Development of Electrospun Films from Wastewater Treatment Plant Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Electrospinning Process
2.2.2. Material Characterization
Viscosity Determination
Fourier-Transform Infrared Spectroscopy (FTIR)
Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR)
Differential Scanning Calorimetry (DSC)
Tensile Properties
Contact Angle
Surface Characterization with a Scanning Electron Microscope (SEM)
3. Results and Discussion
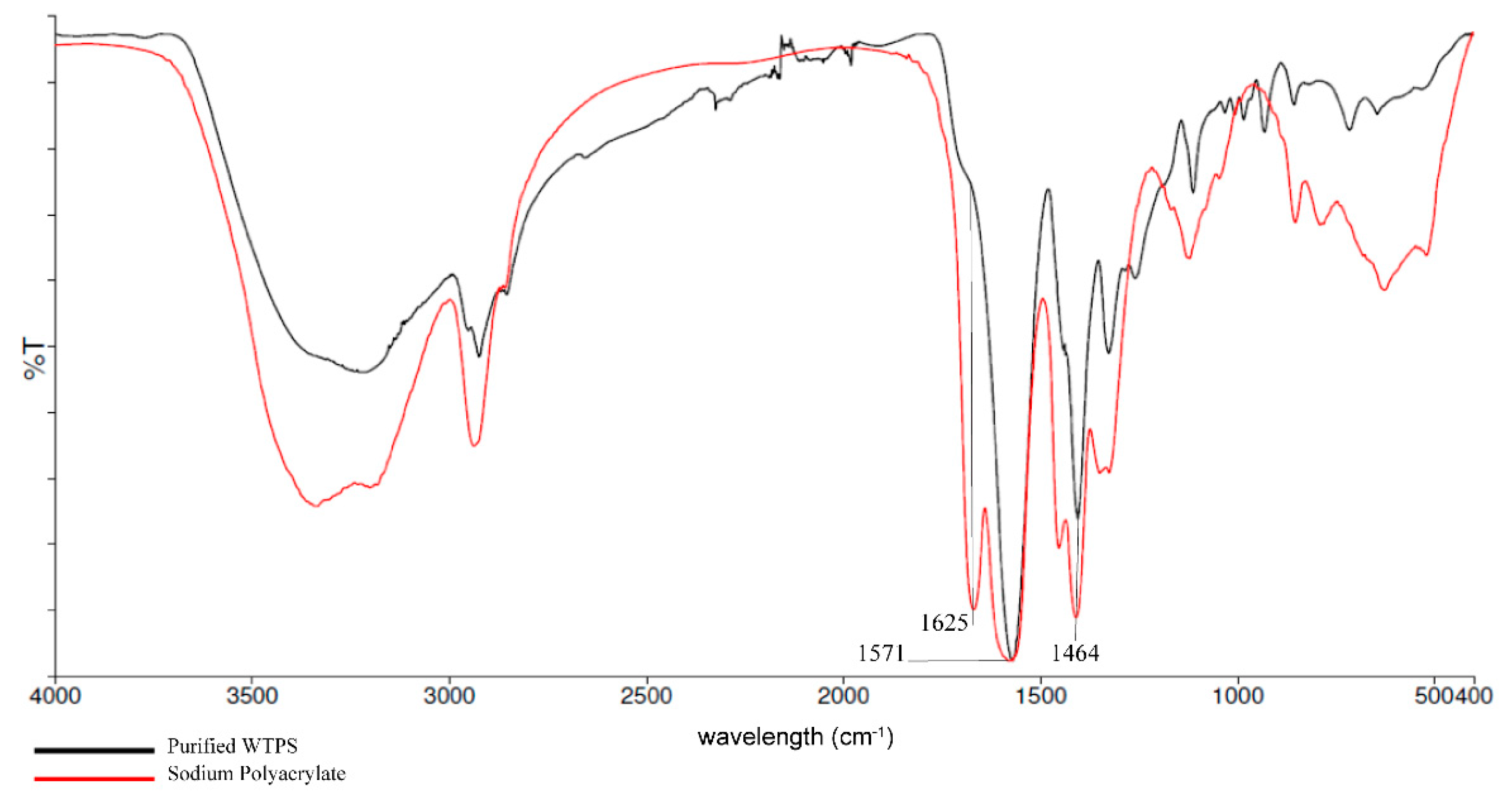
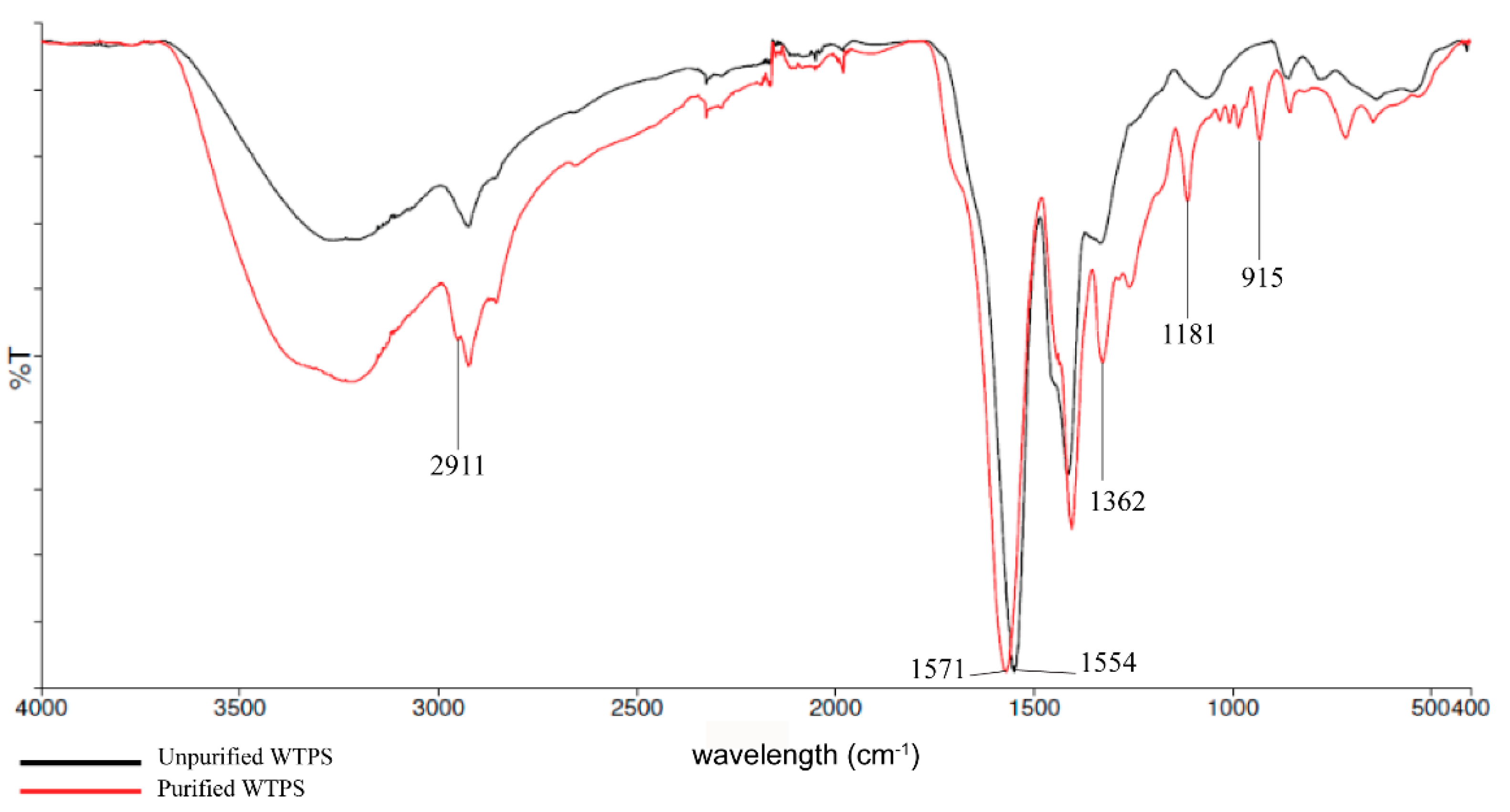
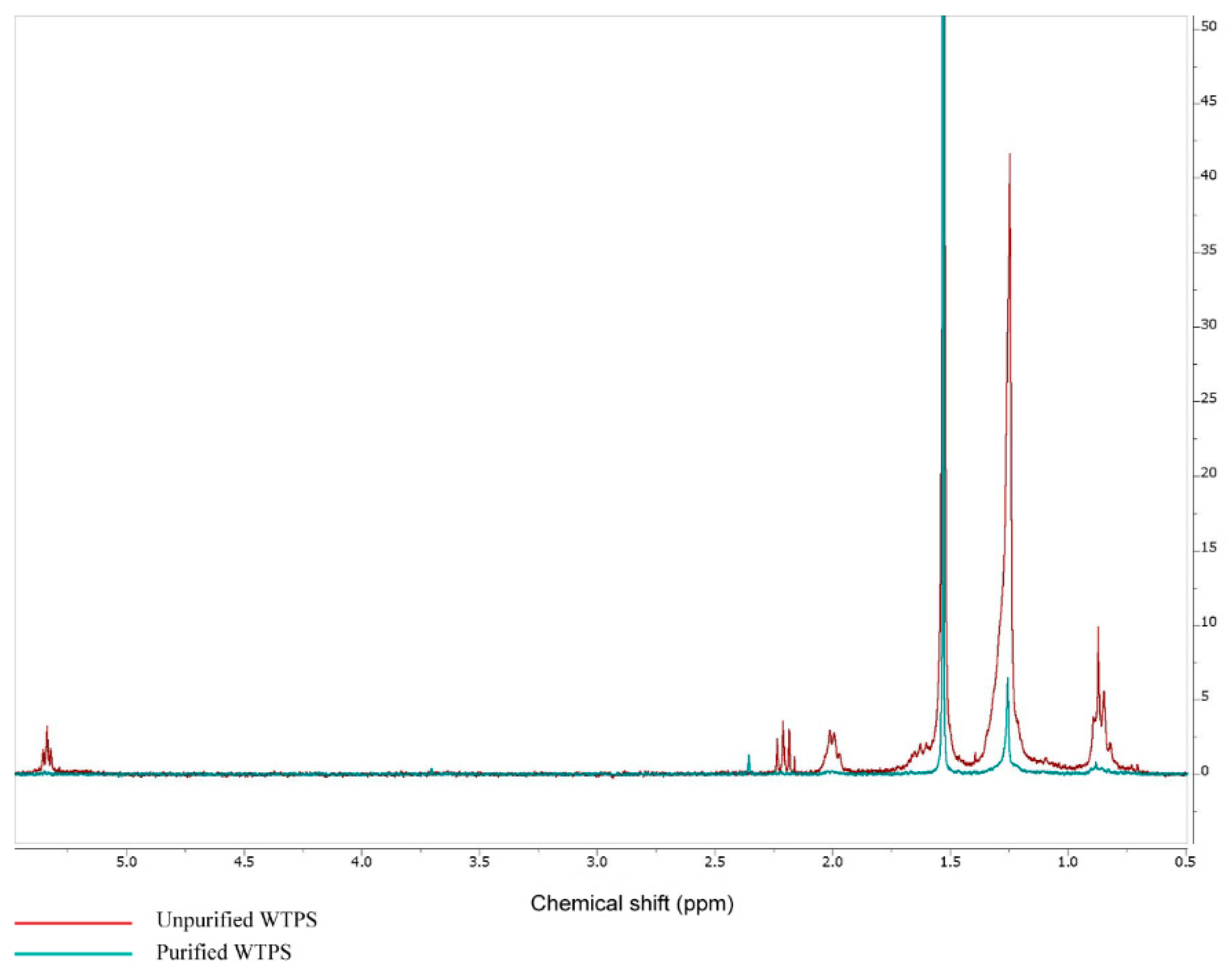
3.1. FTIR and 1H NMR Analysis of Wastewater Treatment Plant Sludge
3.2. Determination of Optimal Viscosity and Electrospinning Parameters
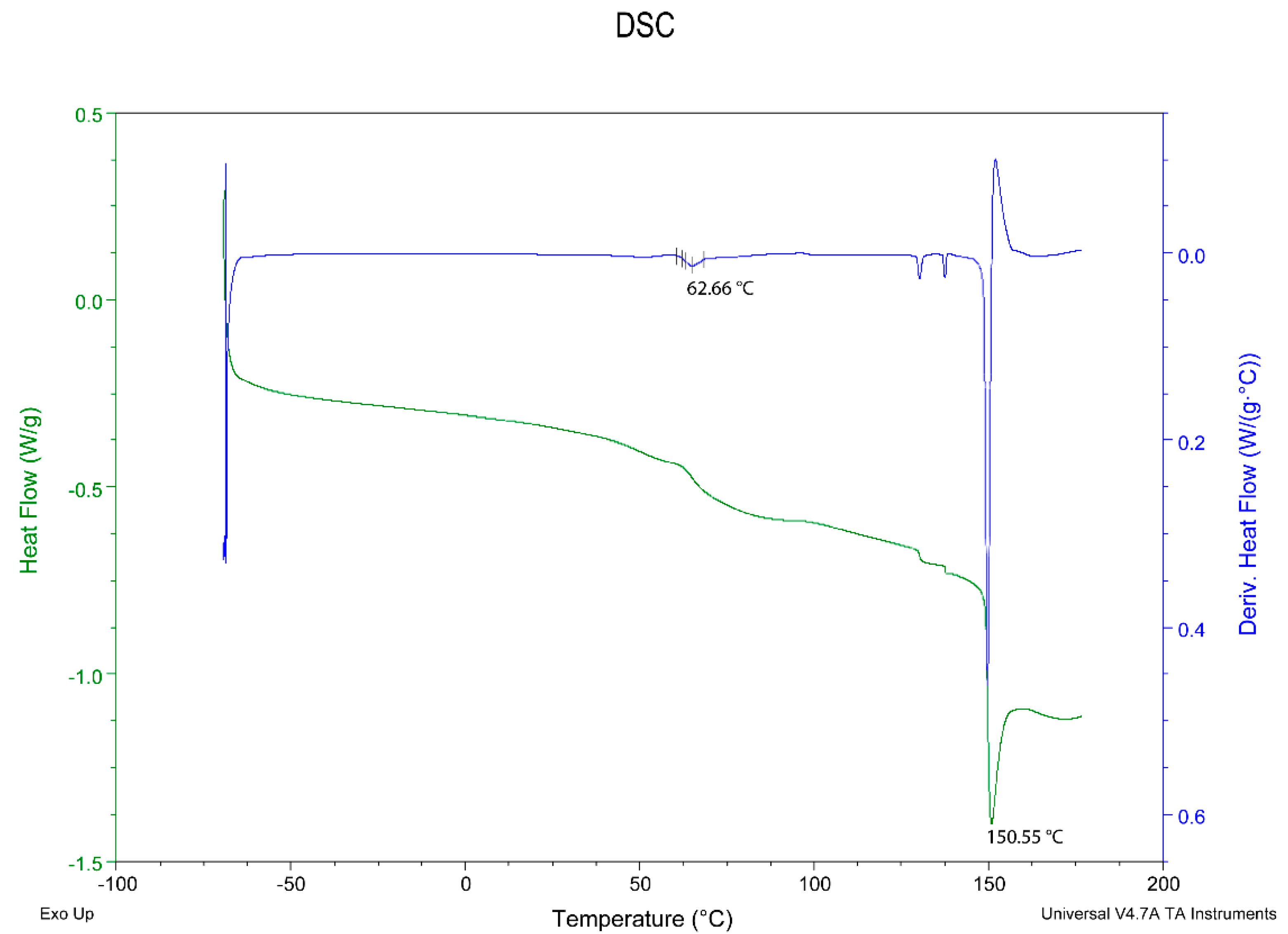
3.3. Differential Scanning Calorimetry Characterization of the Final Material
3.4. Tensile Properties and Contact Angle Analysis of Final Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smithers Pira. Value Estimations & Global Packaging Trends in 2018 and Beyond|Smithers Pira. Available online: https://www.smitherspira.com/resources/2018/january/value-estimations-for-packaging-in-2018-and-beyond (accessed on 8 August 2020).
- World Economic Forum. The New Plastics Economy Rethinking the Future of Plastics; World Economic Forum: Geneva, Switzerland, 2016; 14p. [Google Scholar]
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Plastics Strategy. Available online: https://ec.europa.eu/environment/strategy/plastics-strategy_en (accessed on 24 April 2021).
- Schettini, E.; Santagata, G.; Malinconico, M.; Immirzi, B.; Scarascia Mugnozza, G.; Vox, G. Recycled wastes of tomato and hemp fibres for biodegradable pots: Physico-chemical characterization and field performance. Resour. Conserv. Recycl. 2013, 70, 9–19. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Suárez-Iglesias, O.; Collado, S.; Oulego, P.; Díaz, M. Graphene-family nanomaterials in wastewater treatment plants. Chem. Eng. J. 2017, 313, 121–135. [Google Scholar] [CrossRef]
- Tyagi, V.; Lo, S. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 228, 244–263. [Google Scholar] [CrossRef]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef]
- Figueroa, G.A.; Homann, T.; Rawel, H.M. Coffee production wastes: Potentials and perspectives. Austin Food Sci 2016, 1, 1–5. [Google Scholar]
- Malara, A.; Paone, E.; Frontera, P.; Bonaccorsi, L.; Panzera, G.; Mauriello, F. Sustainable exploitation of coffee silverskin in water remediation. Sustainability 2018, 10, 3547. [Google Scholar] [CrossRef]
- Weston, M.; Phan, M.A.T.; Arcot, J.; Chandrawati, R. Anthocyanin-based sensors derived from food waste as an active use-by date indicator for milk. Food Chem. 2020, 326, 127017. [Google Scholar] [CrossRef] [PubMed]
- Mannu, A.; Garroni, S.; Ibanez Porras, J.; Mele, A. Available technologies and materials for waste cooking oil recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Singhabhandhu, A.; Tezuka, T. The waste-to-energy framework for integrated multi-waste utilization: Waste cooking oil, waste lubricating oil, and waste plastics. Energy 2010, 35, 2544–2551. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, D.G.K.; Weerasinghe, D.U.; Thebuwanage, L.M.; Bandara, U.A.A.N. An environmentally friendly sound insulation material from post-industrial textile waste and natural rubber. J. Build. Eng. 2021, 33, 101606. [Google Scholar] [CrossRef]
- Girard, E.B.; Kaliwoda, M.; Schmahl, W.W.; Wörheide, G.; Orsi, W.D. Biodegradation of textile waste by marine bacterial communities enhanced by light. Environ. Microbiol. Rep. 2020, 12, 406–418. [Google Scholar] [CrossRef]
- Kumar, V.; Pathak, P.; Bhardwaj, N.K. Waste paper: An underutilized but promising source for nanocellulose mining. Waste Manag. 2020, 102, 281–303. [Google Scholar] [CrossRef]
- Zakarya, I.A.; Fazhil, N.S.A.; Izhar, T.N.T.; Zaaba, S.K.; Jamaluddin, M.N.F. Municipal Solid Waste Characterization and Quantification as A Measure Towards Effective Solid Waste Management in UniMAP. IOP Conf. Ser. Earth Environ. Sci. 2020, 616, 12047. [Google Scholar] [CrossRef]
- Zhang, Q.; Khan, M.U.; Lin, X.; Yi, W.; Lei, H. Green-composites produced from waste residue in pulp and paper industry: A sustainable way to manage industrial wastes. J. Clean. Prod. 2020, 262, 121251. [Google Scholar] [CrossRef]
- Albuquerque, P.; Malafaia, C. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef]
- Kumar, M.; Ghosh, P.; Khosla, K.; Thakur, I. Recovery of polyhydroxyalkanoates from municipal secondary wastewater sludge. Bioresour. Technol. 2018, 255, 111–115. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Suárez-Ojeda, M. Bioplastic recovery from wastewater: A new protocol for polyhydroxyalkanoates (PHA) extraction from mixed microbial cultures. Bioresour. Technol. 2018, 282, 361–369. [Google Scholar] [CrossRef]
- Liu, H.; Gough, C.R.; Deng, Q.; Gu, Z.; Wang, F.; Hu, X. Recent advances in electrospun sustainable composites for biomedical, environmental, energy, and packaging applications. Int. J. Biol. Macromol. 2020, 21, 4019. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Vicini, S.; Castellano, M. Preparation of composite alginate-based electrospun membranes loaded with ZnO nanoparticles. Carbohydr. Polym. 2020, 227, 115371. [Google Scholar] [CrossRef] [PubMed]
- de Souza, E.J.D.; Kringel, D.H.; Dias, A.R.G.; da Rosa Zavareze, E. Polysaccharides as wall material for the encapsulation of essential oils by electrospun technique. Carbohydr. Polym. 2021, 265, 118068. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.P.; Jia, X.S. Extraction of extracellular polymer from anaerobic sludges. Biotechnol. Tech. 1996, 10, 803–808. [Google Scholar] [CrossRef]
- Hsiao, M.H.; Lin, K.H.; Liu, D.M. Improved pH-responsive amphiphilic carboxymethyl-hexanoyl chitosan-poly (acrylic acid) macromolecules for biomedical applications. Soft Matter 2013, 9, 2458–2466. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, F.; Wu, D.; Zhu, H. Roles of polyacrylate dispersant in the synthesis of well-dispersed BaSO4 nanoparticles by simple precipitation. Particuology 2014, 14, 33–37. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion resistance of graphene-reinforced waterborne epoxy coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Alexandre e Silva, L.M.; Ferreira, A.G. Advancements in waste water characterization through NMR spectroscopy. Magn. Reson. Chem. 2015, 53, 648–657. [Google Scholar] [CrossRef]
- Tan, G.Y.A.; Chen, C.L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Mo, Y.; Wang, J.Y. Start a research on biopolymer polyhydroxyalkanoate (PHA): A review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Nkrumah-Agyeefi, S.; Scholz, C. Chemical modification of functionalized polyhydroxyalkanoates via “Click” chemistry: A proof of concept. Int. J. Biol. Macromol. 2017, 95, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Lorini, L.; Martinelli, A.; Pavan, P.; Majone, M.; Valentino, F. Downstream processing and characterization of polyhydroxyalkanoates (PHAs) produced by mixed microbial culture (MMC) and organic urban waste as substrate. Biomass Convers. Biorefinery 2021, 11, 693–703. [Google Scholar] [CrossRef]
- de Oliveira Santos, R.P.; Ramos, L.A.; Frollini, E. Bio-based electrospun mats composed of aligned and nonaligned fibers from cellulose nanocrystals, castor oil, and recycled PET. Int. J. Biol. Macromol. 2020, 163, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Ramos, L.A.; Frollini, E. Renewable resources and a recycled polymer as raw materials: Mats from electrospinning of lignocellulosic biomass and PET solutions. Polymers 2018, 10, 538. [Google Scholar]
- Santos, R.P.; Rodrigues, B.V.; Ramires, E.C.; Ruvolo-Filho, A.C.; Frollini, E. Bio-based materials from the electrospinning of lignocellulosic sisal fibers and recycled PET. Ind. Crops Prod. 2015, 72, 69–76. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrič, G.; Miletić, A.; Pilić, B.; Medvešček, D.; Nastran, S.; Vrabič-Brodnjak, U. Development of Electrospun Films from Wastewater Treatment Plant Sludge. Coatings 2021, 11, 733. https://doi.org/10.3390/coatings11060733
Lavrič G, Miletić A, Pilić B, Medvešček D, Nastran S, Vrabič-Brodnjak U. Development of Electrospun Films from Wastewater Treatment Plant Sludge. Coatings. 2021; 11(6):733. https://doi.org/10.3390/coatings11060733
Chicago/Turabian StyleLavrič, Gregor, Aleksandra Miletić, Branka Pilić, Daša Medvešček, Saša Nastran, and Urška Vrabič-Brodnjak. 2021. "Development of Electrospun Films from Wastewater Treatment Plant Sludge" Coatings 11, no. 6: 733. https://doi.org/10.3390/coatings11060733
APA StyleLavrič, G., Miletić, A., Pilić, B., Medvešček, D., Nastran, S., & Vrabič-Brodnjak, U. (2021). Development of Electrospun Films from Wastewater Treatment Plant Sludge. Coatings, 11(6), 733. https://doi.org/10.3390/coatings11060733








