High Pressure X-ray Diffraction as a Tool for Designing Doped Ceria Thin Films Electrolytes
Abstract
1. Introduction
2. Rare Earth-Doped Ceria Thin Films: A Versatile Material to Be Used in Many Fields
3. Ionic Conductivity in Doped Ceria Bulk and Thin Films: Correlations between Structure, Microstructure and Transport Properties
3.1. Ionic Conductivity in Bulk Doped Ceria
3.2. Ionic Conductivity in Doped Ceria Thin Films
3.3. Thin Films Deposition Processes
4. High Pressure Diffraction Studies on Bulk Doped Ceria: A Bridge between Bulk and Thin Films
4.1. Why High Pressure X-ray Diffraction?
4.2. Fundamentals on the Experimental Method
4.3. Fundamentals on Data Analysis
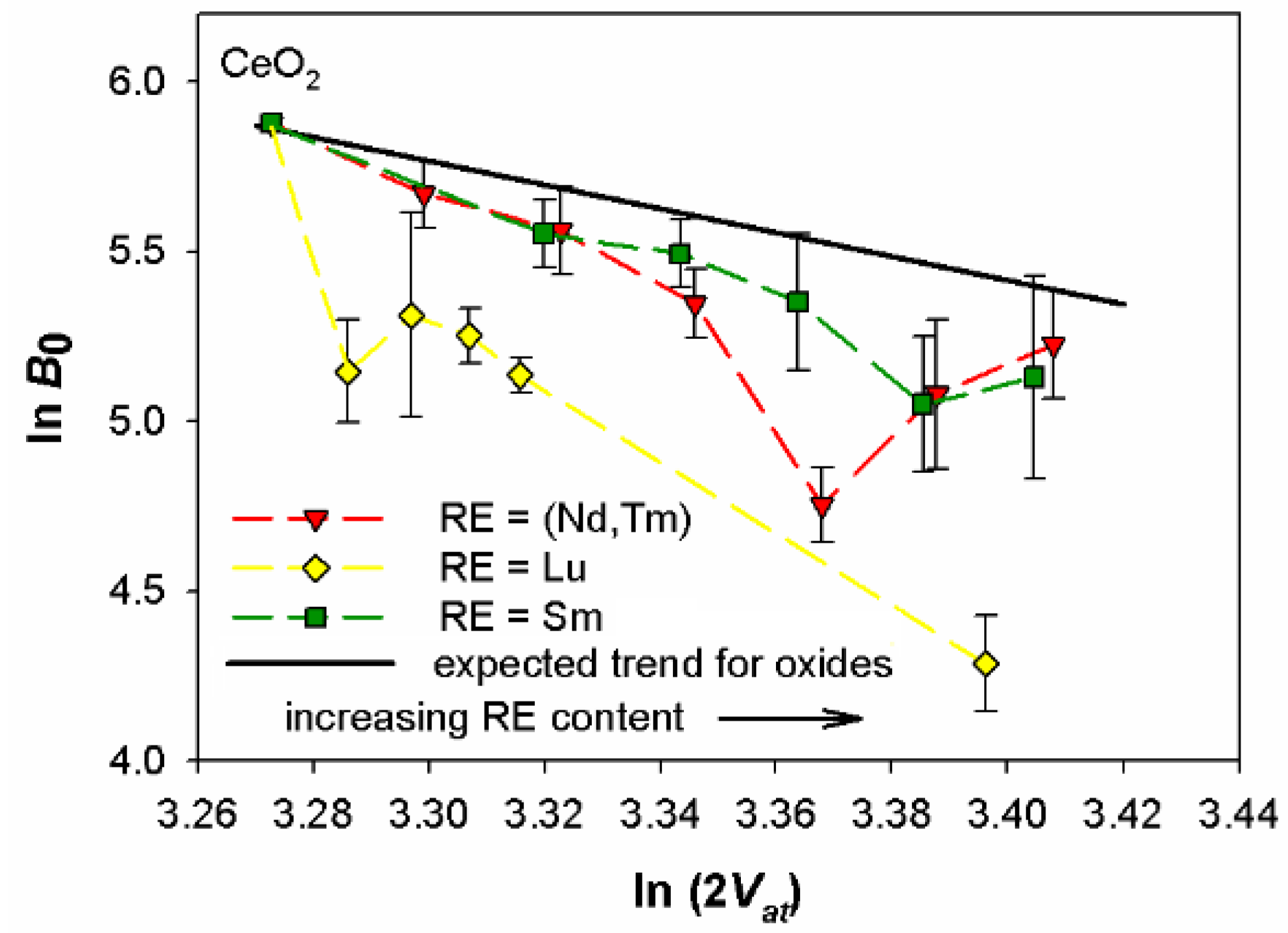
4.4. High Pressure X-ray Diffraction Studies on Sm-, Lu-, and (Nd,Tm)-Doped Ceria: How They Can Drive the Design of Thin Films
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Steele, B.C.H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ion. 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Di Bartolomeo, E.; Dell’Agli, G.; Zurlo, F. GDC-based infiltrated electrodes for solid oxide electrolyzer cells (SOECs). Appl. Sci. 2020, 10, 3882. [Google Scholar] [CrossRef]
- Beie, H.-J.; Gnörich, A. Oxygen gas sensors based on CeO2 thick and thin films. Sens. Actuators B Chem. 1991, 4, 393–399. [Google Scholar] [CrossRef]
- Gerblinger, J.; Lohwasser, W.; Lampe, U.; Meixner, H. High temperature oxygen sensor based on sputtered cerium oxide. Sens. Actuators B Chem. 1995, 26, 93–96. [Google Scholar] [CrossRef]
- Yang, J.J.; Strukov, D.B.; Stewart, D.R. Memristive devices for computing. Nat. Nanotechnol. 2013, 8, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kant, K.M.; Esposito, V.; Pryds, N. Strain induced ionic conductivity enhancement in epitaxial Ce0.9Gd0.1O2−δ thin films. Appl. Phys. Lett. 2012, 100, 033105. [Google Scholar] [CrossRef]
- Burbano, M.; Marrocchelli, D.; Watson, G.W. Strain effects on the ionic conductivity of Y-doped ceria: A simulation study. J. Electroceramics 2014, 32, 28–36. [Google Scholar] [CrossRef]
- Rupp, J.L.; Infortuna, A.; Gauckler, L.J. Thermodynamic stability of gadolinia-doped ceria thin film electrolytes for micro-solid oxide fuel cells. J. Am. Ceram. Soc. 2007, 90, 1792–1797. [Google Scholar] [CrossRef]
- Rainwater, B.H.; Velisavljevic, N.; Park, C.; Sun, H.; Waller, G.H.; Tsoi, G.M.; Vohra, Y.K.; Liu, M. High pressure structural study of samarium doped CeO2 oxygen vacancy conductor—Insight into the dopant concentration relationship to the strain effect in thin film ionic conductors. Solid State Ion. 2016, 292, 59–65. [Google Scholar] [CrossRef]
- Artini, C.; Joseph, B.; Costa, G.A.; Pani, M. Crystallographic properties of the Ce1-xLuxO2−x/2 system at pressures up to 7 GPa. Solid State Ion. 2018, 320, 152–158. [Google Scholar] [CrossRef]
- Artini, C.; Massardo, S.; Carnasciali, M.M.; Joseph, B.; Pani, M. In situ high pressure structural investigation of sm-doped ceria. Energies 2020, 13, 1558. [Google Scholar] [CrossRef]
- Artini, C.; Massardo, S.; Carnasciali, M.M.; Joseph, B.; Pani, M. Evaluation of the defect cluster content in singly and doubly doped ceria through in situ high-pressure X-ray diffraction. Inorg. Chem. 2021, 60, 7306–7314. [Google Scholar] [CrossRef]
- Artini, C. RE-doped ceria systems and their performance as solid electrolytes: A puzzling tangle of structural issues at the average and local scale. Inorg. Chem. 2018, 57, 13047–13062. [Google Scholar] [CrossRef]
- Inaba, H.; Tagawa, H. Ceria-based solid electrolytes. Solid State Ion. 1996, 83, 1–16. [Google Scholar] [CrossRef]
- Kumar, S.A.; Kuppusami, P.; Yen-Pei, F. Structural, morphological and electrical properties of Sm-Gd Co-doped ceria thin films for micro-solid oxide fuel cells. Mater. Lett. 2020, 275, 128110. [Google Scholar] [CrossRef]
- Bastakys, L.; Kalyk, F.; Marcinauskas, L.; Čyvienė, J.; Abakevičienė, B. Structural investigation of gadolinia-ceria multilayered thin films deposited by reactive magnetron sputtering. Mater. Lett. 2020, 271, 127762. [Google Scholar] [CrossRef]
- Sanna, C.; Zhang, W.; Costamagna, P.; Holtappels, P. Synthesis and electrochemical characterization of La0.6Sr0.4Co0.2Fe0.8O3–δ/Ce0.9Gd0.1O1.95 co-electrospun nanofiber cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 13818–13831. [Google Scholar] [CrossRef]
- Brito, M.E.; Morishita, H.; Yamada, J.; Nishino, H.; Uchida, H. Further improvement in performances of La0.6Sr0.4Co0.2Fe0.8O3−δ—doped ceria composite oxygen electrodes with infiltrated doped ceria nanoparticles for reversible solid oxide cells. J. Power Sour. 2019, 427, 293–298. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium cobalt ferrite perovskite electrodes of solid oxide fuel cells—A review. Int. J. Hydrog. Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J. Mater. Sci. 2008, 43, 6799–6833. [Google Scholar] [CrossRef]
- Marcucci, A.; Zurlo, F.; Sora, I.N.; Placidi, E.; Casciardi, S.; Licoccia, S.; Di Bartolomeo, E. A redox stable Pd-doped perovskite for SOFC applications. J. Mater. Chem. A 2019, 7, 5344–5352. [Google Scholar] [CrossRef]
- Kumar, K.L.A.; Durgajanani, S.; Jeyaprakash, B.G.; Rayappan, J.B.B. nanostructured ceria thin film for ethanol and triethylamine sensing. Sens. Actuators B Chem. 2013, 177, 19–26. [Google Scholar] [CrossRef]
- Gupta, S.; Kuchibhatla, S.; Engelhard, M.; Shutthanandan, V.; Nachimuthu, P.; Jiang, W.; Saraf, L.; Thevuthasan, S.; Prasad, S. Influence of samaria doping on the resistance of ceria thin films and its implications to the planar oxygen sensing devices. Sens. Actuators B Chem. 2009, 139, 380–386. [Google Scholar] [CrossRef]
- Ueda, T.; Defferriere, T.; Hyodo, T.; Shimizu, Y.; Tuller, H.L. Nanostructured Pr-doped Ceria (PCO) thin films as sensing electrodes in solid-electrolyte type gas sensors with enhanced toluene sensitivity. Sens. Actuators B Chem. 2020, 317, 128037. [Google Scholar] [CrossRef]
- Liu, L.; Zang, C.; Wang, B.; Su, W.; Xiao, H.; Zhang, D.; Zhang, Y. Ceria thin film memristive device by magnetron sputtering method. Vacuum 2020, 173, 109128. [Google Scholar] [CrossRef]
- Schmitt, R.; Spring, J.; Korobko, R.; Rupp, J.L. Design of oxygen vacancy configuration for memristive systems. Acs Nano 2017, 11, 8881–8891. [Google Scholar] [CrossRef]
- Schweiger, S.; Pfenninger, R.; Bowman, W.J.; Aschauer, U.; Rupp, J.L.M. Designing strained interface heterostructures for memristive devices. Adv. Mater. 2017, 29, 1605049. [Google Scholar] [CrossRef]
- Eyring, L. The binary rare earth oxides. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Jr., Eyring, L., Eds.; North Holland: Amsterdam, The Netherlands, 1979; Volume 3, pp. 337–399. [Google Scholar]
- Costa, G.A.; Artini, C.; Ubaldini, A.; Carnasciali, M.M.; Mele, P.; Masini, R. Phase stability study of the pseudobinary system Gd2O3-Nd2O3 (T≤1350 °C). J. Therm. Anal. Calorim. 2008, 92, 101–104. [Google Scholar] [CrossRef]
- Artini, C.; Costa, G.A.; Pani, M.; Lausi, A.; Plaisier, J. Structural characterization of the CeO2/Gd2O3 mixed system by synchrotron X-ray diffraction. J. Solid State Chem. 2012, 190, 24–28. [Google Scholar] [CrossRef]
- Artini, C.; Presto, S.; Massardo, S.; Pani, M.; Carnasciali, M.M.; Viviani, M. Transport properties and high temperature raman features of heavily Gd-doped ceria. Energies 2019, 12, 4148. [Google Scholar] [CrossRef]
- Artini, C.; Presto, S.; Viviani, M.; Massardo, S.; Carnasciali, M.M.; Gigli, L.; Pani, M. The role of defects association in structural and transport properties of the Ce1-x(Nd0.74Tm0.26)xO2−x/2 system. J. Energy Chem. 2021, 60, 494–502. [Google Scholar] [CrossRef]
- Omar, S.; Wachsman, E.D.; Jones, J.L.; Nino, J.C. Crystal structure-ionic conductivity relationships in doped ceria systems. J. Am. Ceram. Soc. 2009, 92, 2674–2681. [Google Scholar] [CrossRef]
- Gupta, M.; Shirbhate, S.; Ojha, P.; Acharya, S. Processing and conductivity behavior of La, Sm, Fe singly and doubly doped ceria: As electrolytes for IT-SOFCs. Solid State Ion. 2018, 320, 199–209. [Google Scholar] [CrossRef]
- Minervini, L.; Zacate, M.O.; Grimes, R.W. Defect cluster formation in M2O3-doped CeO2. Solid State Ion. 1999, 116, 339–349. [Google Scholar] [CrossRef]
- Li, Z.-P.; Mori, T.; Zou, J.; Drennan, J. Defects clustering and ordering in di- and trivalently doped ceria. Mater. Res. Bull. 2013, 48, 807–812. [Google Scholar] [CrossRef]
- Presto, S.; Artini, C.; Pani, M.; Carnasciali, M.M.; Massardo, S.; Viviani, M. Ionic conductivity and local structural features in Ce1-xSmxO2−x/2. Phys. Chem. Chem. Phys. 2018, 20, 28338–28345. [Google Scholar] [CrossRef] [PubMed]
- Koettgen, J.; Dück, G.; Martin, M. The oxygen ion conductivity of Lu doped ceria. J. Phys. Condens. Matter 2020, 32, 265402. [Google Scholar] [CrossRef]
- Artini, C.; Pani, M.; Lausi, A.; Masini, R.; Costa, G.A. High temperature structural study of Gd-doped ceria by synchrotron X-ray Diffraction (673 K ≤ T ≤ 1073 K). Inorg. Chem. 2014, 53, 10140–10149. [Google Scholar] [CrossRef]
- Artini, C.; Pani, M.; Carnasciali, M.M.; Buscaglia, M.T.; Plaisier, J.R.; Costa, G.A. Structural features of Sm- and Gd-doped ceria studied by synchrotron X-ray diffraction and μ-Raman spectroscopy. Inorg. Chem. 2015, 54, 4126–4137. [Google Scholar] [CrossRef]
- Artini, C.; Pani, M.; Carnasciali, M.M.; Plaisier, J.R.; Costa, G.A. Lu-, Sm-, and Gd-doped ceria: A comparative approach to their structural properties. Inorg. Chem. 2016, 55, 10567–10579. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhou, H.; Liu, Z.; Wang, Y.; Zhang, J. High performance Gd3+ and Y3+ co-doped ceria-based electrolytes for intermediate temperature solid oxide fuel cells. Mater. Res. Bull. 2008, 43, 1046–1054. [Google Scholar] [CrossRef]
- Coles-Aldridge, A.V.; Baker, R. Ionic conductivity in multiply substituted ceria-based electrolytes. Solid State Ion. 2018, 316, 9–19. [Google Scholar] [CrossRef]
- Omar, S.; Wachsman, E.D.; Nino, J.C. Higher conductivity Sm3+ and Nd3+ co-doped ceria-based electrolyte materials. Solid State Ion. 2008, 178, 1890–1897. [Google Scholar] [CrossRef]
- Arabaci, A.; Altınçekiç, T.G.; Der, M.; Öksüzömer, M.A.F. Preparation and properties of ceramic electrolytes in the Nd and Gd co-doped ceria systems prepared by polyol method. J. Alloys Compd. 2019, 792, 1141–1149. [Google Scholar] [CrossRef]
- Venkataramana, K.; Madhuri, C.; Madhusudan, C.; Reddy, Y.; Bhikshamaiah, G.; Reddy, C. Investigation on La3+ and Dy3+ co-doped ceria ceramics with an optimized average atomic number of dopants for electrolytes in IT-SOFCs. Ceram. Int. 2018, 44, 6300–6310. [Google Scholar] [CrossRef]
- Venkataramana, K.; Madhuri, C.; Madhusudan, C.; Reddy, Y.S.; Reddy, C.V. Investigation on micro-structural, structural, electrical and thermal properties of La3+, Sm3+ & Gd3+ triple-doped ceria as solid-electrolyte for intermediate temperature-solid oxide fuel cell applications. J. Appl. Phys. 2019, 126, 144901. [Google Scholar] [CrossRef]
- Rai, A.; Mehta, P.; Omar, S. Ionic conduction behavior in SmxNd0.15−xCe0.85O2−δ. Solid State Ion. 2014, 263, 190–196. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Dell’Agli, G.; Marocco, A.; Accardo, G.; Pansini, M.; Yoon, S.P.; Ham, H.C.; Frattini, D. Engineered coeprecipitation chemistry with ammonium carbonate for scalable synthesis and sintering of improved Sm0.2Ce0.8O1.90 and Gd0.16Pr0.04Ce0.8O1.90 electrolytes for IT-SOFCs. J. Ind. Eng. Chem. 2018, 59, 17–27. [Google Scholar] [CrossRef]
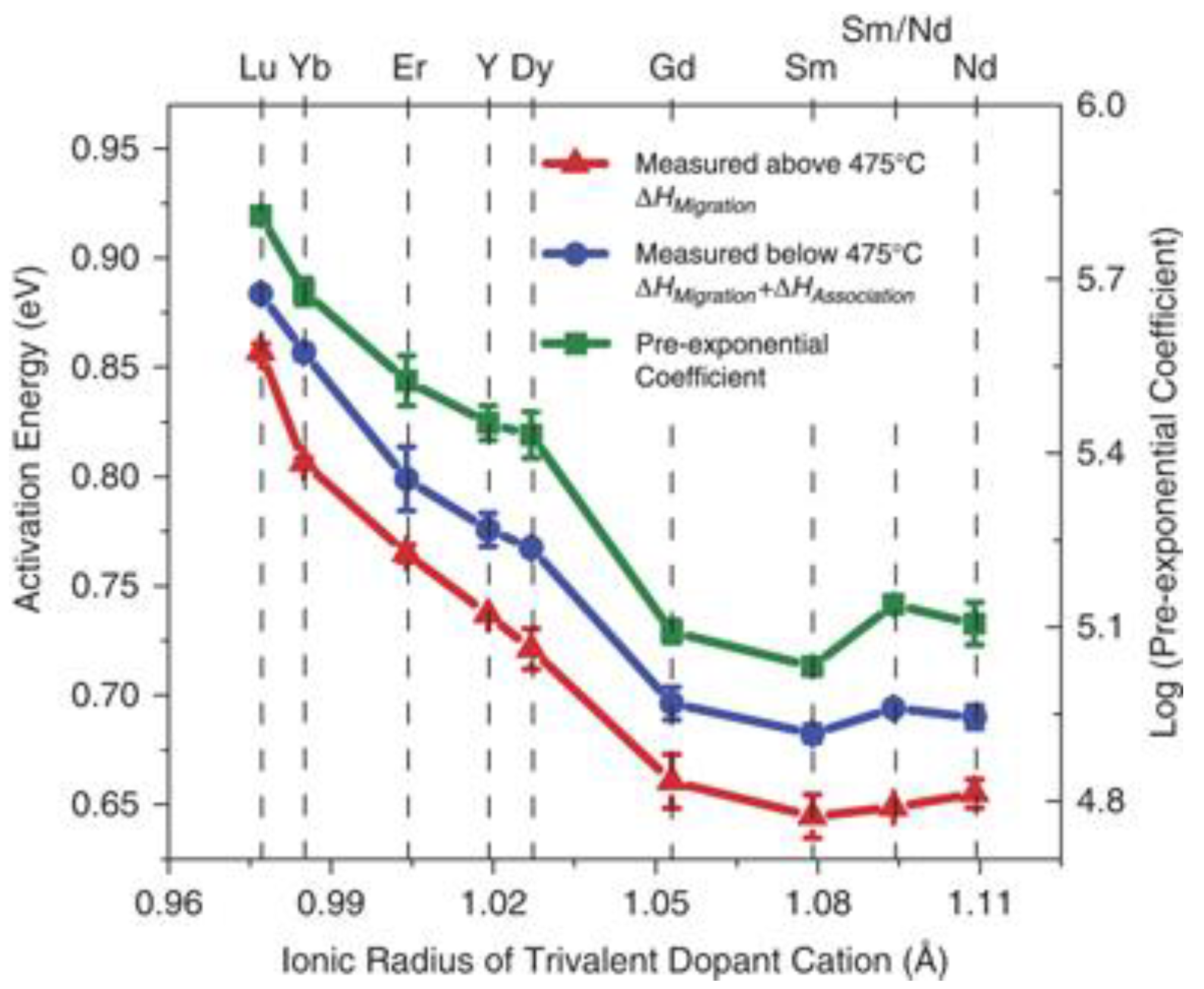
- Harrington, G.F.; Kim, S.; Sasaki, K.; Tuller, H.L.; Grieshammer, S. Strain-modified ionic conductivity in rare-earth substituted ceria: Effects of migration direction, barriers, and defect-interactions. J. Mater. Chem. A 2021, 9, 8630–8643. [Google Scholar] [CrossRef]
- De Souza, R.A.; Ramadan, A.; Hörner, S. Modifying the barriers for oxygen-vacancy migration in fluorite-structured CeO2 electrolytes through strain: A computer simulation study. Energy Environ. Sci. 2011, 5, 5445–5453. [Google Scholar] [CrossRef]
- Shi, Y.; Garbayo, I.; Muralt, P.; Rupp, J.L.M. Micro-solid state energy conversion membranes: Influence of doping and strain on oxygen ion transport and near order for electrolytes. J. Mater. Chem. A 2017, 5, 3900–3908. [Google Scholar] [CrossRef]
- Schweiger, S.; Kubicek, M.; Messerschmitt, F.; Murer, C.; Rupp, J.L.M. A microdot multilayer oxide device: Let us tune the strain-ionic transport interaction. ACS Nano 2014, 8, 5032–5048. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Marrocchelli, D.; Yildiz, B. Edge dislocation slows down oxide ion diffusion in doped CeO2 by segregation of charged defects. Nat. Commun. 2015, 6, 6294. [Google Scholar] [CrossRef] [PubMed]
- Sanna, S.; Esposito, V.; Pergolesi, D.; Orsini, A.; Tebano, A.; Licoccia, S.; Balestrino, G.; Traversa, E. Fabrication and electrochemical properties of epitaxial samarium-doped ceria films on SrTiO3-buffered MgO substrates. Adv. Funct. Mater. 2009, 19, 1713–1719. [Google Scholar] [CrossRef]
- Korte, C.; Keppner, J.; Peters, A.; Schichtel, N.; Aydin, H.; Janek, J. Coherency strain and its effect on ionic conductivity and diffusion in solid electrolytes—An improved model for nanocrystalline thin films and a review of experimental data. Phys. Chem. Chem. Phys. 2014, 16, 24575–24591. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef]
- Kilner, J.A. Feel the strain. Nat. Mater. 2008, 7, 838–839. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.L.; Chen, X.; Donner, W.; Liu, S.W.; Lin, Y.; Huang, D.X.; Jacobson, A.J. Electrical properties of a highly oriented, textured thin film of the ionic conductor Gd:CeO2-δ on (001) MgO. Appl. Phys. Lett. 2003, 83, 4737–4739. [Google Scholar] [CrossRef]
- Sanna, S.; Esposito, V.; Tebano, A.; Licoccia, S.; Traversa, E.; Balestrino, G. Enhancement of ionic conductivity in Sm-doped ceria/yttria-stabilized zirconia heteroepitaxial structures. Small 2010, 6, 1863–1867. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, W.; Hertz, J. Structure and ionic conductivity of nanoscale gadolinia-doped ceria thin films. Solid State Ion. 2013, 249, 139–143. [Google Scholar] [CrossRef]
- Christie, G.M.; van Berkel, F.P.F. Microstructure—ionic conductivity relationships in ceria-gadolinia electrolytes. Solid State Ion. 1996, 83, 17–27. [Google Scholar] [CrossRef]
- Suzuki, T.; Kosacki, I.; Anderson, H.U. Microstructure—electrical conductivity relationships in nanocrystalline ceria thin films. Solid State Ion. 2002, 151, 111–121. [Google Scholar] [CrossRef]
- Rupp, J.L.M.; Gauckler, L.J. Microstructures and electrical conductivity of nanocrystalline ceria-based thin films. Solid State Ion. 2006, 177, 2513–2518. [Google Scholar] [CrossRef]
- Karageorgakis, N.I.; Heel, A.; Rupp, J.L.M.; Aguirre, M.H.; Graule, T.; Gauckler, L.J. Properties of flame sprayed Ce0.8Gd0.2O1.9-δ electrolyte thin films. Adv. Funct. Mater. 2010, 21, 532–539. [Google Scholar] [CrossRef]
- Joo, J.H.; Choi, G.M. Electrical conductivity of thin film ceria grown by pulsed laser deposition. J. Eur. Ceram. Soc. 2007, 27, 4273–4277. [Google Scholar] [CrossRef]
- Chiodelli, G.; Malavasi, L.; Massarotti, V.; Mustarelli, P.; Quartarone, E. Synthesis and characterization of Ce0.8Gd0.2O2−y polycrystalline and thin film materials. Solid State Ion. 2005, 176, 1505–1512. [Google Scholar] [CrossRef]
- Schlupp, M.V.F.; Kurlov, A.; Hwang, J.; Yáng, Z.; Döbeli, M.; Martynczuk, J.; Prestat, M.; Son, J.W.; Gauckler, L.J. Gadolinia doped ceria thin films prepared by aerosol assisted chemical vapor deposition and applications in intermediate-temperature solid oxide fuel cells. Fuel Cells 2013, 5, 658–665. [Google Scholar] [CrossRef]
- Lair, V.; Živković, L.S.; Lupan, O.; Ringuedé, A. Synthesis and characterizathion of electrodeposited samaria and samaria-doped ceria thin films. Electrochim. Acta 2011, 56, 4638–4644. [Google Scholar] [CrossRef]
- Lee, Y.H.; Chang, I.; Cho, G.Y.; Park, J.; Yu, W.; Tanveer, W.H.; Cha, S.W. Thin film solid oxide fuel cells operating below 600 C: A review. Int. J. Precis. Eng. Manuf. 2018, 5, 441–453. [Google Scholar] [CrossRef]
- Artini, C.; Cingolani, A.; Anselmi Tamburini, U.; Valenza, F.; Latronico, G.; Mele, P. Effect of the sintering pressure on structure and microstructure of the filled skutterudite Smy (FexNi1-x)4Sb12. Mater. Res. Bull. 2021, 139, 111261. [Google Scholar] [CrossRef]
- Ghadiri, M.; Kang, A.K.; Gorji, N.E. XRD characterization of graphene-contacted perovskite solar cells: Moisture degradation and dark-resting recovery. Superlattices Microstruct. 2020, 146, 106677. [Google Scholar] [CrossRef]
- Shen, Y.; Kumar, R.S.; Pravica, M.; Nicol, M.F. Characteristics of silicone fluid as a pressure transmitting medium in diamond anvil cells. Rev. Sci. Instrum. 2004, 75, 4450–4454. [Google Scholar] [CrossRef]
- Artini, C.; Carnasciali, M.; Viviani, M.; Presto, S.; Plaisier, J.; Costa, G.; Pani, M. Structural properties of Sm-doped ceria electrolytes at the fuel cell operating temperatures. Solid State Ion. 2018, 315, 85–91. [Google Scholar] [CrossRef]
- Ubaldini, A.; Artini, C.; Costa, G.A.; Carnasciali, M.M.; Masini, R. Synthesis and thermal decomposition of mixed Gd-Nd oxalates. J. Therm. Anal. Calorim. 2008, 91, 797–803. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D: An Introduction and Overview. ESRF Internal Report; ESRF: Grenoble, France, 1997; ESRF97HA02T. [Google Scholar]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Artini, C.; Carnasciali, M.M.; Plaisier, J.R.; Costa, G.A.; Pani, M. A novel method for the evaluation of the Rare Earth (RE) coordination number in RE-doped ceria through Raman spectroscopy. Solid State Ion. 2017, 311, 90–97. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef]
- Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Poirier, J.-P.; Tarantola, A. A logarithmic equation of state. Phys. Earth Planet. Inter. 1998, 109, 1–8. [Google Scholar] [CrossRef]
- Vinet, P.; Smith, J.R.; Ferrante, J.; Rose, J.H. Temperature effects on the universal equation of state of solids. Phys. Rev. B 1987, 35, 1945–1953. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. EosFit7-GUI: A new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Cryst. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Anderson, O.L.; Nafe, J.E. The bulk modulus-volume relationship for oxides compounds and related geophysical problems. J. Geophys. Res. 1965, 70, 3951–3963. [Google Scholar] [CrossRef]
- Hill, S.; Catlow, C. A hartree-fock periodic study of bulk ceria. J. Phys. Chem. Solids 1993, 54, 411–419. [Google Scholar] [CrossRef]

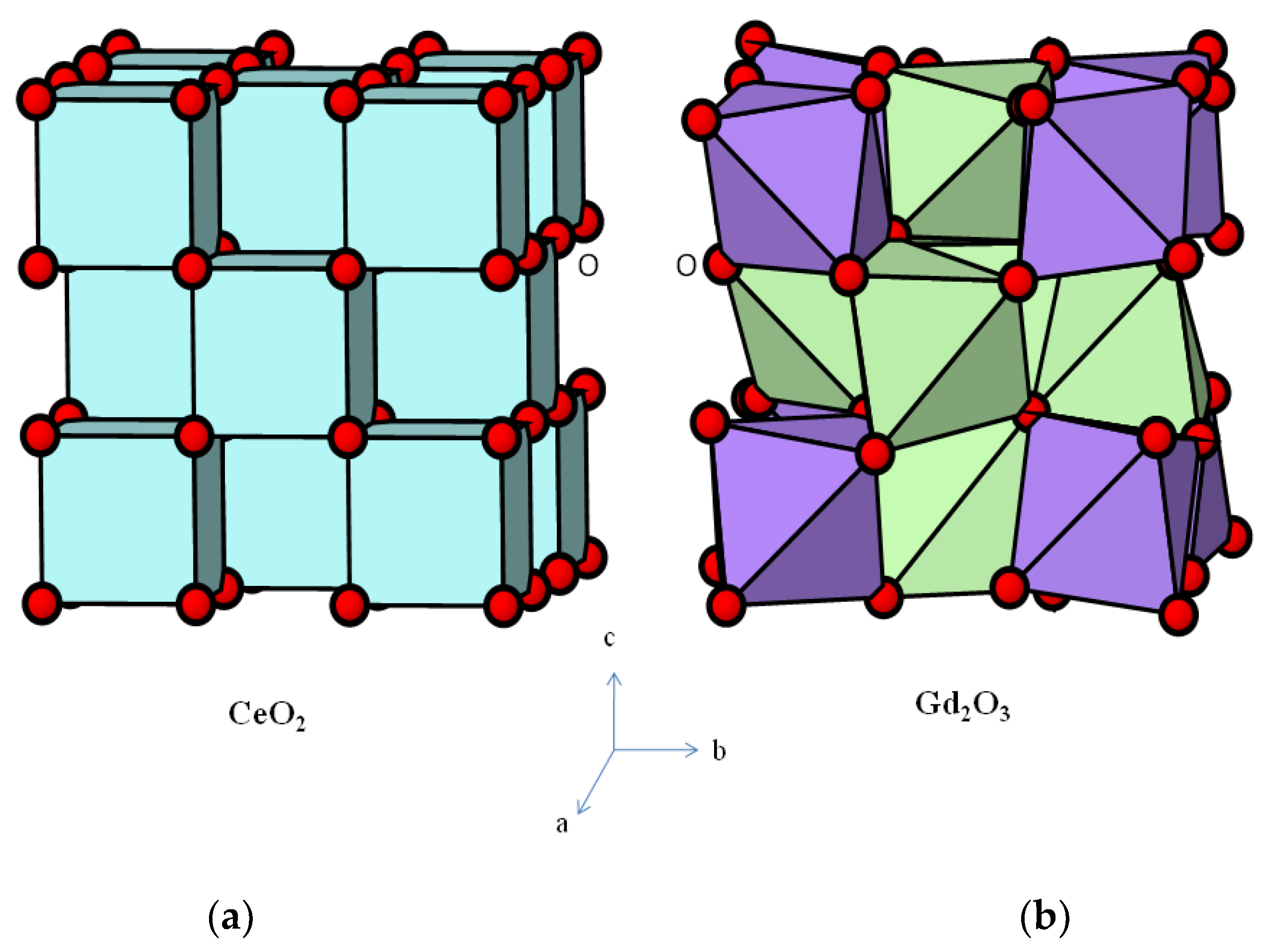

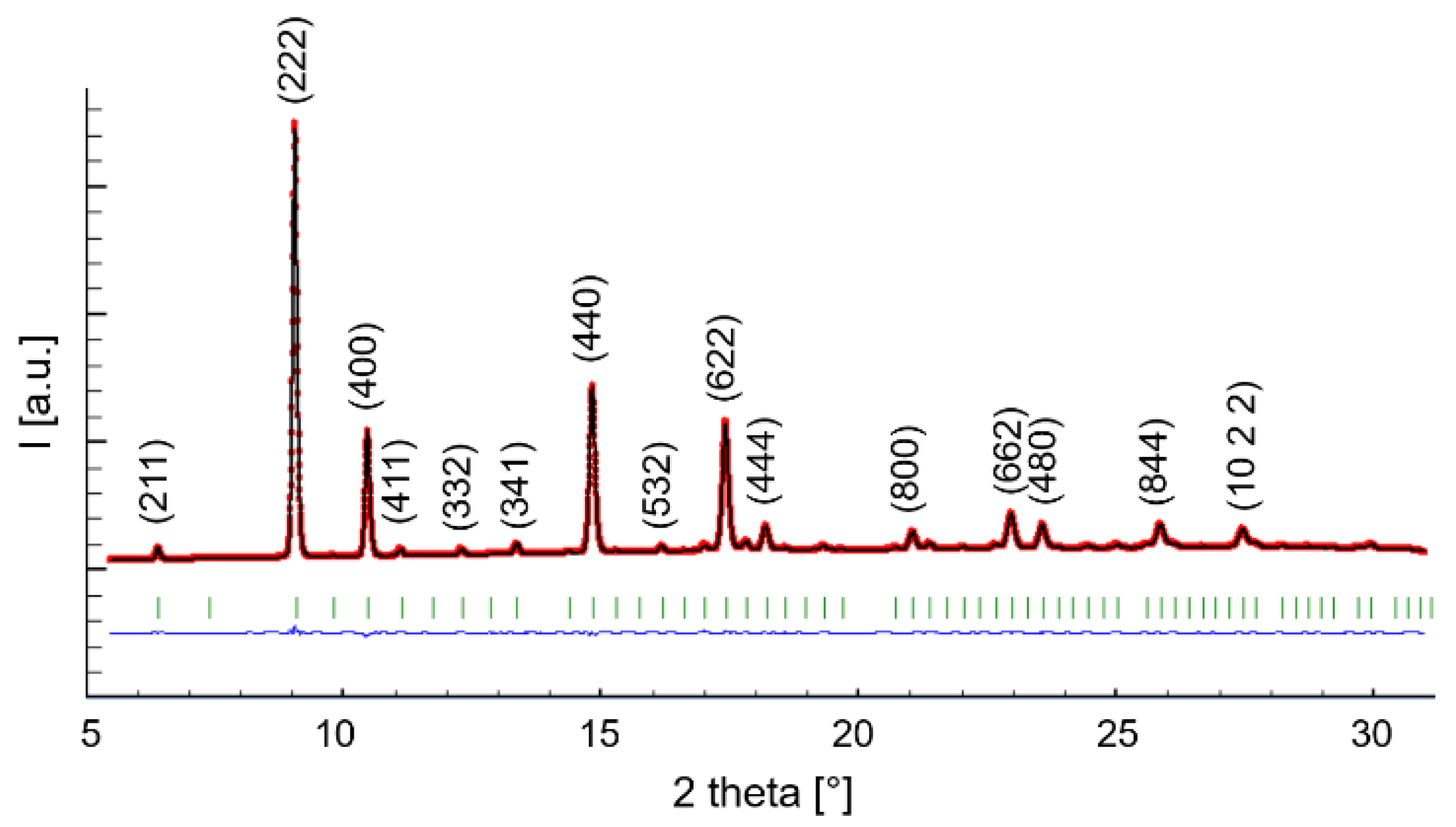

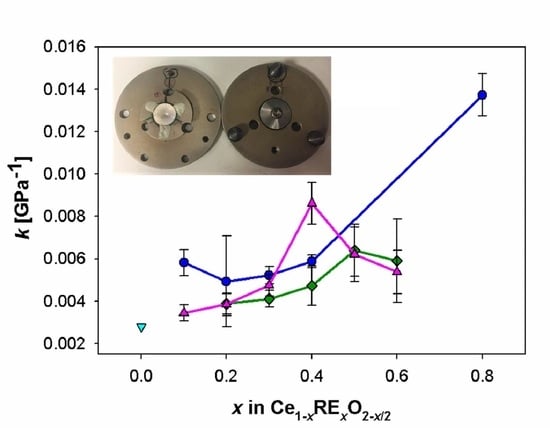
| Sample | ||
|---|---|---|
| Sm20 | 258(31) | −2(2) |
| Sm30 | 243(23) | 23(2) |
| Sm40 | 211(40) | 68(6) |
| Sm50 | 156(29) | 39(2) |
| Sm60 | 169(51) | 46(2) |
| Lu10 | 172(18) | 24(6) |
| Lu20 | 203(76) | 13(2) |
| Lu30 | 191(15) | 7(1) |
| Lu40 | 170(9) | 5(3) |
| Lu80 | 73(10) | 85(4) |
| NdTm10 | 290(32) | 34(14) |
| NdTm20 | 260(35) | 18(3) |
| NdTm30 | 194(20) | 31(12) |
| NdTm40 | 107(11) | 121(17) |
| NdTm50 | 161(32) | 7(9) |
| NdTm60 | 186(34) | 37(16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massardo, S.; Cingolani, A.; Artini, C. High Pressure X-ray Diffraction as a Tool for Designing Doped Ceria Thin Films Electrolytes. Coatings 2021, 11, 724. https://doi.org/10.3390/coatings11060724
Massardo S, Cingolani A, Artini C. High Pressure X-ray Diffraction as a Tool for Designing Doped Ceria Thin Films Electrolytes. Coatings. 2021; 11(6):724. https://doi.org/10.3390/coatings11060724
Chicago/Turabian StyleMassardo, Sara, Alessandro Cingolani, and Cristina Artini. 2021. "High Pressure X-ray Diffraction as a Tool for Designing Doped Ceria Thin Films Electrolytes" Coatings 11, no. 6: 724. https://doi.org/10.3390/coatings11060724
APA StyleMassardo, S., Cingolani, A., & Artini, C. (2021). High Pressure X-ray Diffraction as a Tool for Designing Doped Ceria Thin Films Electrolytes. Coatings, 11(6), 724. https://doi.org/10.3390/coatings11060724