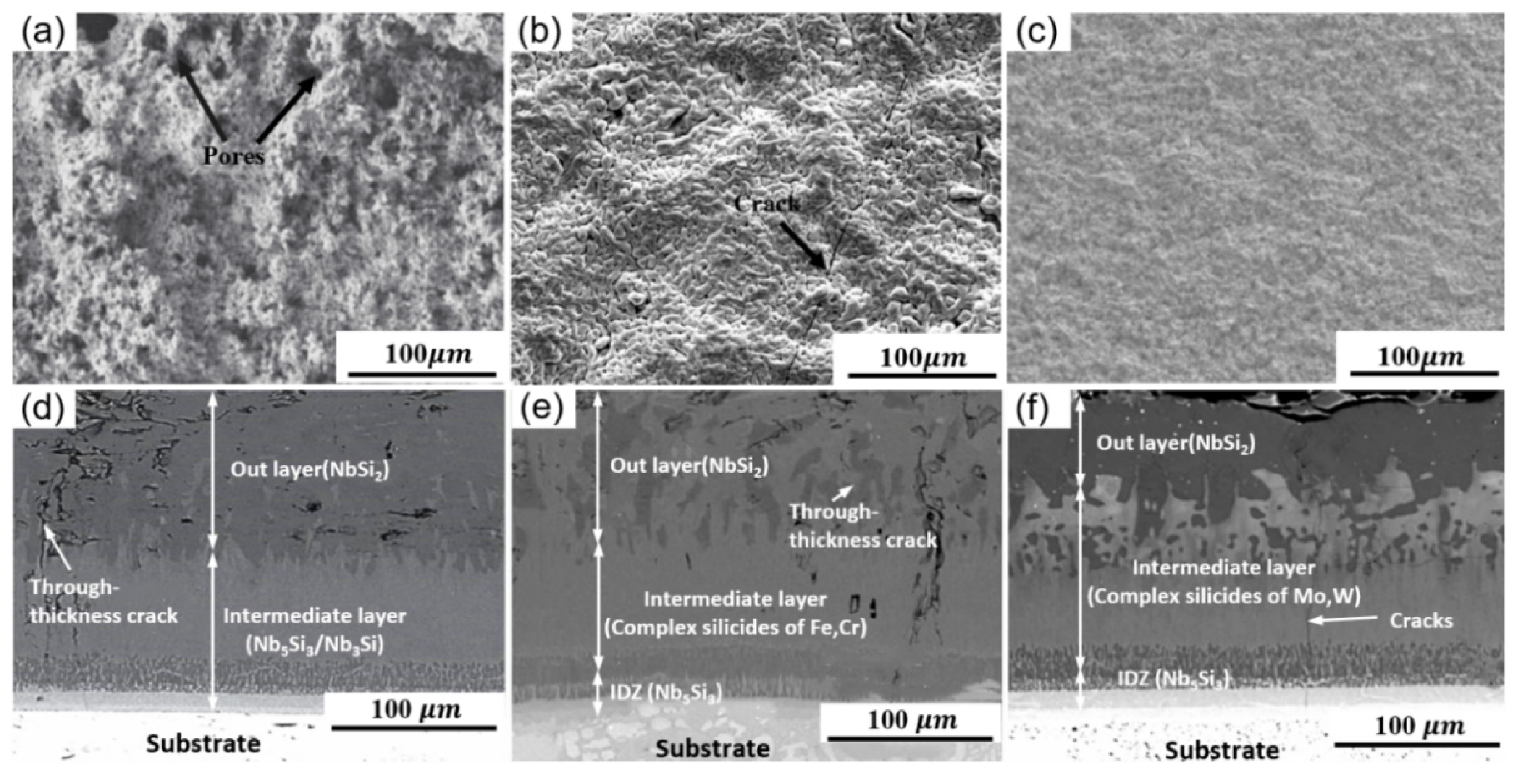

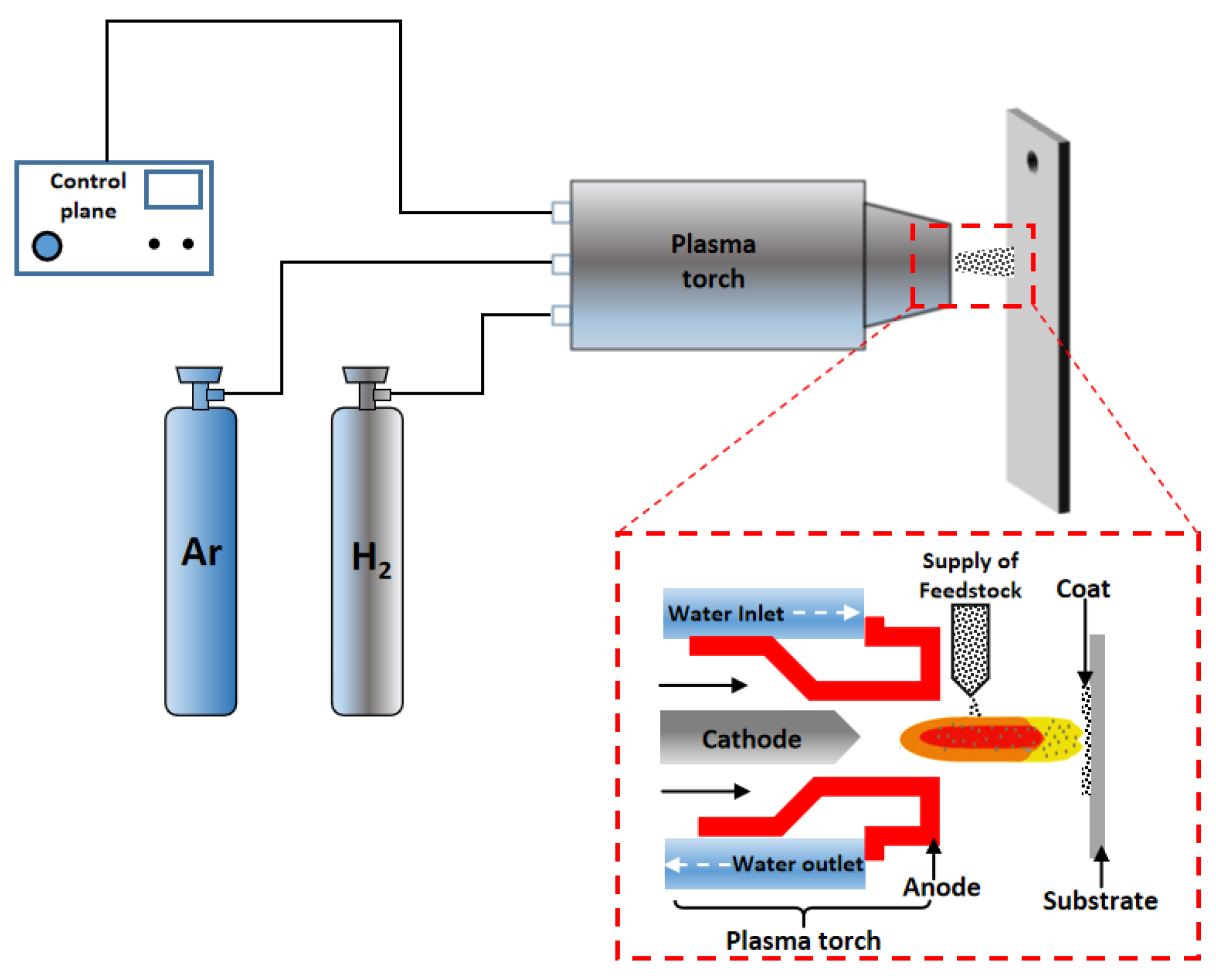

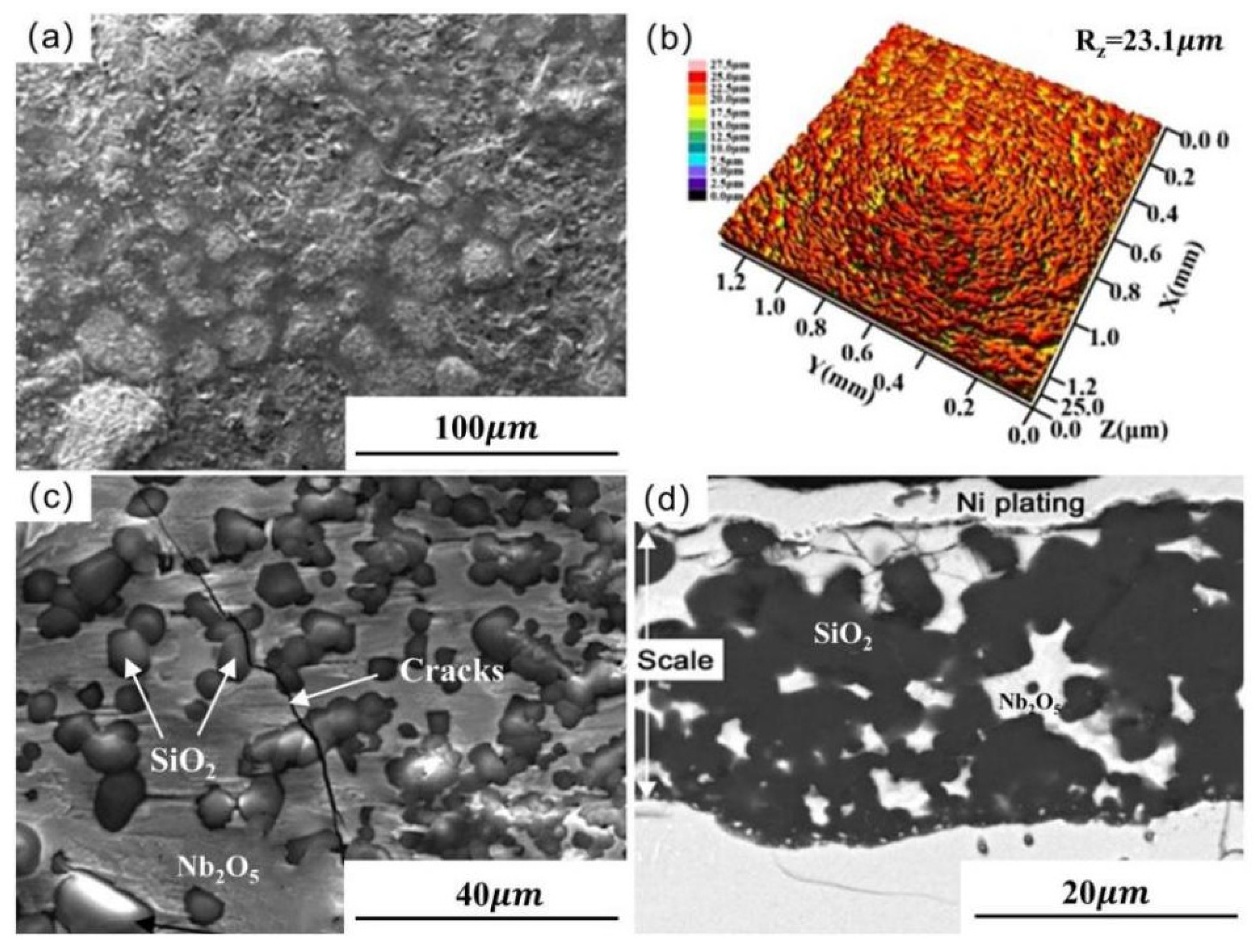
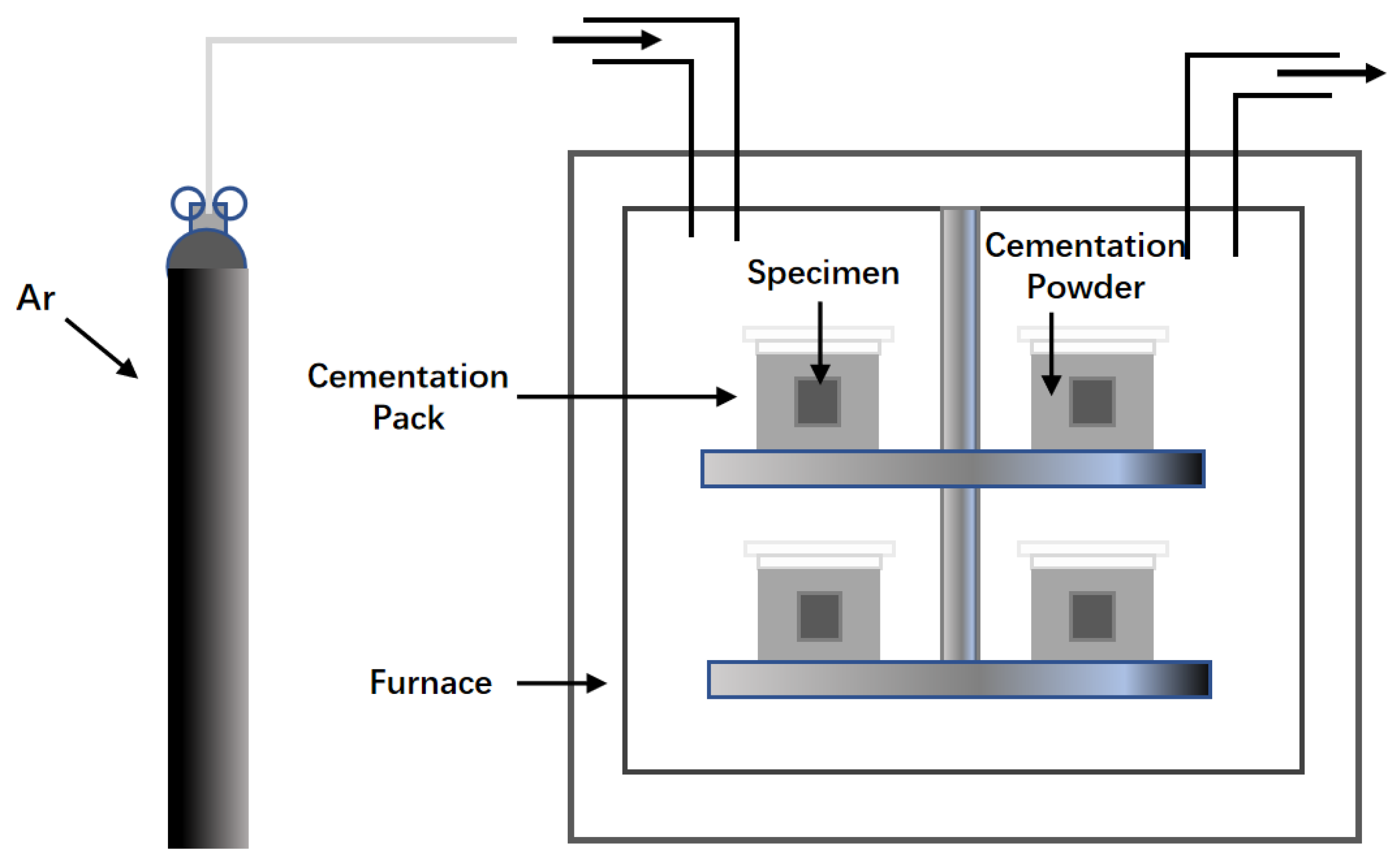
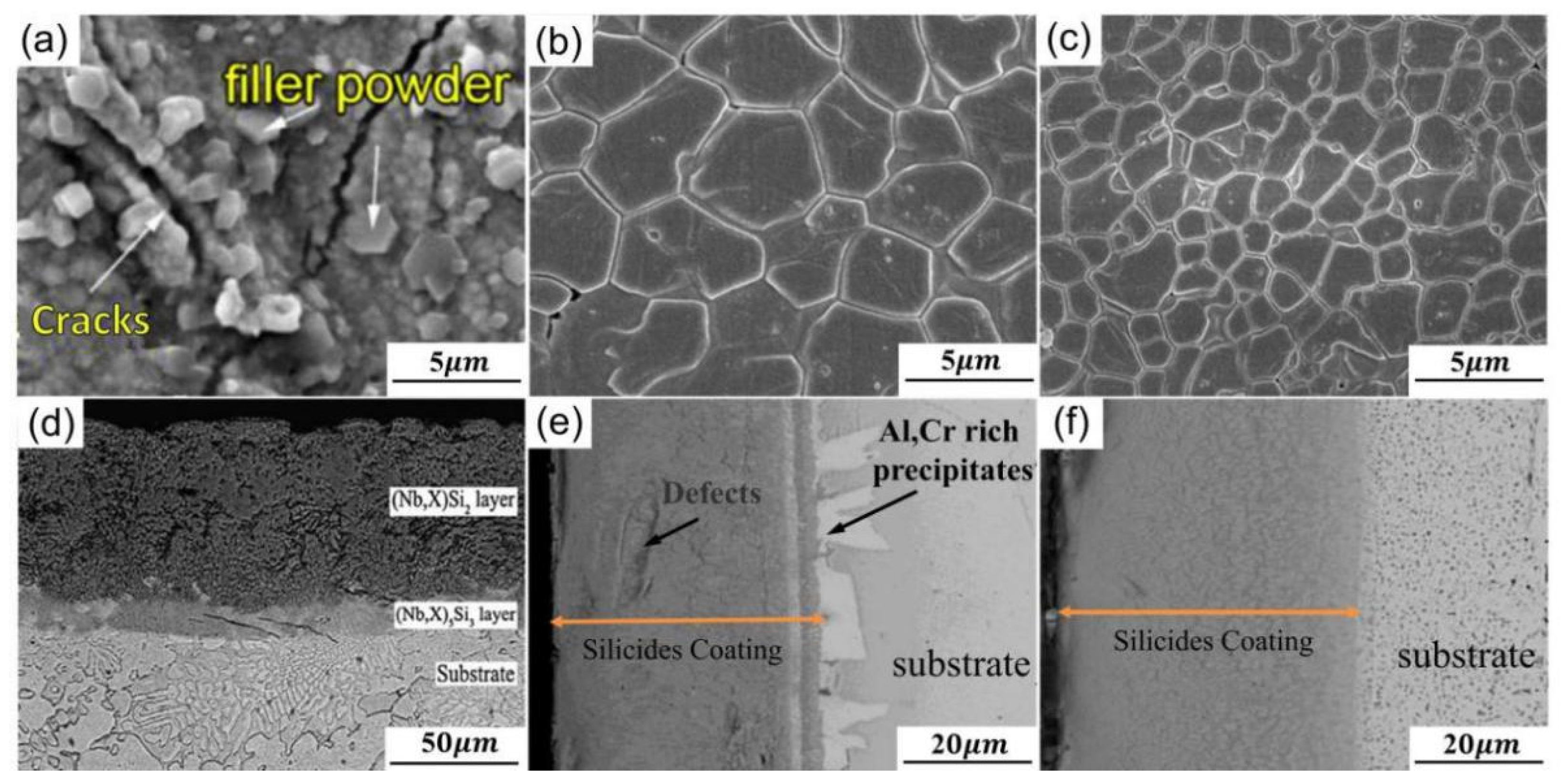
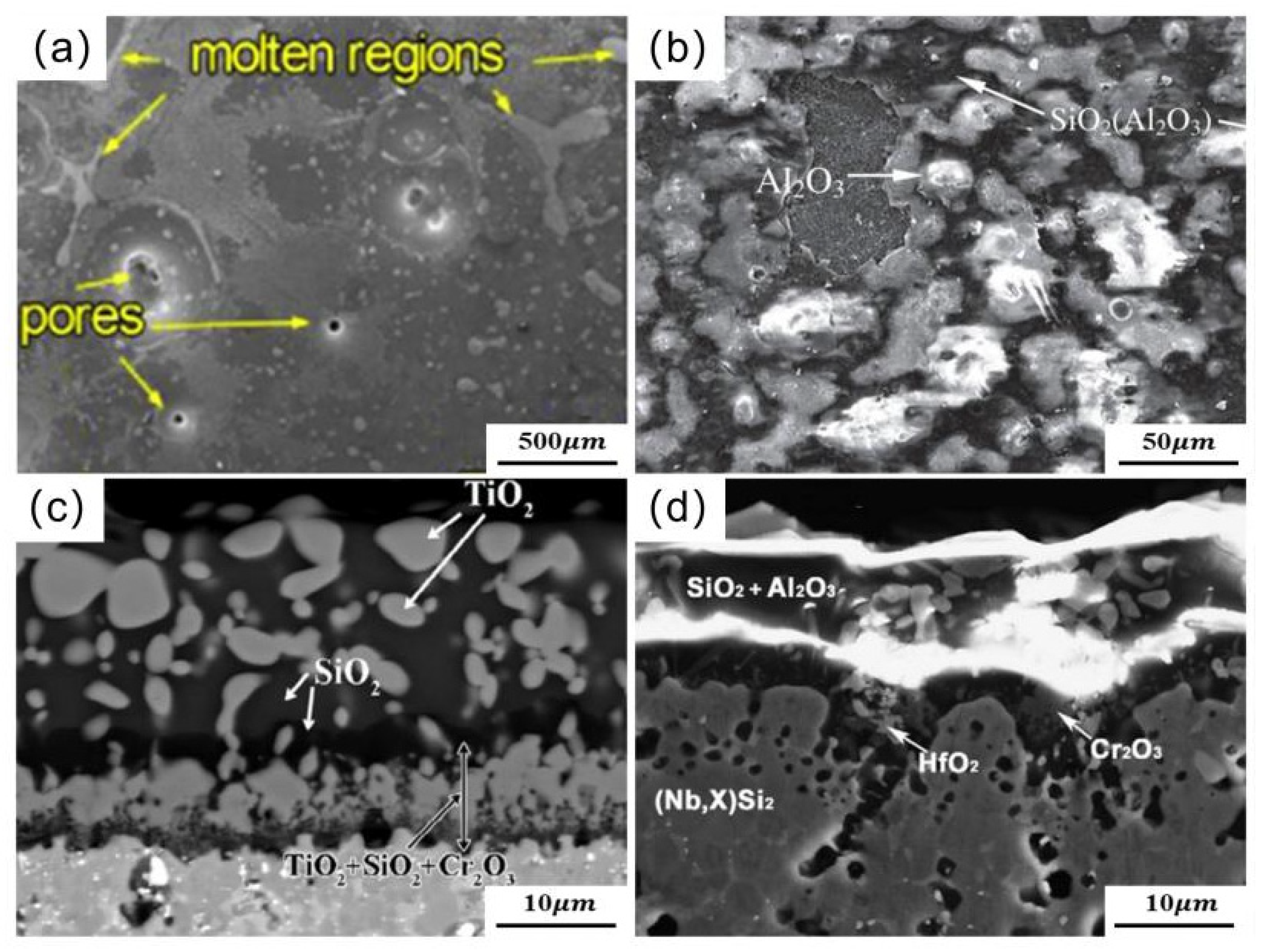

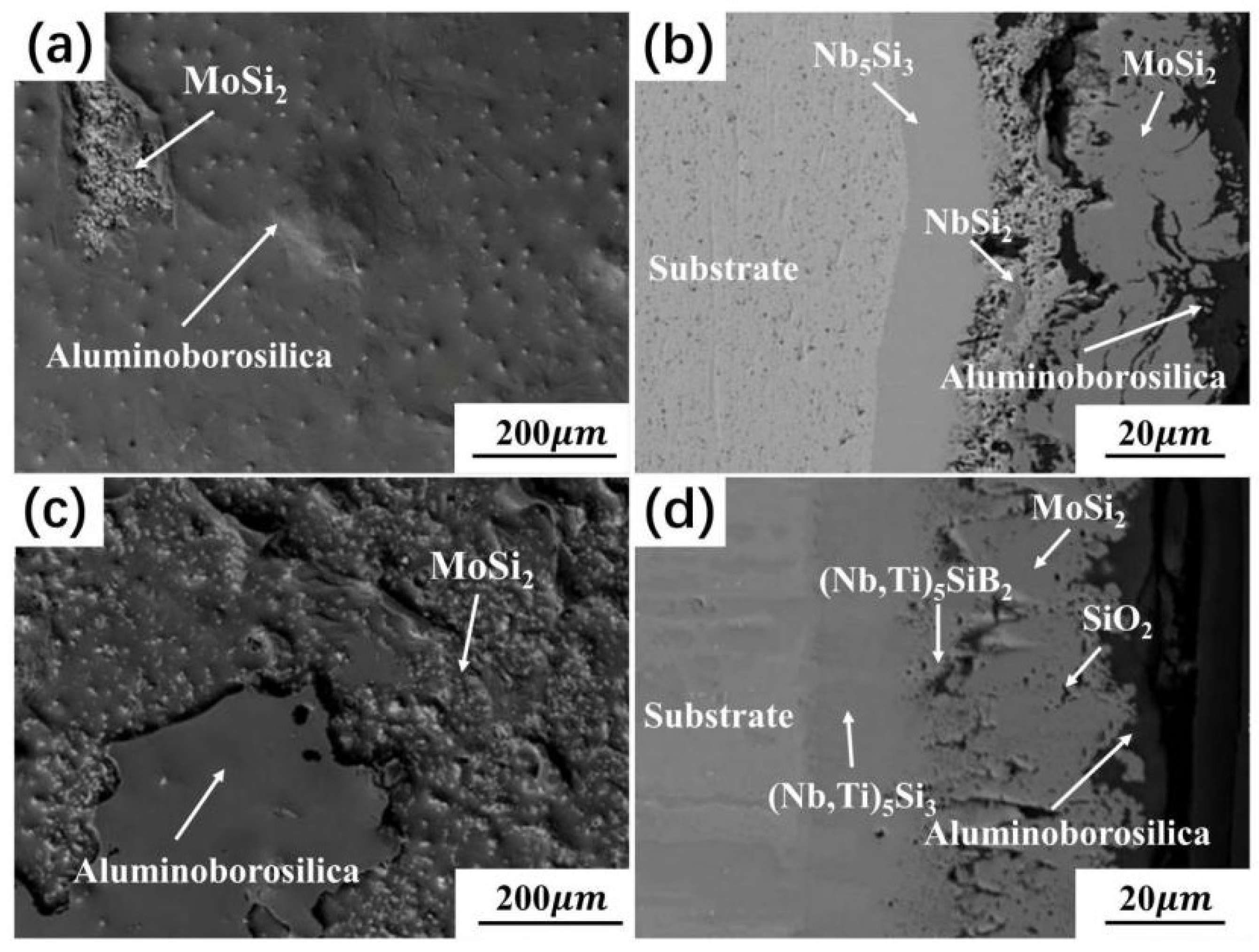
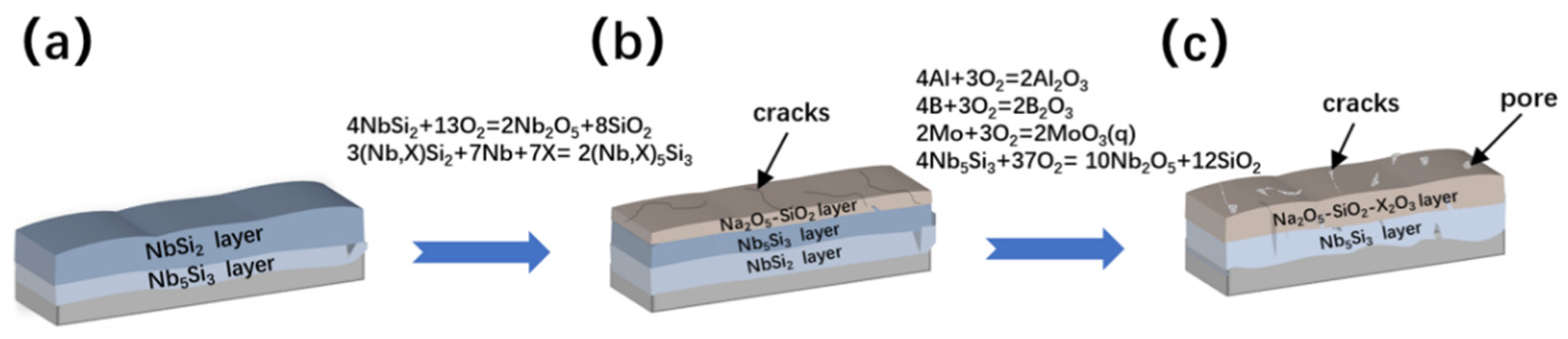
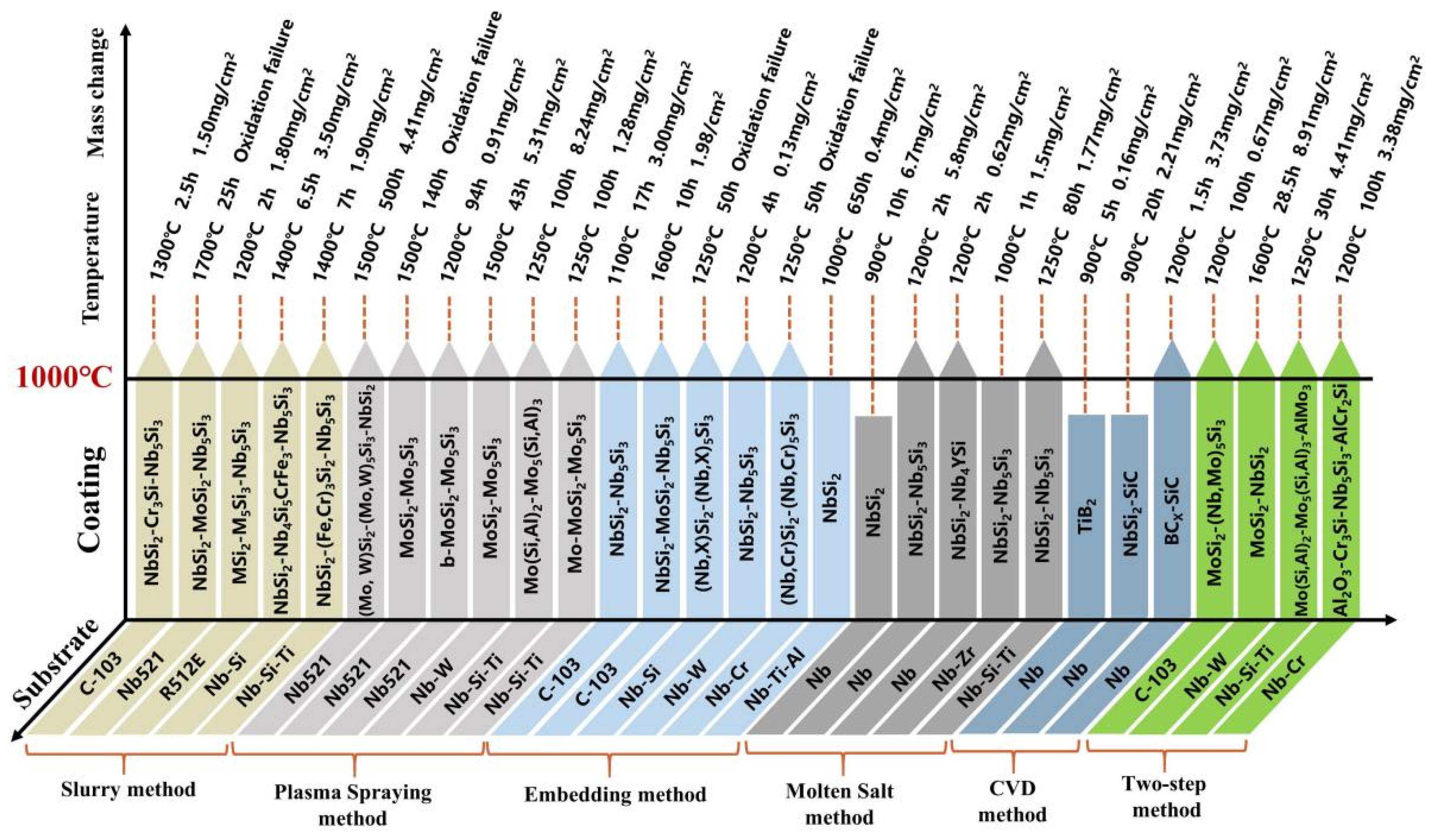
| Substrate | Slurry Composition (wt.%) | Process Conditions | Coating Composition and Thickness (μm) | Oxidation System | Oxidation Products | Quality Change (mg/cm2) | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Outer Layer | Interface Layer | Oxidation Temperature (°C) | Oxidation Time (h) | ||||||
| C-103 | 60Si-15Fe-20Cr-5NaF | 1400 °C/2 h vacuum | NbSi2 Cr3Si Fe3Si2 (200) | Nb5Si3 (30) | 1300 | 2.5 | Nb2O5 SiO2 Fe2O3 | 1.5 | [17] |
| R512E | 20Si-35Fe-35Cr-10NaF | 1250 °C/4 h vacuum | MoSi2 M5Si3 (135) | Nb5Si3 (15) | 1200 | 2 | Nb2O5 Cr2O3 SiO2 CrNbO4 | 1.8 | [18] |
| Nb521 | 45Mo-45Si-10NaF | 1500 °C/1 h Ar | NbSi2 MoSi2 (110) | Nb5Si3 (25) | 1700 | 25 | SiO2 MoSi2 | −9.5 | [19] |
| Nb-Si | 60Si-15Fe-20Cr-5NaF | 1400 °C/2 h vacuum | NbSi2 Nb4Si5CrFe3 Fe4Nb4Si7 (250) | Nb5Si3 (50) | 1400 | 6.5 | Nb2O5 SiO2 | 3.5 | [20] |
| Nb-Si-Ti | 20Fe-20Cr-50Si-10NaF | 1400 °C/2 h vacuum | NbSi2 (Fe, Cr)3Si2 (170) | Nb5Si3 (20) | 1400 | 7 | Nb2O5 SiO2 | 1.9 | [21] |
| Substrate | Experimental Conditions | Embedding Components (wt.%) | Coating Composition and Thickness (μm) | Oxidation System | Oxidation Products | Quality Change (mg/cm2) | References | |
|---|---|---|---|---|---|---|---|---|
| Outer Layer | Interface Layer | |||||||
| C-103 | 1100 °C/6 h Ar | 25Si-5NaF-70Al2O3 | NbSi2 Nb5Si3 (60) | Nb5Si3 (5) | 1100 °C/17 h | Nb2O5 SiO2 | 3 | [52] |
| Nb-Cr | 1150 °C/5 h Ar | 10Si-10Al-5NaF- 75Al2O3 | Al2O3 Cr3Si Nb5Si3 (35) | Nb5Si3 Cr3Si (5) | 1200 °C/100 h | SiO2 Al2O3 | 3.38 | [53] |
| 1250 °C/8 h Ar | 10Si- 2Y2O3-5NaF- 83Al2O3 | (Nb, Cr)Si2 (Cr, Nb)Si2 (185) | (Nb, Cr)5Si3 (15) | 1250 °C/50 h | SiO2 Cr2O3 CrNbO4 | 13.1 | [54] | |
| Nb-Si | 1150 °C/10 h Ar | 10Si-10Al-5NaF- 2Y2O3-73Al2O3 | (Nb, X)Si2 (Nb, X)5Si3 (40) | (Nb,Ti)3(Al, X) (5) | 1250 °C/50 h | TiO2 Al2O3 SiO2 | 12.5 | [55] |
| 1300 °C/10 h Ar | 16Si-8Ge-Y2O3- 5NaF-70Al2O3 | (Nb, X)(Si, Ge)2 (180) | (Ti, Nb)5(Si, Ge)4 (Nb, X)5(Si, Ge)3 (12) | 1250 °C/100 h | SiO2 GeO2 TiO2 Cr2O3 | 2.78 | [56] | |
| Nb-Ti-Al | 850 °C/25 h vacuum | 60Al2O3-40Al | NbAl3 (160) | 1000 °C/650 h | NbAl3 (α-Al2O3) | 1.5 | [57] | |
| 1050 °C/25 h vacuum | 60Al2O3-40Si | NbSi2 (50) | 1000 °C/650 h | SiO2 TiO2 | 0.4 | |||
| Substrate | Process Conditions | Coating System | Coating Composition and Thickness (μm) | Oxidation System | Oxidation Products | Quality Change (mg/cm2) | References | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Outer Layer | Interface Layer | Oxidation Temperature (°C) | Oxidation Time (h) | |||||||
| Nb | SPS SD: 100 mm SR: 20 g/min | HAPC Ar 1000 °C/50 h | Mo-Si–B | MoSi2 NbB2 NbSi2 (70) | Nb5Si3 NbB2 (10) | 1300 | 24 | Nb2O5 SiO2 | 0.44 | [76] |
| C-103 | HAPC 1100 °C/6 h | HAPC 1050 °C/4 h | Si-B | NbSi2 NbB2 (125) | NbB2 (13) | 1300 | 100 | Nb2O5 NbO2 B2O3 | 1.44 | [77] |
| Nb-Si | SS 1550 °C/2 h | HAPC Ar 1200 °C/5 h | Mo-Si-Ce | NbSi2 MoSi2 (80) | Nb5Si3 (4) | 1600 | 24.7 | SiO2 | 3.57 | [78] |
| Nb-Si-Ti | PVD 300 °C/2 h | HAPC Ar 1450 °C/12 h | Mo-Si–B | MoSi2 (Nb,Ti)5SiB2 (50) | (Nb,Ti)Si2 (Nb, X)5Si3 (5) | 1300 | 24 | MoO3 SiO2 | −0.55 | [79] |
| Nb-Si-Ti | SPS SD: 60 mm SR: 90 g/min | HAPC Ar 1250 °C/4 h | Si-Y-Zr | (Nb,Ti)5Si4 (110) | (Nb, X)5Si3 (5) | 1250 | 100 | Nb2O5 TiO2 SiO2 | 1.6 | [80] |
| Nb-Si-Ti | SPS SD: 90 mm SR: 20 g/min | HAPC 1000 °C/40 h | Mo-Si-B | MoSi2 MoB (115) | Mo (45) | 1250 | 100 | B2O3 SiO2 | 0.92 | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fu, T.; Cui, K.; Zhang, Y.; Shen, F.; Wang, J.; Yu, L.; Mao, H. The Protection, Challenge, and Prospect of Anti-Oxidation Coating on the Surface of Niobium Alloy. Coatings 2021, 11, 742. https://doi.org/10.3390/coatings11070742
Zhang X, Fu T, Cui K, Zhang Y, Shen F, Wang J, Yu L, Mao H. The Protection, Challenge, and Prospect of Anti-Oxidation Coating on the Surface of Niobium Alloy. Coatings. 2021; 11(7):742. https://doi.org/10.3390/coatings11070742
Chicago/Turabian StyleZhang, Xu, Tao Fu, Kunkun Cui, Yingyi Zhang, Fuqiang Shen, Jie Wang, Laihao Yu, and Haobo Mao. 2021. "The Protection, Challenge, and Prospect of Anti-Oxidation Coating on the Surface of Niobium Alloy" Coatings 11, no. 7: 742. https://doi.org/10.3390/coatings11070742
APA StyleZhang, X., Fu, T., Cui, K., Zhang, Y., Shen, F., Wang, J., Yu, L., & Mao, H. (2021). The Protection, Challenge, and Prospect of Anti-Oxidation Coating on the Surface of Niobium Alloy. Coatings, 11(7), 742. https://doi.org/10.3390/coatings11070742






