Effects of Organic Solvent on Curing Behavior and Storage Stability for Waterborne Paint Containing Catalyst Encapsulated in Micelles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Paint
2.1.1. Paint without Organic Solvents
2.1.2. Paints Containing Organic Solvents
2.2. Measurement of Paint Curing Behavior
2.3. Measurement of Changes in Molecular Weight of Micelles
2.4. Evaluation of Storage Stability
3. Results and Discussion
3.1. Curing Behavior of Paint
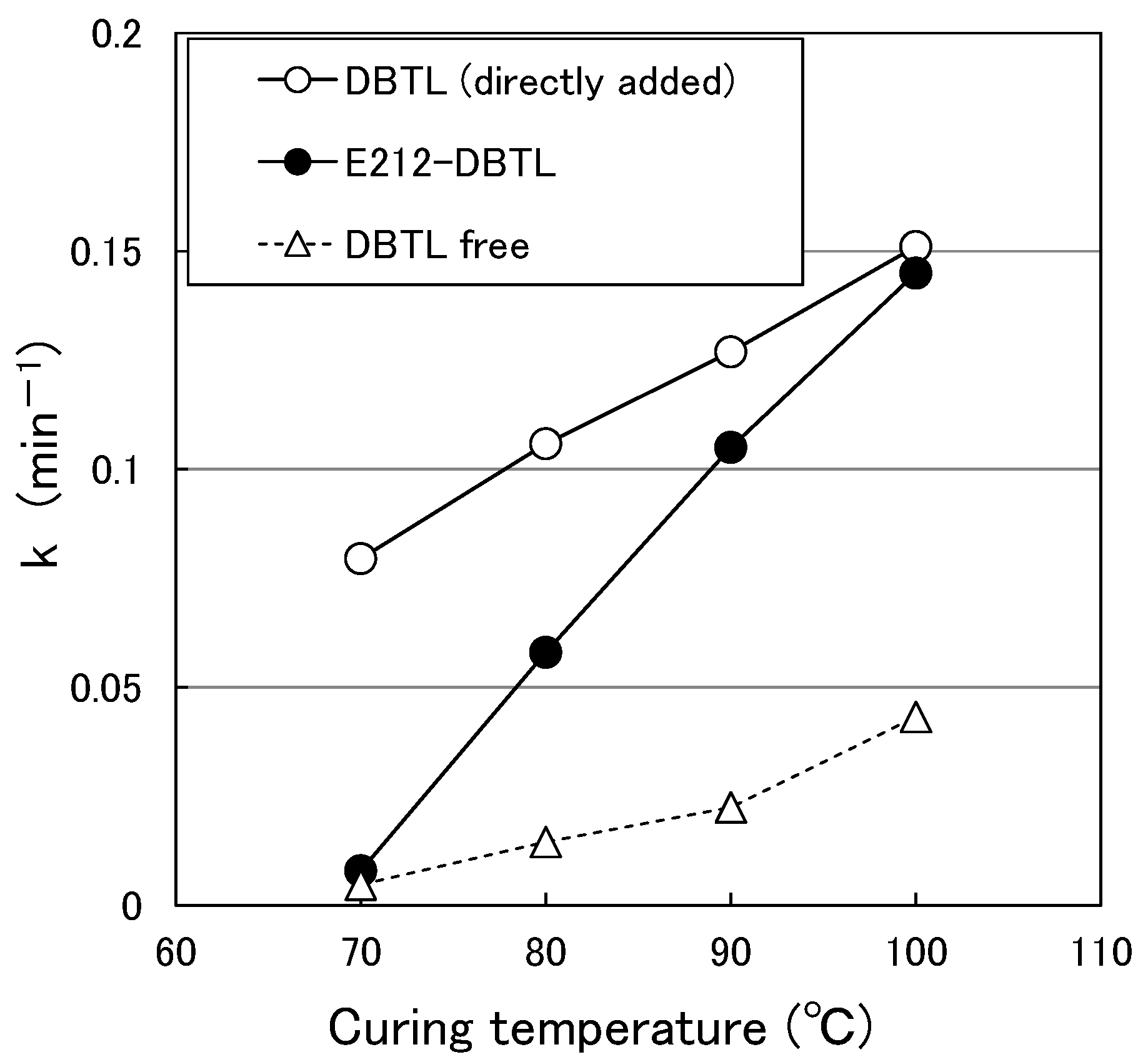
3.1.1. Temperature Dependence of k for the Paint without Organic Solvent
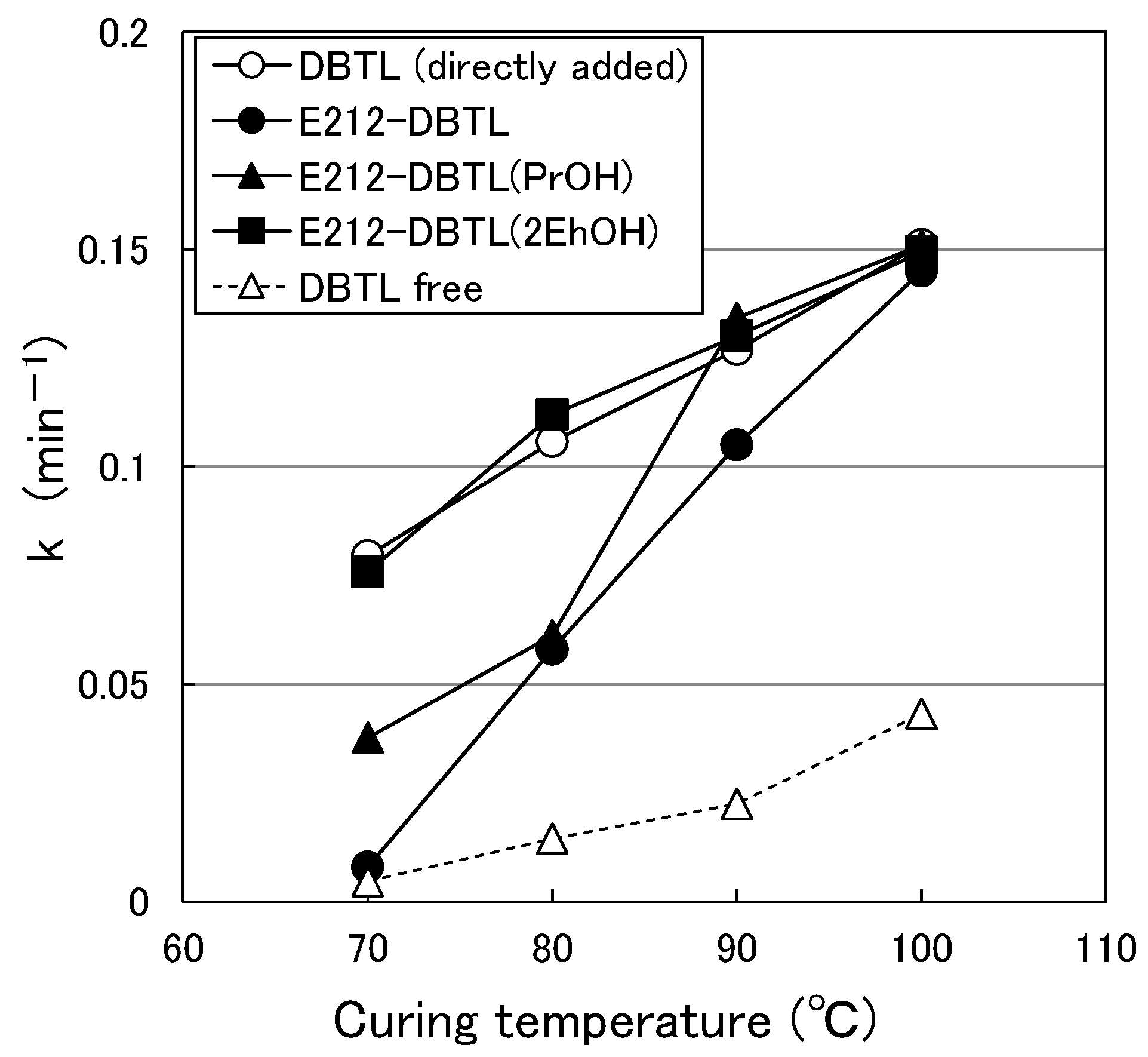
3.1.2. Temperature Dependence of k for the Paint Containing Organic Solvent
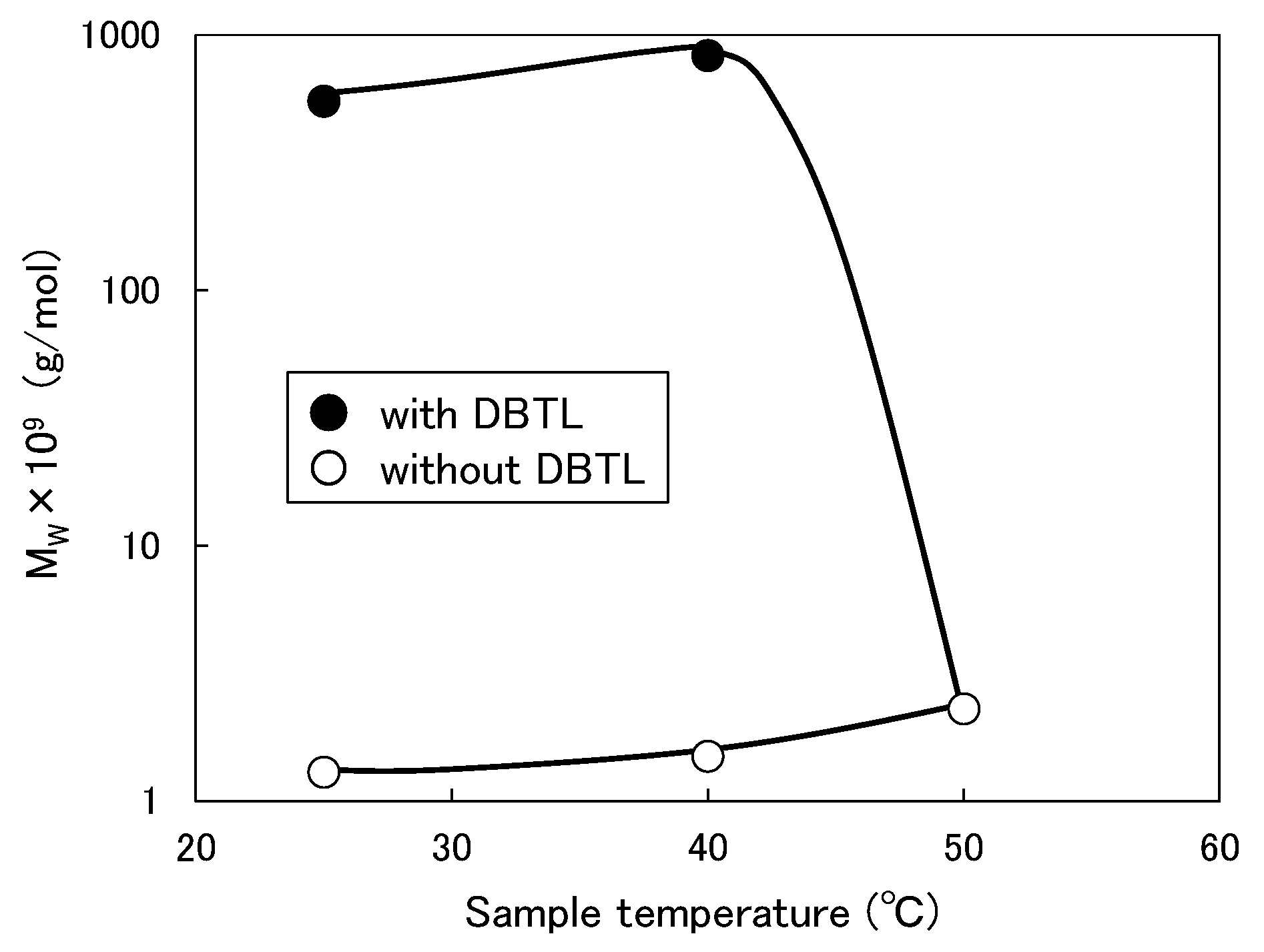
3.2. Effect of Organic Solvents on the Mw of Micelle with Catalyst
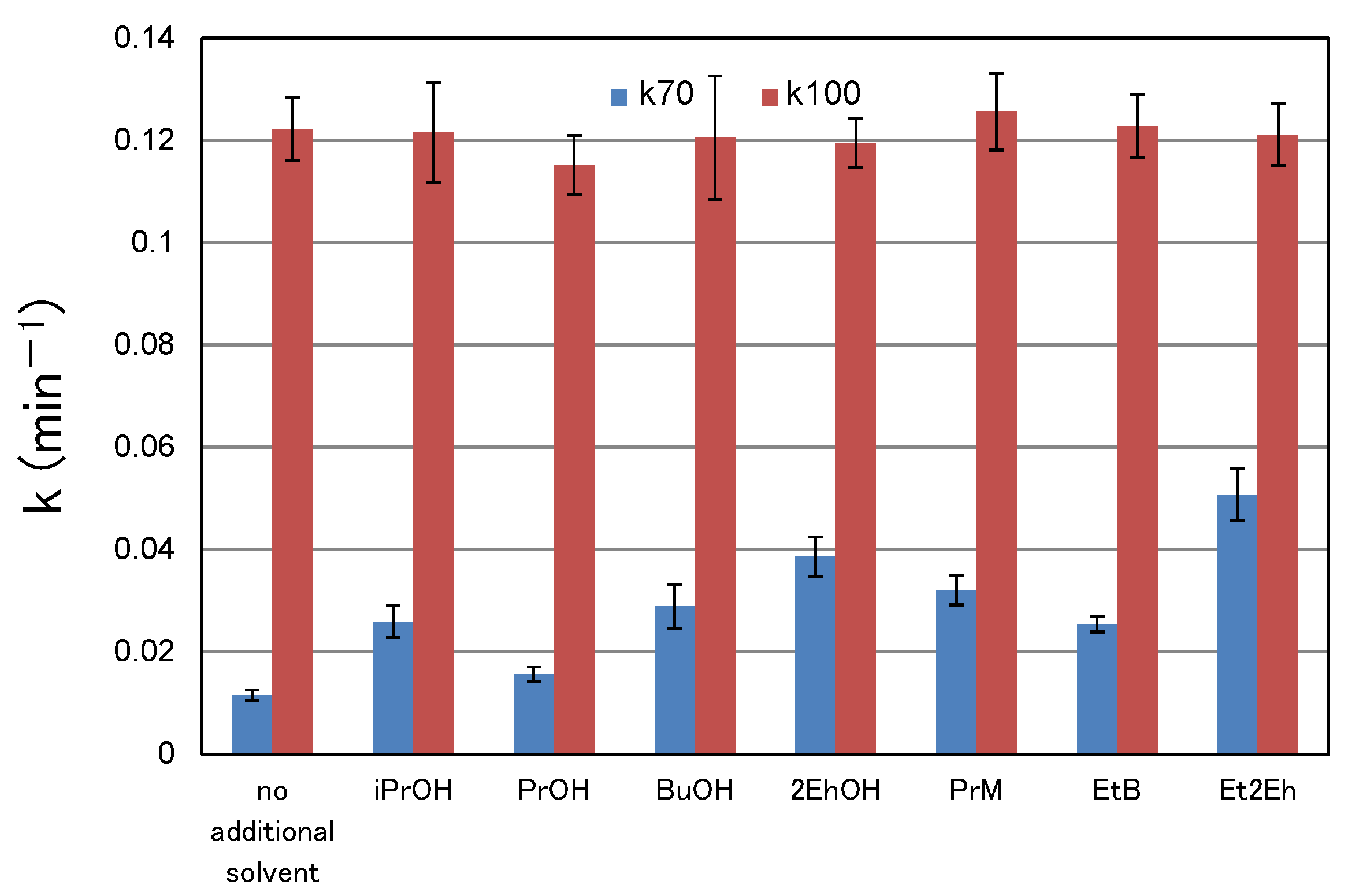
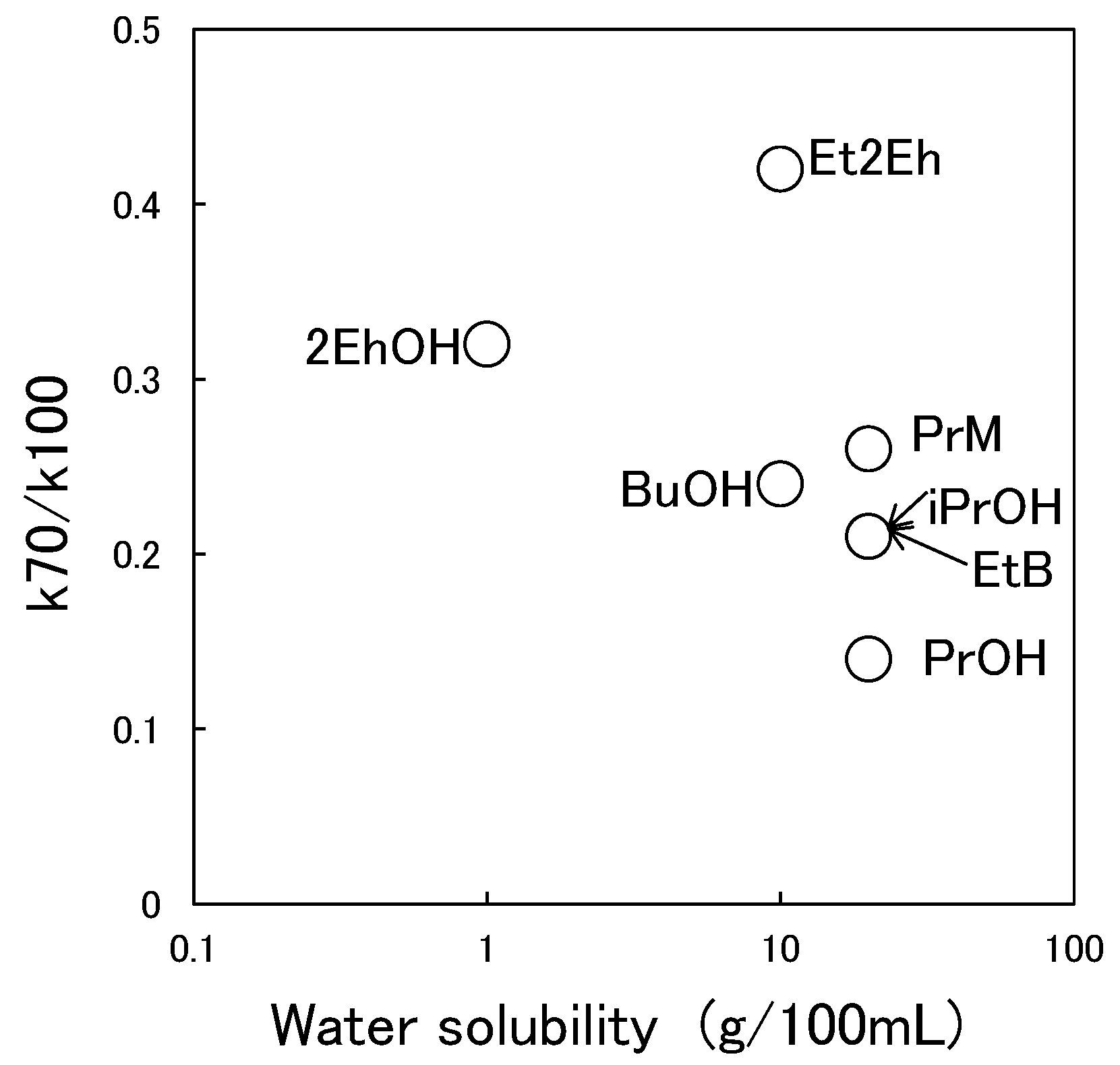
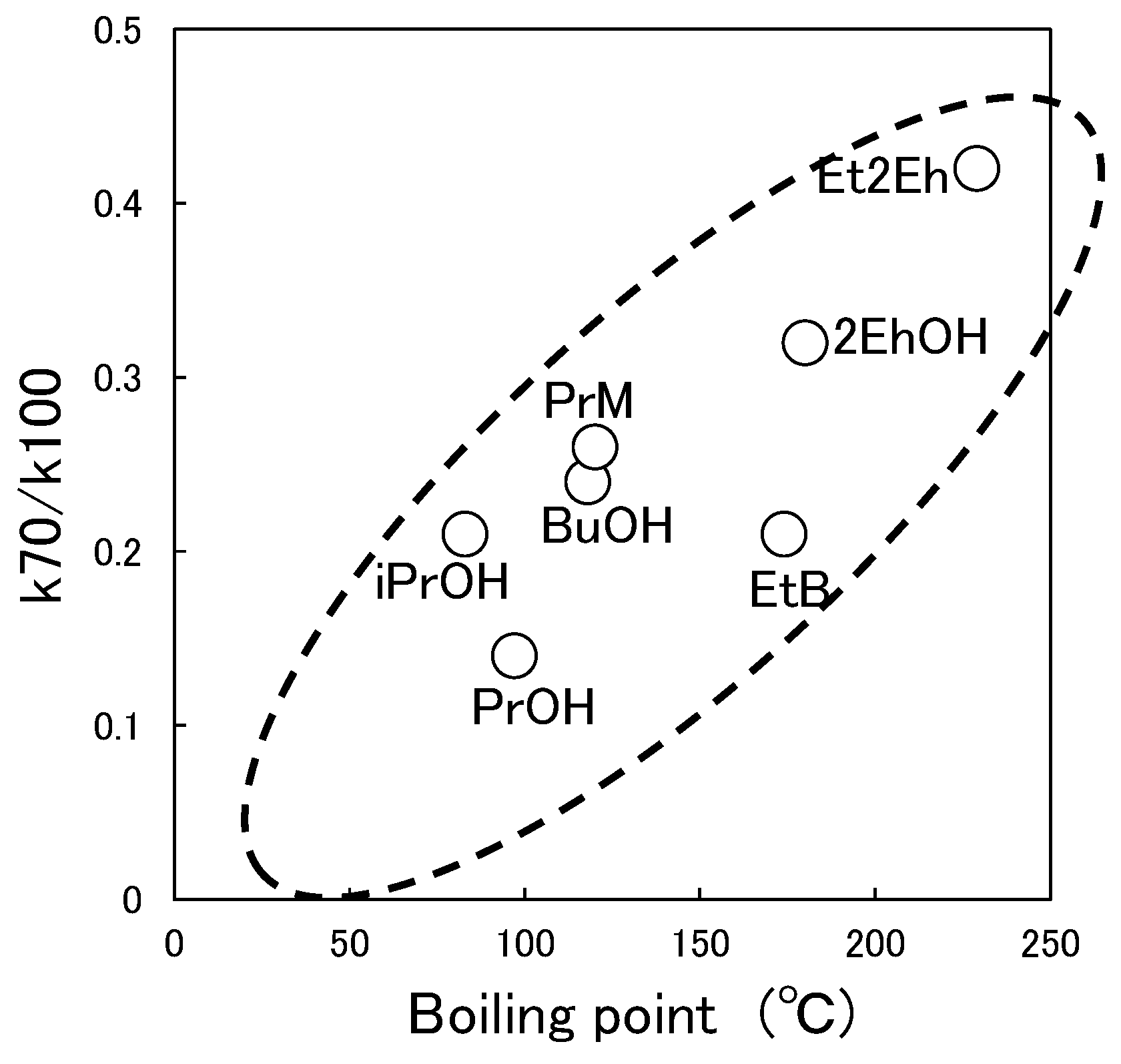
3.3. Relationship between Characteristic Values of Organic Solvents and Switching Functionality
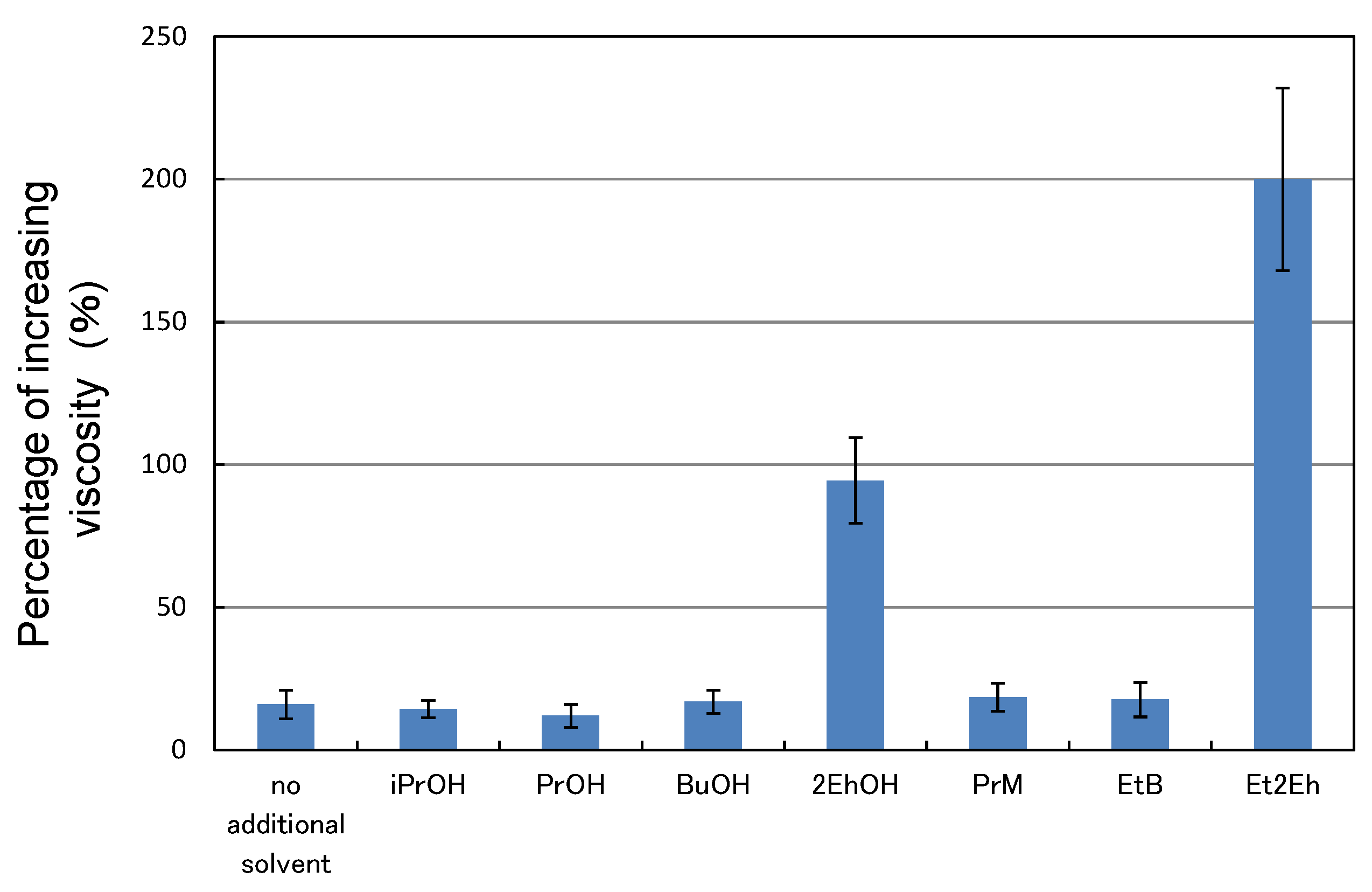
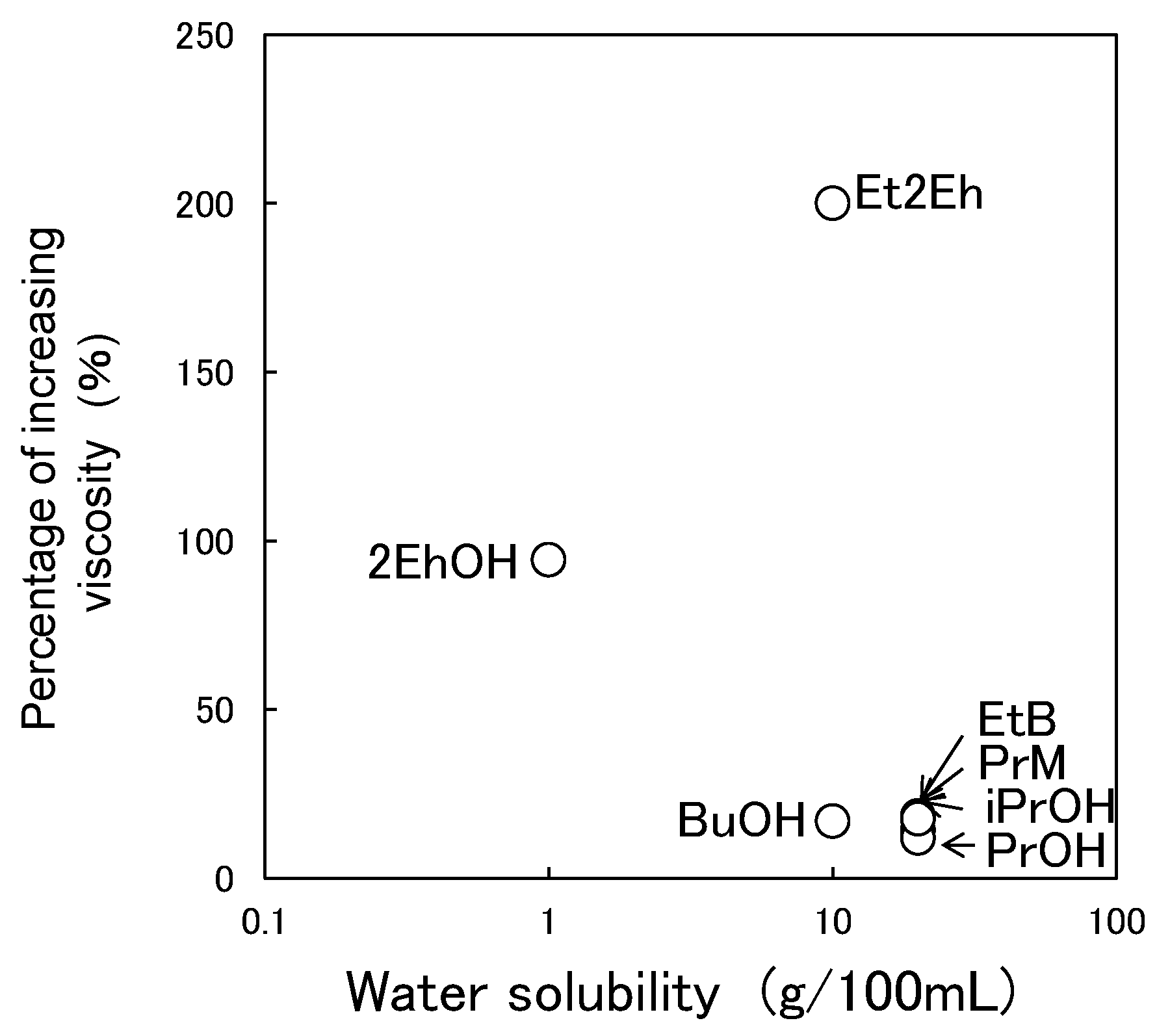
3.4. Storage Stability of Paint
3.5. Switchability and Storage Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, M.E.; Kirss, V.; Spaunburgh, R.G. Reactivity of Organic Isocyanates. Ind. Eng. Chem. 1956, 48, 794. [Google Scholar] [CrossRef]
- Wicks, D.A.; Wicks, Z.W., Jr. Blocked isocyanates III Part B: Uses and applications of blocked isocyanates. Prog. Org. Coat. 2001, 41, 1–83. [Google Scholar] [CrossRef]
- Modi, S.; Spubler, A.; Jin, J. Impact of Automated, Connected, Electric, and Shared (ACES) Vehicles on Design, Materials, Manufacturing and Business Models; Center for Automotive Research: Ann Arbor, MI, USA, 2018. [Google Scholar]
- Melchiors, M.; Sonntag, M.; Kobusch, C.; Juergens, E. Recent developments in aqueous two-component polyurethane (2K-PUR) coatings. Prog. Org. Coat. 2020, 40, 99–109. [Google Scholar] [CrossRef]
- Yomo, S.; Tachi, K.; Narita, T. Improving the appearance of 3-coat-l-bake multilayer films on automotive bodies. Prog. Org. Coat. 2018, 123, 299. [Google Scholar] [CrossRef]
- Wicks, D.A.; Wicks, Z.W., Jr. Blocked isocyanates III: Part A. Mechanisms and chemistry. Prog. Org. Coat. 1999, 36, 148–172. [Google Scholar] [CrossRef]
- Cram, D.J.; Cram, J.M. Monographs in Supramolecular Chemistry; The Royal Society of Chemistry: Cambridge, UK, 1994; Volume 4, p. 223. [Google Scholar]
- June, Y.G.; Jung, K.I.; Choi, M.; Lee, T.H.; Noh, S.M.; Jung, H.W. Effect of Urethane Crosslinking by Blocked Isocyanates with Pyrazole-Based Blocking Agents on Rheological and Mechanical Performance of Clearcoats. Coatings 2020, 10, 961. [Google Scholar] [CrossRef]
- Wang, L.; Zou, H.; Dong, Z.; Zhou, L.; Li, J.; Luo, Q.; Zhu, J.; Xu, J.; Liu, J. Temperature-Driven Switching of the Catalytic Activity of Artificial Glutathione Peroxidase by the Shape Transition between the Nanotubes and Vesicle-like Structures. Langmuir 2014, 30, 4013–4018. [Google Scholar] [CrossRef] [PubMed]
- Kremer, C.; Schnakenburg, G.; Lutzen, A. Towards allosteric receptors—Synthesis of beta-cyclodextrin-functionalised 2,2’-bipyridines and their metal complexes. Beilstein. J. Org. Chem. 2014, 10, 814–824. [Google Scholar]
- Zhu, X.; Xu, G.; Chamoreau, L.; Zhang, Y.; Mouries-Mansuy, V.; Fensterbank, L.; Bistri-Aslanoff, O.; Roland, S.; Sollogoub, M. Permethylated NHC-Capped Alpha- and Beta-Cyclodextrins (ICyDMe) Regioselective and Enantioselective Gold-Catalysis in Pure Water. Chem. Eur. J. 2020, 26, 15901–15909. [Google Scholar]
- Quach, Q.; Biehler, E.; Elzamzami, A.; Huff, C.; Long, J.L.M.; Abdei-Fattah, T.M. Catalytic Activity of Beta-Cyclodextrin-Gold Nanoparticles Network in Hydrogen Evolution Reaction. Catalysts 2021, 11, 118. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Liu, C.Q.; Chen, C.C.; Yao, Y.G. One-Pot Synthesis of Cyclodextrin-Doped Cu-SiO2 Catalysts for Efficient Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Chemcatchem 2017, 9, 4587–4597. [Google Scholar] [CrossRef]
- Ikeda, A.; Shinkai, S. Novel cavity design using calix[n]arene skeletons: Toward molecular recognition and metal binding. Chem. Rev. 1997, 97, 1713–1734. [Google Scholar] [CrossRef] [PubMed]
- Duchene, D. New Trends in Cyclodextrins and Derivatives; Edition de Sante: Paris, France, 1991; p. 449. [Google Scholar]
- Yomo, S. Curing Behavior of Waterborne Paint Containing Catalyst Encapsulated in Micelle. Coatings 2021, 11, 375. [Google Scholar] [CrossRef]
- Yomo, S.; Tachi, K. Improving appearance of 3-coat-1-bake multilayer films on automotive bodies through solvent composition design. Prog. Org. Coat. 2019, 137, 105318. [Google Scholar] [CrossRef]
- Howard, P.H.; Meylan, W.M. Handbook of Physical Properties of Organic Chemicals; CRC Lewis Publishers: Boca Raton, FL, USA; New York, NY, USA; London, UK; Tokyo, Japan, 1997. [Google Scholar]
- Mori, K. Method for Estimating Crosslink Density in Curing Process on Coating Films-Proportionality between Storage Modulus and Crosslink Density. J. Jpn. Soc. Color Mater. 2013, 86, 123. [Google Scholar] [CrossRef] [Green Version]
- Zimm, B.H. The Scattering of Light and the Radial Distribution Function of High Polymer Solutions. J. Chem. Phys. 1948, 16, 1093–1099. [Google Scholar] [CrossRef]




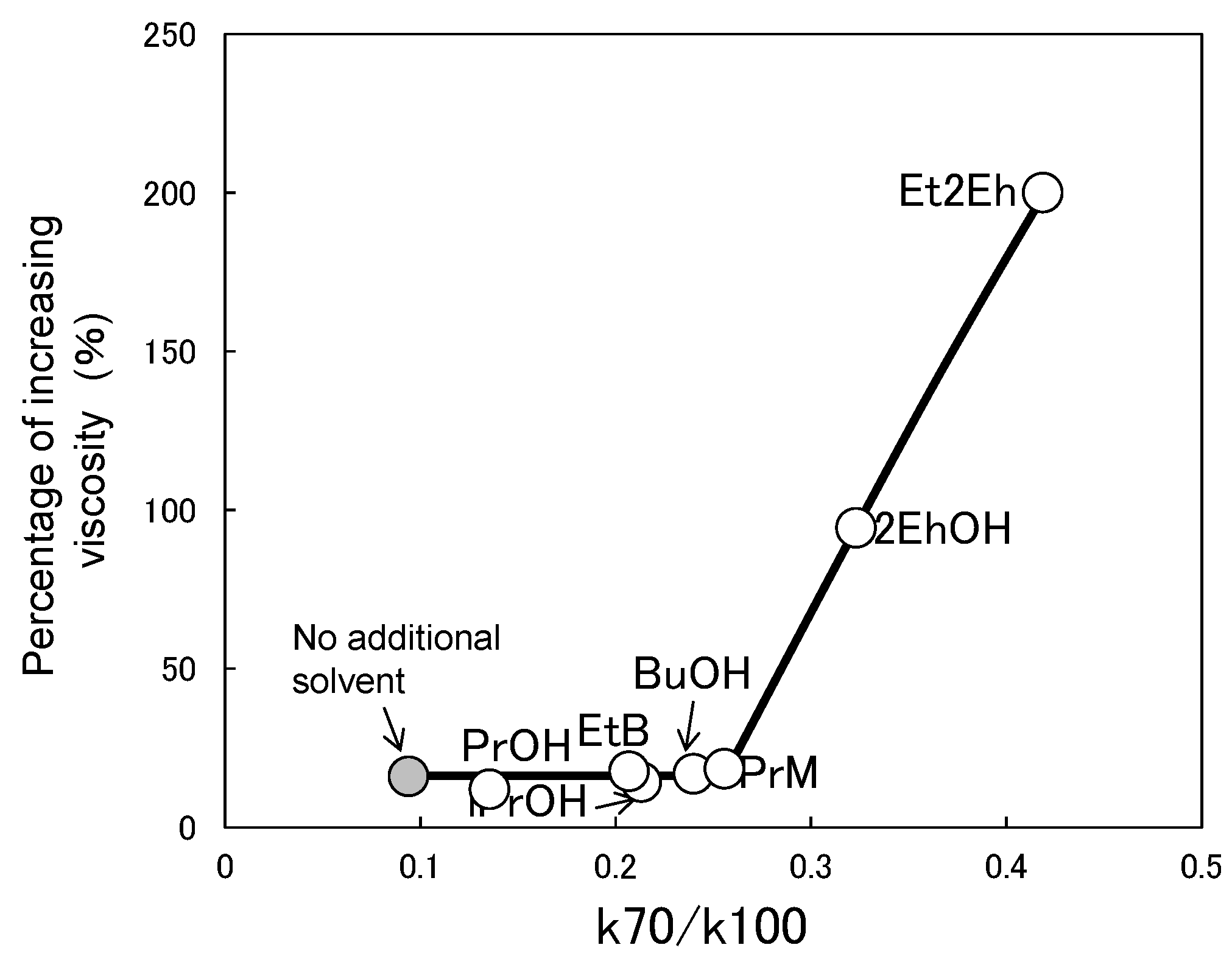
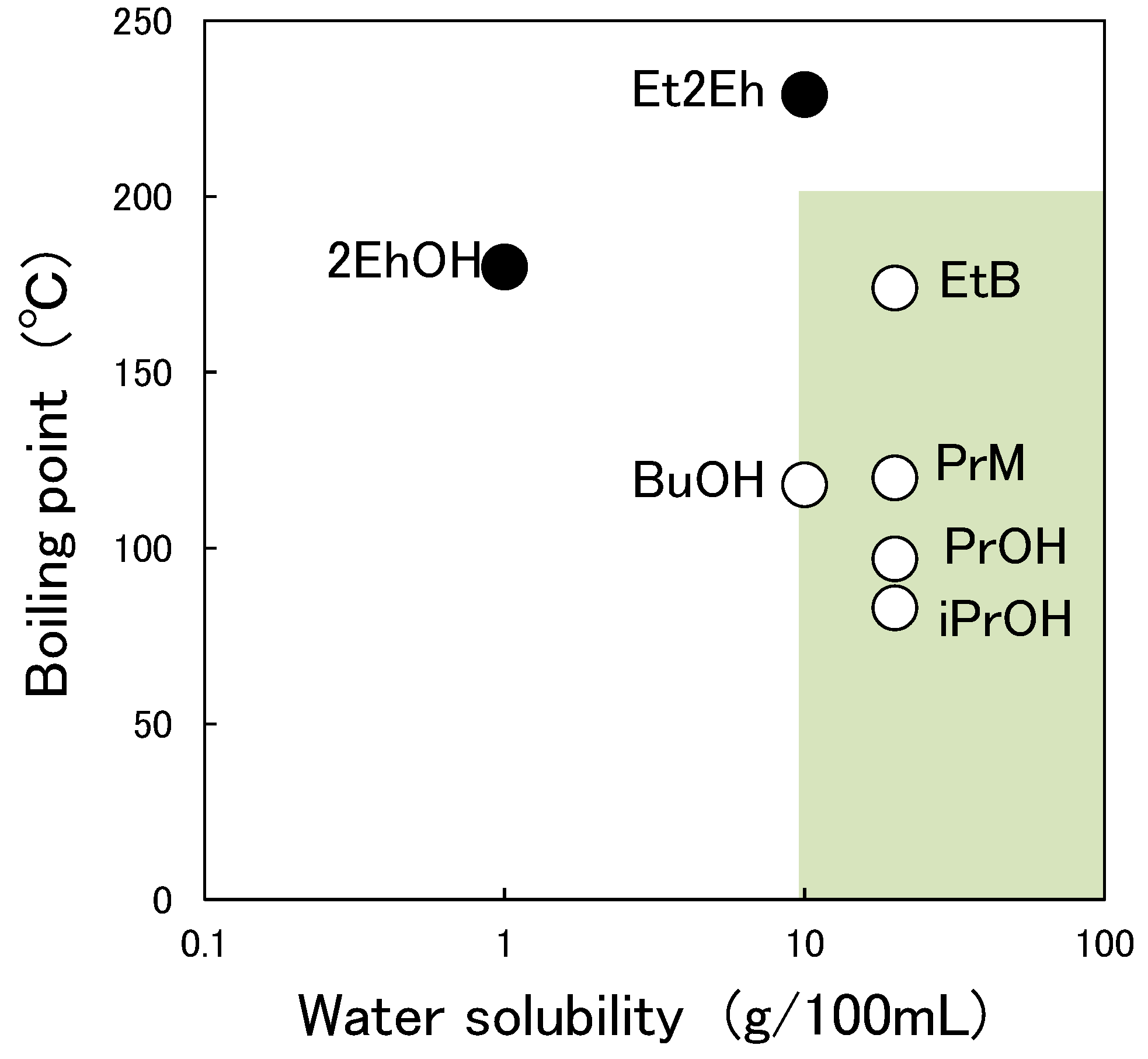
| Abbreviation of Organic Solvent | Chemical Name | Water Solubility (g/100 mL, 20 °C) | Boiling Point (°C) [18] | Abbreviation of Paint |
|---|---|---|---|---|
| iPrOH | 2-propanol | ≥10 | 83 | E212–DBTL(iPrOH) |
| PrOH | 1-propanol | ≥10 | 97 | E212–DBTL(PrOH) |
| BuOH | 1-butanol | 10 | 118 | E212–DBTL(BuOH) |
| 2EhOH | 2-ethylhexanol | ≤1 | 184 | E212–DBTL(2EhOH) |
| PrM | 1-methoxy-2-propanol | ≥10 | 120 | E212–DBTL(PrM) |
| EtB | ethylene glycol mono butyl ether | ≥10 | 171 | E212–DBTL(EtB) |
| Et2Eh | ethylene glycol 2-ethylhexyl ether | ≤1 | 229 | E212–DBTL(Et2Eh) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yomo, S. Effects of Organic Solvent on Curing Behavior and Storage Stability for Waterborne Paint Containing Catalyst Encapsulated in Micelles. Coatings 2021, 11, 722. https://doi.org/10.3390/coatings11060722
Yomo S. Effects of Organic Solvent on Curing Behavior and Storage Stability for Waterborne Paint Containing Catalyst Encapsulated in Micelles. Coatings. 2021; 11(6):722. https://doi.org/10.3390/coatings11060722
Chicago/Turabian StyleYomo, Shuji. 2021. "Effects of Organic Solvent on Curing Behavior and Storage Stability for Waterborne Paint Containing Catalyst Encapsulated in Micelles" Coatings 11, no. 6: 722. https://doi.org/10.3390/coatings11060722
APA StyleYomo, S. (2021). Effects of Organic Solvent on Curing Behavior and Storage Stability for Waterborne Paint Containing Catalyst Encapsulated in Micelles. Coatings, 11(6), 722. https://doi.org/10.3390/coatings11060722






