Effect of Sulfate-Reducing Bacteria (SRB) on the Corrosion of Buried Pipe Steel in Acidic Soil Solution
Abstract
1. Introduction
2. Experimental
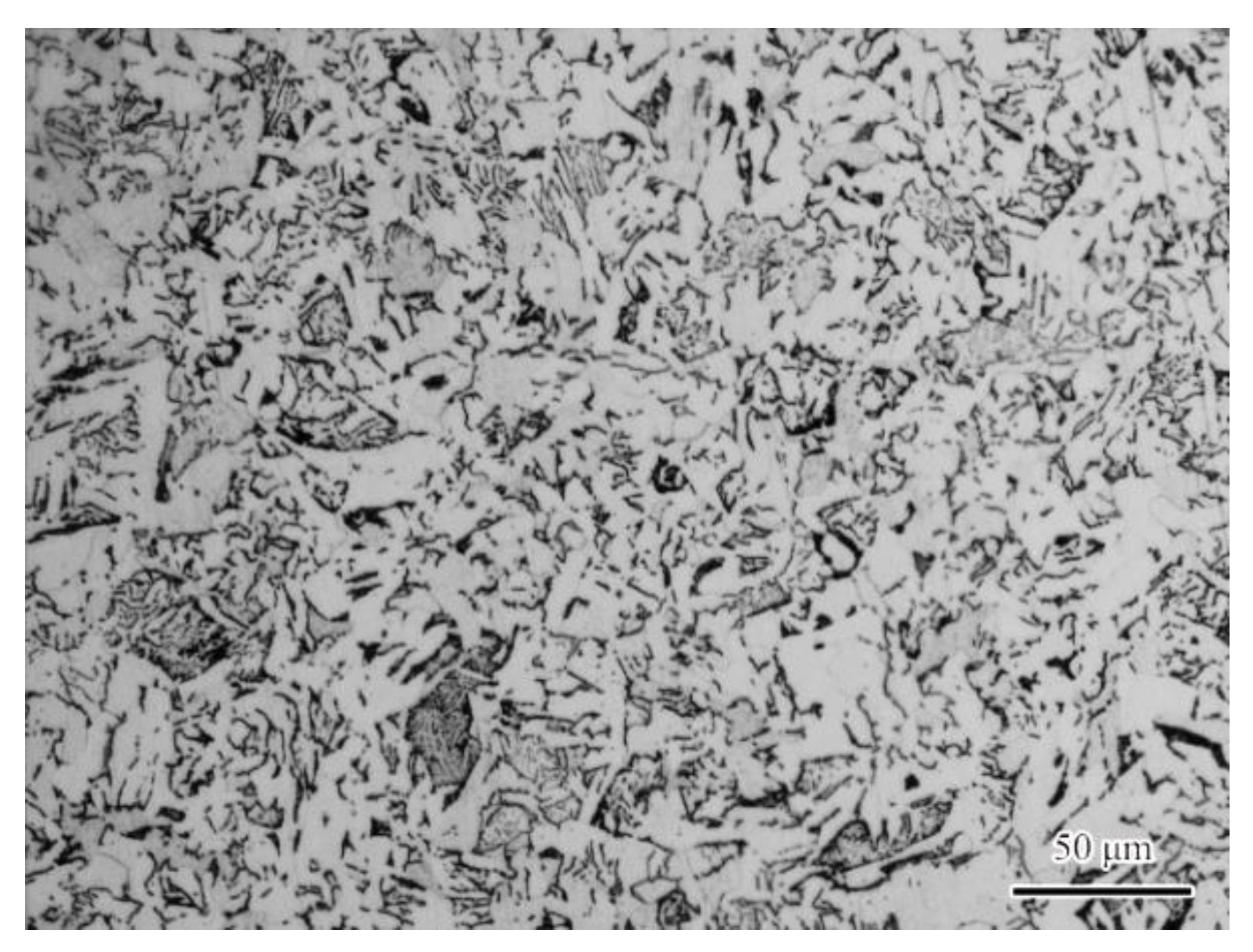
2.1. Materials and Solutions
2.2. SRB Culturing and Inoculation
2.3. Electrochemical Measurements
2.4. Surface Analysis
3. Result
3.1. Live/Dead Staining
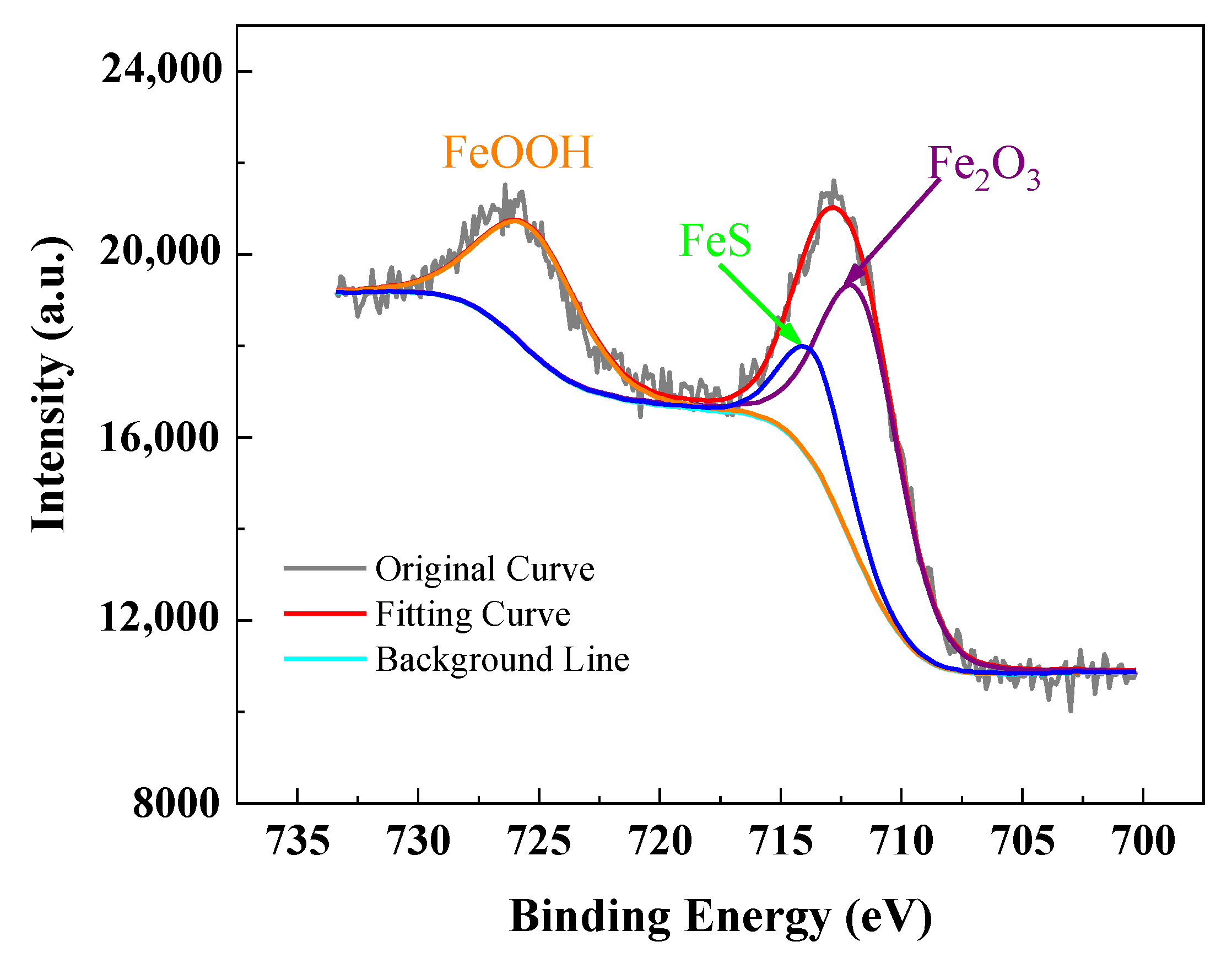
3.2. Characterization of Corrosion Products on Specimen Surface
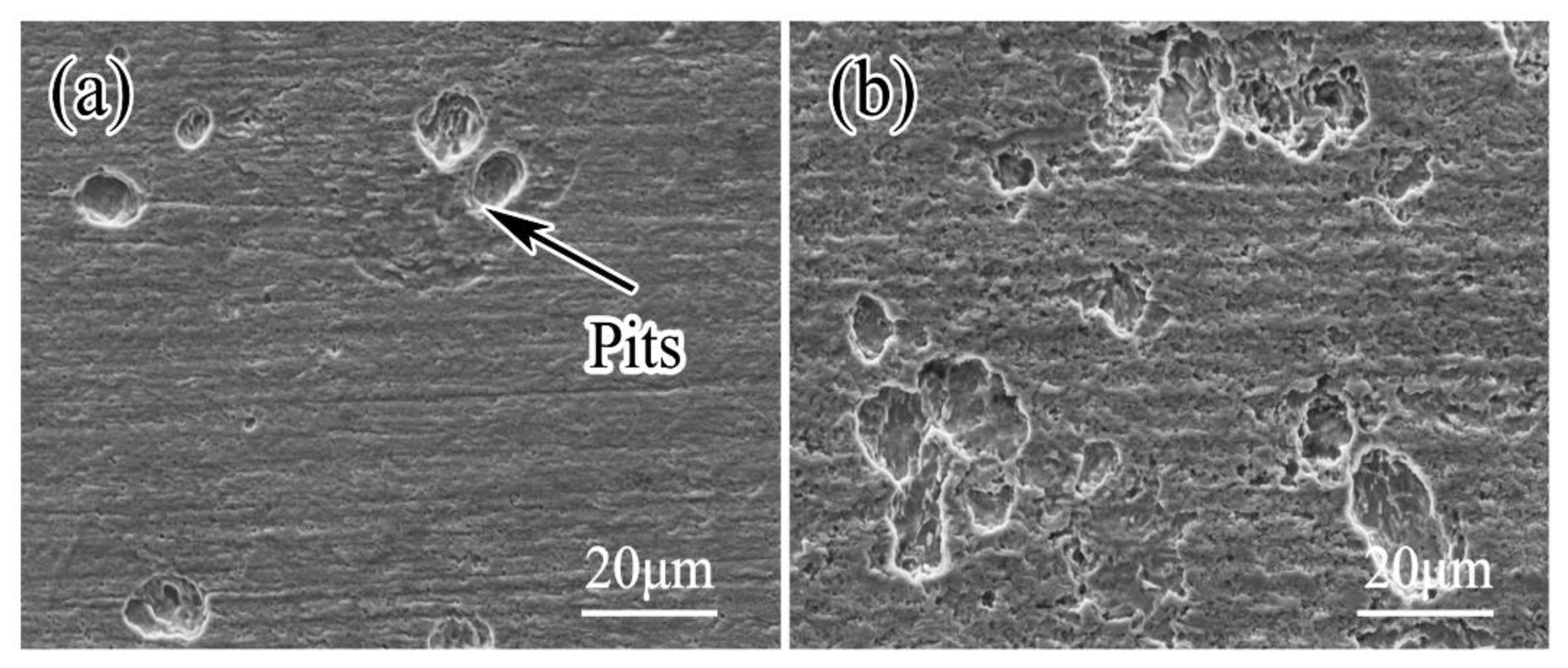
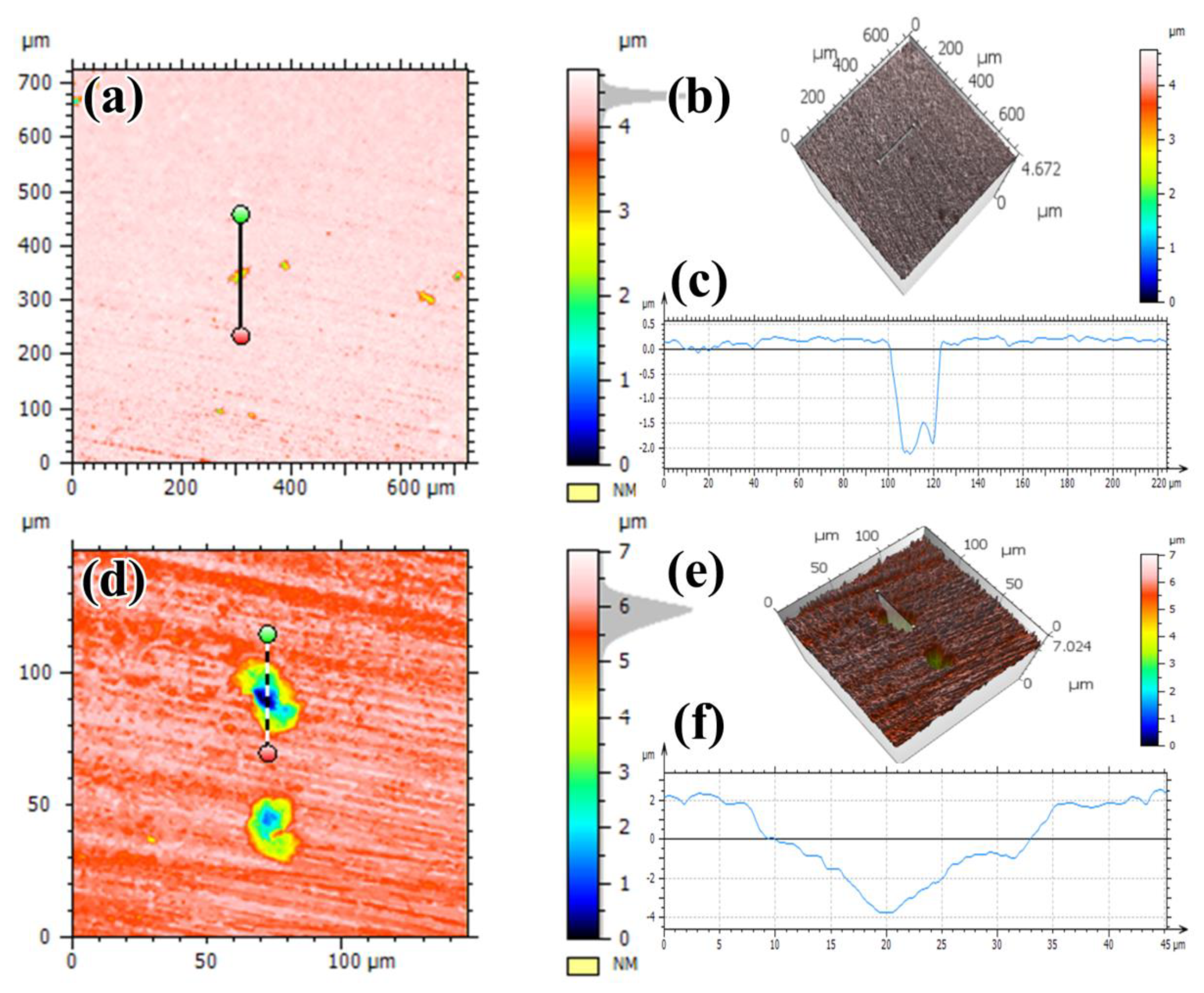
3.3. Pitting Morphologies
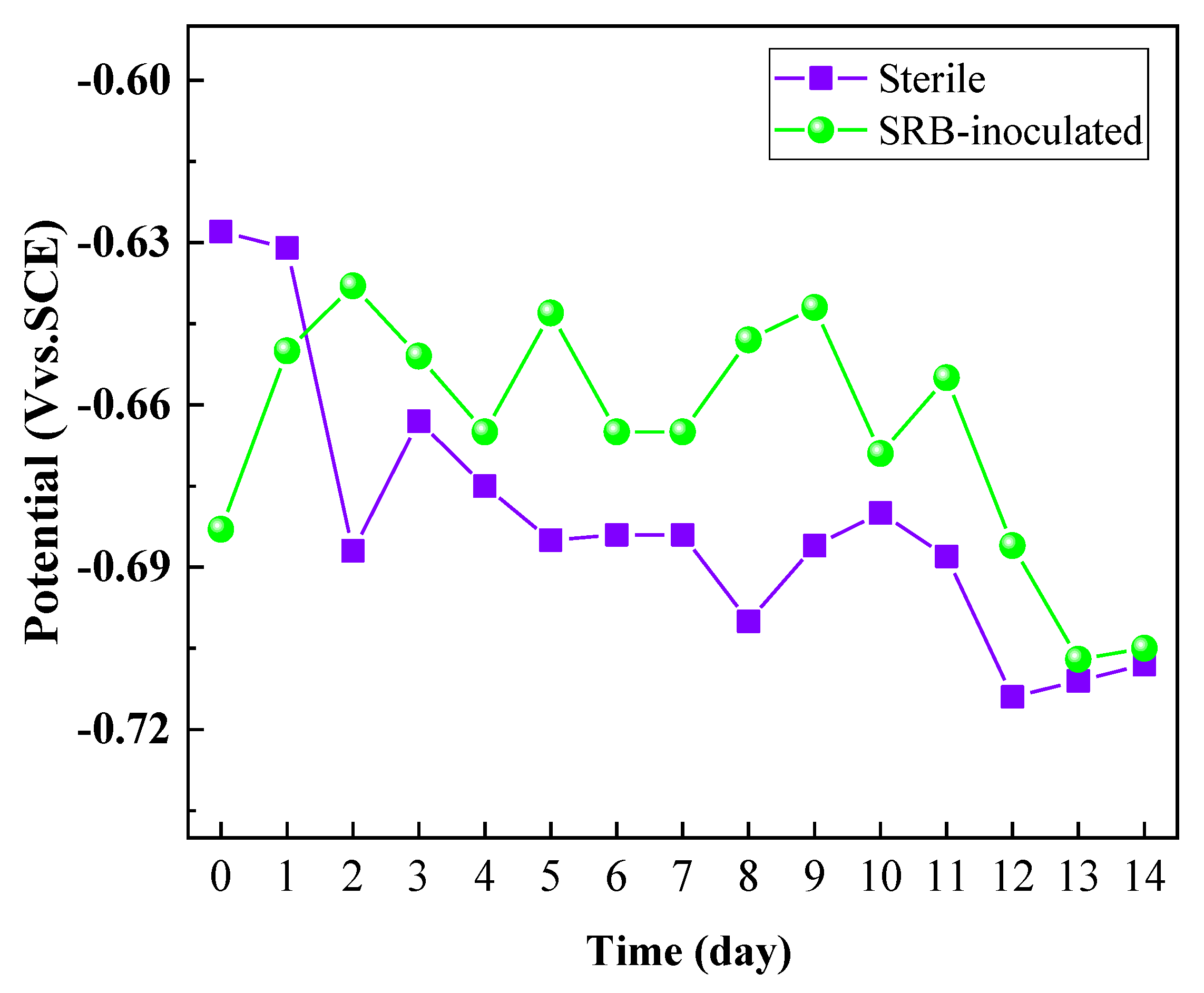
3.4. OCP Measurements
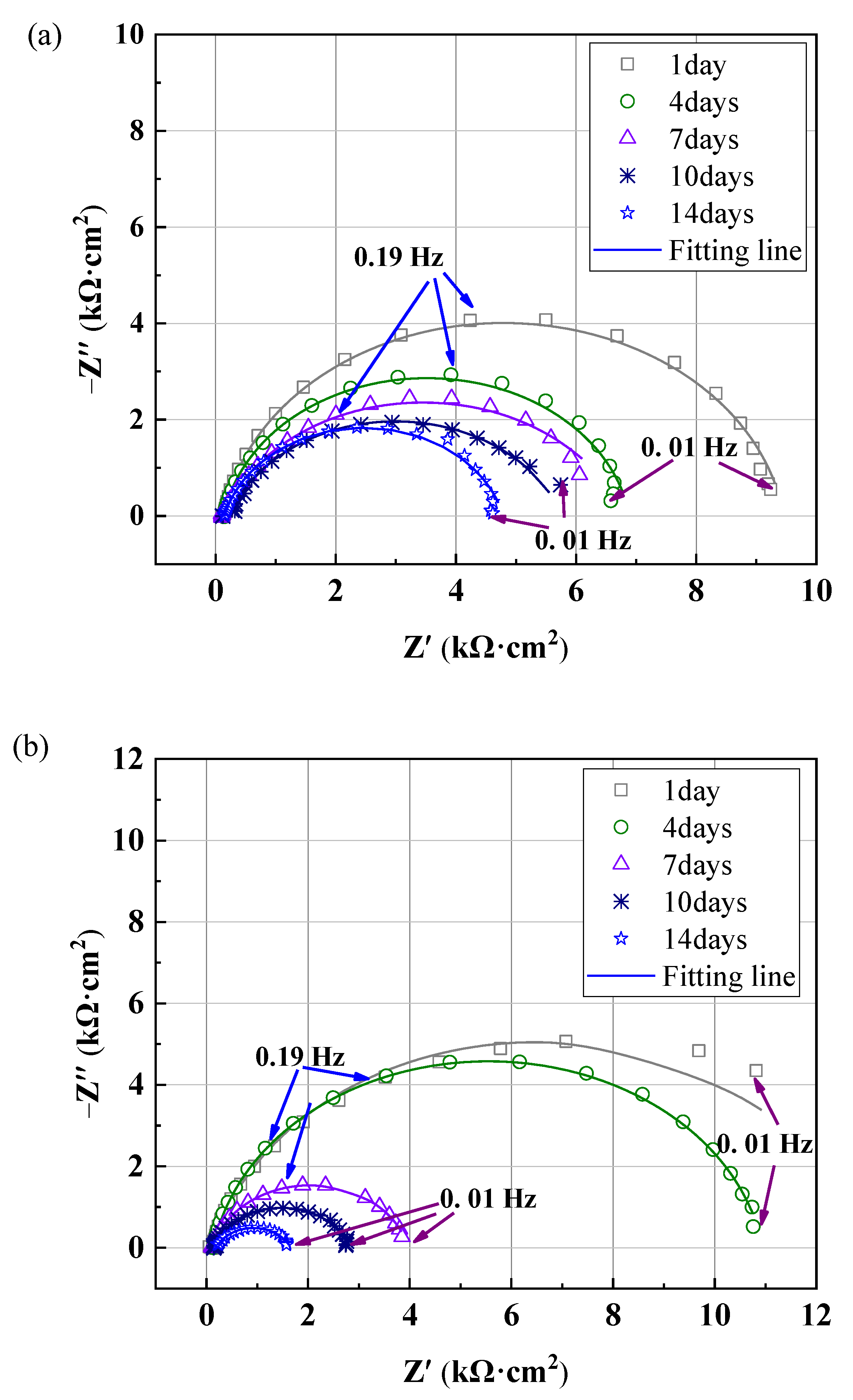
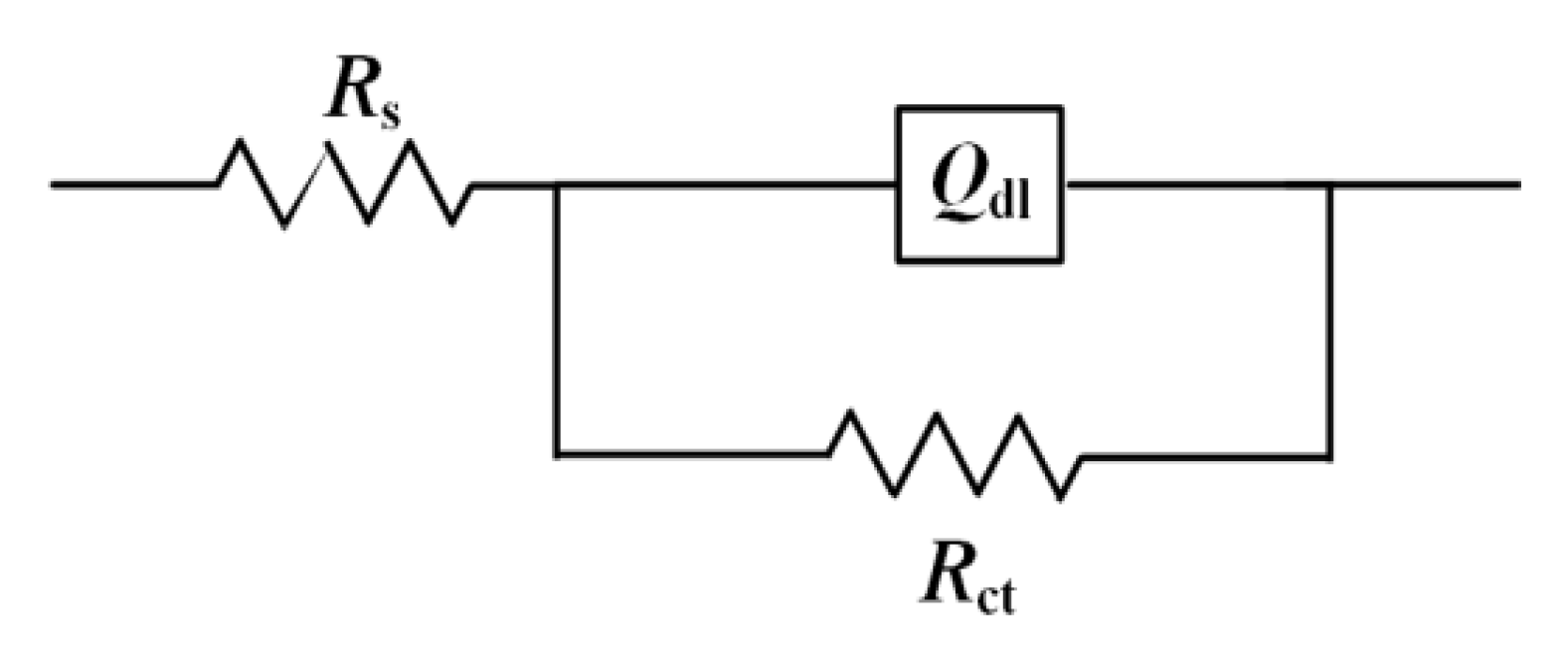
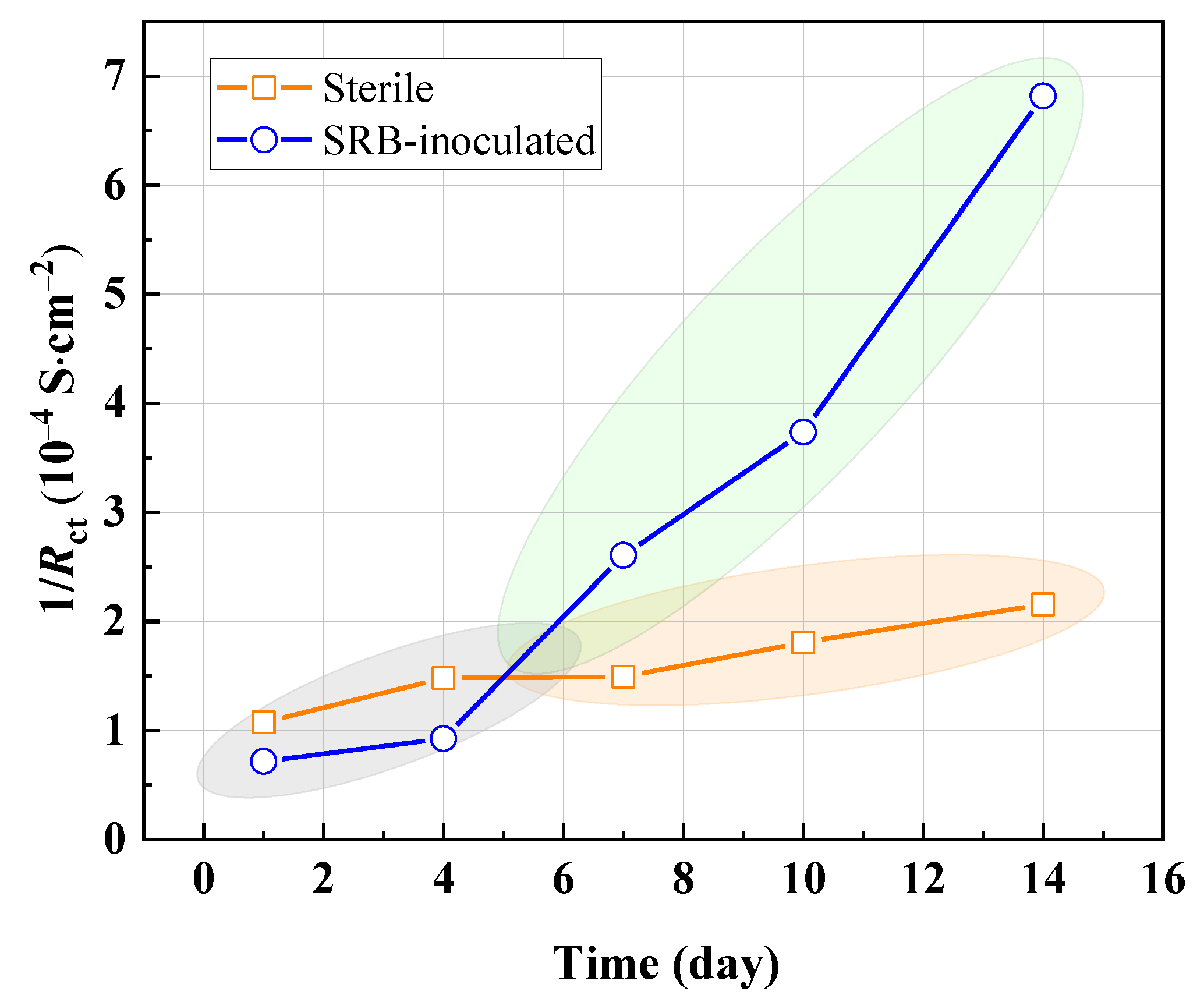
3.5. EIS Results Analysis
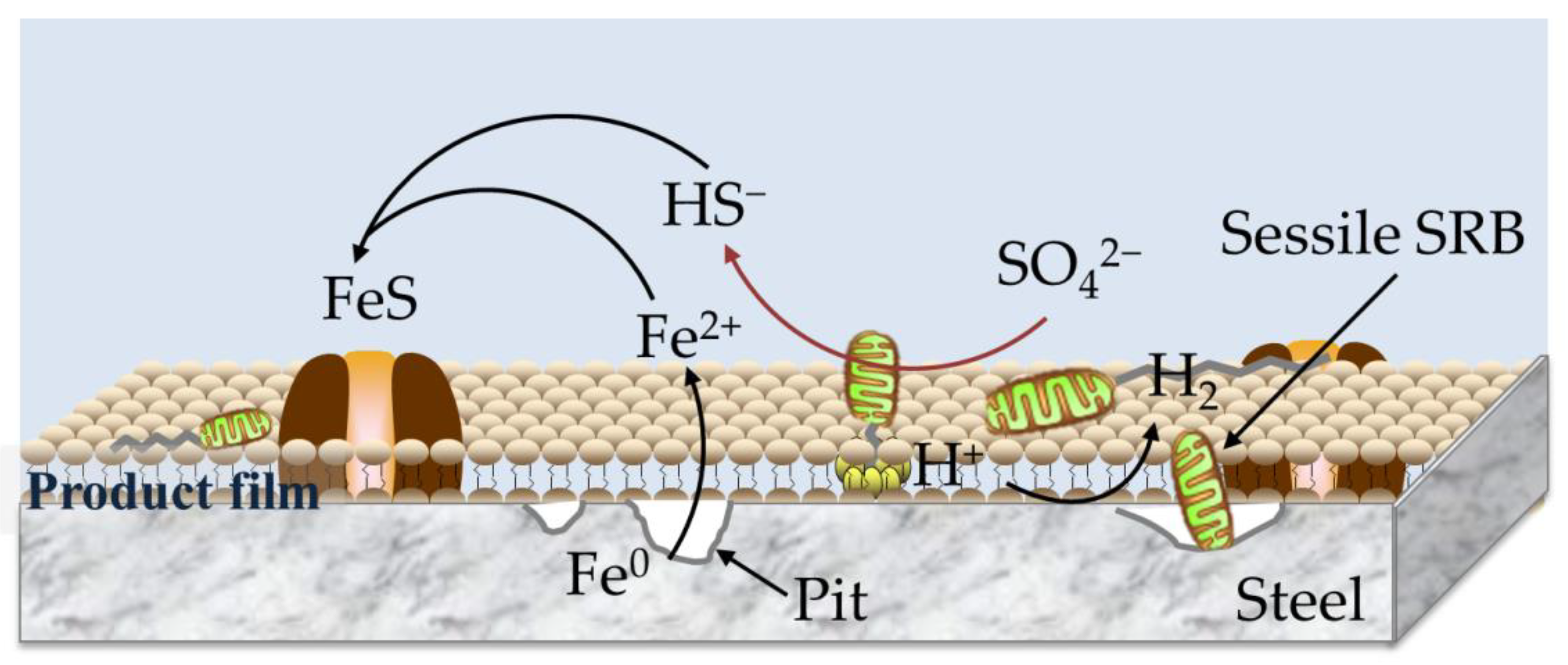
4. Discussion
5. Conclusion
- (1)
- The biofilm distributed on the specimen surface, and the biofilm thickness was 20 μm after 14 days.
- (2)
- Both the SRB activity and metabolite significantly affect the corrosion behavior of X80 steel. SRB can form a biofilm on the steel surface in the inoculated soil solution and play a vital role in the corrosion of X80 steel.
- (3)
- EIS results indicate that the corrosion of steel is inhibited by the activity of SRB in the first 4 days and enhanced in the rest of the experiment.
- (4)
- Localized corrosion dominates in the soil solution, and pitting corrosion of steel is severe in the inoculated soil solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Y.; Cheng, Y.F. An intelligent coating doped with inhibitor-encapsulated nanocontainers for corrosion protection of pipeline steel. Chem. Eng. J. 2017, 315, 537–551. [Google Scholar] [CrossRef]
- Wei, B.X.; Pang, J.Y.; Xu, J.; Sun, C.; Zhang, H.W.; Wang, Z.Y.; Ke, W. Microbiologically influenced corrosion of TiZrNb medium-entropy alloys by Desulfovibrio desulfuricans. J. Alloys Compd. 2021, 875, 160020. [Google Scholar] [CrossRef]
- Xu, D.K.; Huang, W.; Ruschau, G.; Hornemann, J.; Wen, J.; Gu, T.Y. Laboratory investigation of MIC threat due to hydrotest using untreated seawater and subsequent exposure to pipeline fluids with and without SRB spiking. Eng. Fail. Anal. 2013, 28, 149–159. [Google Scholar] [CrossRef]
- Starosvetsky, J.; Starosvetsky, D.; Armon, R. Identification of microbiologically influenced corrosion (MIC) in industrial equipment failures. Eng. Fail. Anal. 2007, 14, 1500–1511. [Google Scholar] [CrossRef]
- Hou, B.R. The Cost of Corrosion in China; Science Press: Beijing, China, 2019. [Google Scholar]
- Huang, L.Y.; Huang, Y.; Lou, Y.T.; Qian, H.C.; Xu, D.K.; Ma, L.W.; Jiang, C.Y.; Zhang, D.W. Pyocyanin-modifying genes phzM and phzS regulated the extracellular electron transfer in microbiologically-influenced corrosion of X80 carbon steel by Pseudomonas aeruginosa. Corros. Sci. 2020, 164, 108355. [Google Scholar] [CrossRef]
- Brooks, W. Microbiologically–influenced corrosion Riviera Park case study. In Corrosion; NACE International: Houston, TX, USA, 2013. [Google Scholar]
- Tambe, S.P.; Jagtap, S.D.; Chaurasiya, A.K.; Joshi, K.K. Evaluation of microbial corrosion of epoxy coating by using sulphate reducing bacteria. Prog. Org. Coat. 2016, 94, 49–55. [Google Scholar] [CrossRef]
- Sarioǧlu, F.; Javaherdashti, R.; Aksöz, N. Corrosion of a drilling pipe steel in an environment containing sulphate-reducing bacteria. Int. J. Pres. Ves. Pip. 1997, 73, 127–131. [Google Scholar] [CrossRef]
- Bhat, S.; Sharma, V.K.; Thomas, S.; Anto, P.F.; Singh, S.K. 8-in pipeline from group gathering station to Central Tank Farm. Mater Perform. 2011, 50, 50–53. [Google Scholar]
- Abedi, S.S.; Abdolmaleki, A.; Adibi, N. Failure analysis of SCC and SRB induced cracking of a transmission oil products pipeline. Eng. Fail. Anal. 2007, 14, 250–261. [Google Scholar]
- Liu, H.W.; Cheng, Y.F. Mechanistic aspects of microbially influenced corrosion of X52 pipeline steel in a thin layer of soil solution containing sulphate-reducing bacteria under various gassing conditions. Corros. Sci. 2018, 133, 178–189. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.X.; Sun, C.; Wang, F.H.; Li, X.M.; Yang, J.; Yu, C.K. The effects of sulfate reducing bacteria on corrosion of carbon steel Q235 under simulated disbonded coating by using electrochemical impedance spectroscopy. Corros. Sci. 2011, 53, 1554–1562. [Google Scholar] [CrossRef]
- Wei, B.X.; Xu, J.; Fu, Q.; Qin, Q.Y.; Bai, Y.L.; Sun, C.; Wang, C.; Wang, Z.Y.; Ke, W. Effect of sulfate-reducing bacteria on corrosion of X80 pipeline steel under disbonded coating in a red soil solution. J. Mater. Sci. Technol. 2021, 87, 1–17. [Google Scholar] [CrossRef]
- Von Wolzogen Khur, C.A.H.; van der Vlugt, L.S. De grafiteering van gietijzeralselectro biochemichproces in anaerobe Gronden. Water 1934, 18, 147–165. [Google Scholar]
- AlAbbas, F.M.; Bhola, R.; Spear, J.R.; Olson, D.L.; Mishra, B. Electrochemical characterization of microbiologically influenced corrosion on linepipe steel exposed to facultative anaerobic Desulfovibrio sp. Int. J. Electrochem. Sci. 2013, 8, 859–871. [Google Scholar]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef]
- Iverson, W.P. Biological corrosion. In Advances in Corrosion Science and Technology; Fontana, M.G., Staehle, R.W., Eds.; Springer: Boston, MA, USA, 1972; pp. 1–42. [Google Scholar]
- Dong, Z.H.; Liu, T.; Liu, H.F. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling 2011, 27, 487–495. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeterior. Biodegrad. 2009, 63, 896–900. [Google Scholar] [CrossRef]
- ZDong, H.; Shi, W.; Ruan, H.M.; Zhang, G.A. Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique. Corros. Sci. 2011, 53, 2978–2987. [Google Scholar]
- Gu, T.Y.; Jia, R.; Unsal, T.; Xu, D.K. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Xu, D.K.; Gu, T. Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm. Int. Biodeterior. Biodegrad. 2014, 91, 74–81. [Google Scholar] [CrossRef]
- Liu, H.W.; Cheng, Y.F. Mechanism of microbiologically influenced corrosion of X52 pipeline steel in a wet soil containing sulfate-reduced bacteria. Electrochim. Acta 2017, 253, 368–378. [Google Scholar] [CrossRef]
- Daia, M.J.; Liu, J.; Huang, F.; Zhang, Y.H.; Cheng, Y.F. Effect of cathodic protection potential fluctuations on pitting corrosion of X100 pipeline steel in acidic soil environment. Corros. Sci. 2018, 143, 428–437. [Google Scholar] [CrossRef]
- Yan, M.C.; Sun, C.; Xu, J.; Ke, W. Anoxic corrosion behavior of pipeline steel in acidic soils. Ind. Eng. Chem. Res. 2014, 53, 17615–17624. [Google Scholar] [CrossRef]
- Yan, M.C.; Sun, C.; Xu, J.; Dong, J.H.; Ke, W. Role of Fe oxides in corrosion of pipeline steel in a red clay soil. Corros. Sci. 2014, 80, 309–317. [Google Scholar] [CrossRef]
- Wei, B.X.; Qin, Q.Y.; Fu, Q.; Bai, Y.L.; Xu, J.; Yu, C.; Sun, C.; Ke, W. X80 steel corrosion induced by alternating current in water-saturated acidic soil. Corrosion 2020, 76, 248–267. [Google Scholar] [CrossRef]
- Sun, C.; Xu, J.; Wang, F.H.; Yu, C.K. Effect of sulfate reducing bacteria on corrosion of stainless steel 1Cr18Ni9Ti in soils containing chloride ions. Mater Chem. Phys. 2011, 126, 330–336. [Google Scholar] [CrossRef]
- Wu, T.Q.; Yan, M.C.; Yu, L.B.; Zhao, H.; Sun, C.; Yin, F.C.; Ke, W. Stress corrosion of pipeline steel under disbonded coating in a SRB-containing environment. Corros. Sci. 2019, 157, 518–530. [Google Scholar] [CrossRef]
- Liu, H.W.; Xu, D.; Yang, K.; Liu, H.F.; Cheng, Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Zhou, E.Z.; Li, H.; Yang, C.; Wang, J.; Xu, D.K.; Zhang, D.W.; Gu, T.Y. Accelerated corrosion of 2304 duplex stainless steel by marine Pseudomonas aeruginosa biofilm. Int. Biodeterior. Biodegrad. 2018, 127, 1–9. [Google Scholar] [CrossRef]
- Wu, T.Q.; Jin, X.; Yan, M.C.; Cheng, S.; Yu, C.K.; Wei, K. Synergistic effect of sulfate-reducing bacteria and elastic stress on corrosion of X80 steel in soil solution. Corros. Sci. 2014, 83, 38–47. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M.A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina. Chem. Mater. 1990, 2, 186–191. [Google Scholar] [CrossRef]
- Zheng, B.; Li, K.; Liu, H.; Gu, T. Effects of magnetic fields on microbiologically influenced corrosion of 304 stainless steel. Ind. Eng. Chem. Res. 2013, 53, 48–54. [Google Scholar] [CrossRef]
- Li, Y.; Feng, S.; Liu, H.; Tian, X.; Xia, Y.; Li, M.; Xu, K.; Yu, H.; Liu, Q.; Chen, C. Bacterial distribution in SRB biofilm affects MIC pitting of carbon steel studied using FIB-SEM. Corros. Sci. 2020, 167, 108512. [Google Scholar] [CrossRef]
- Zuo, R.; Kus, E.; Mansfeld, F.; Wood, T.K. The importance of live biofilms in corrosion protection. Corros. Sci. 2005, 47, 279–287. [Google Scholar] [CrossRef]
- Michael, D.W. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166. [Google Scholar]
- Yan, M.C.; Sun, C.; Xu, J.; Wu, T.Q.; Yang, S.; Ke, W. Stress corrosion of pipeline steel under occluded coating disbondment in a red soil environment. Corros. Sci. 2015, 93, 27–38. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, A.; Li, Q.; Kong, X.; Ji, G. Acidity regime of the red soils in a subtropical region of southern China under field conditions. Geoderma 2003, 115, 75–84. [Google Scholar] [CrossRef]
- Li, H.W.; Dake, X.K.; Yingchao, L.; Hao, F.; Zhiyong, L.; Xiaogang, L.; Tingyue, G.; Ke, Y.; Zhang, W. Extracellular electron transfer is a bottleneck in the microbiologically influenced corrosion of C1018 carbon steel by the biofilm of sulfate-reducing bacterium Desulfovibrio vulgaris. PLoS ONE 2015, 10, e0136183. [Google Scholar]
- Fatah, M.C.; Ismail, M.C.; Wahjoedi, B.A. Effects of sulphide ion on corrosion behaviour of X52 steel in simulated solution containing metabolic products species: A study pertaining to microbiologically influenced corrosion (MIC). Corros. Eng. Sci. Techn. 2013, 48, 211–220. [Google Scholar] [CrossRef]
- Castaneda, H.; Benetton, X.D. SRB-biofilm influence in active corrosion sites formed at the steel-electrolyte interface when exposed to artificial seawater conditions. Corros. Sci. 2008, 50, 1169–1183. [Google Scholar] [CrossRef]
- Wei, B.X.; Qin, Q.Y.; Bai, Y.L.; Yu, C.K.; Xu, J.; Sun, C.; Ke, W. Short-period corrosion of X80 pipeline steel induced by AC current in acidic red soil. Eng. Fail. Anal. 2019, 105, 156–175. [Google Scholar] [CrossRef]
- Wu, T.Q.; Yan, M.C.; Zeng, D.C.; Xu, J.; Yu, C.K.; Sun, C.; Ke, W. Microbiologically induced corrosion of X80 pipeline steel in a near-neutral pH soil solution. Acta. Metall. Sin. Engl. 2014, 28, 93–102. [Google Scholar] [CrossRef]


| C | Mn | Si | P | S | Cr | Ni | Cu |
| 0.07 | 1.82 | 0.19 | 0.007 | 0.023 | 0.026 | 0.17 | 0.02 |
| Al | Mo | Ti | Nb | V | N | B | Fe |
| 0.028 | 0.23 | 0.012 | 0.056 | 0.002 | 0.004 | 0.0001 | Bal. |
| pH | Ca2+ | Mg2+ | K+ | Na+ | NO3− | Cl− | SO42− | HCO3− |
|---|---|---|---|---|---|---|---|---|
| 4.5 | 5 | 2 | 4 | 8 | 4 | 9 | 10 | 11 |
| Positions | Element (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Al | Si | P | S | Fe | |
| A | 7.41 | 23.45 | 0.12 | 0.68 | 6.53 | – | 61.71 |
| B | 17.53 | 26.94 | 0.03 | 0.51 | 13.09 | 1.83 | 40.38 |
| Time (day) | Sterile | SRB-Inoculated | ||||||
|---|---|---|---|---|---|---|---|---|
| Rs (Ω·cm2) | Qdl | Rct (Ω·cm2) | Rs (Ω·cm2) | Qdl | Rct (Ω·cm2) | |||
| Y0/S sn·cm−2 | n | Y0/S sn·cm−2 | n | |||||
| 1 | 137.2 | 1.017 × 10−4 | 0.913 | 9318 | 129.8 | 4.862 × 10−4 | 0.791 | 13,950 |
| 4 | 137.7 | 1.423 × 10−4 | 0.903 | 6754 | 126.7 | 9.03 × 10−4 | 0.902 | 10,810 |
| 7 | 101.3 | 2.964 × 10−4 | 0.786 | 6699 | 110.1 | 3.173 × 10−4 | 0.866 | 3836 |
| 10 | 217.4 | 1.582 × 10−4 | 0.789 | 5533 | 146 | 1.782 × 10−4 | 0.81 | 2678 |
| 14 | 147.3 | 1.425 × 10−4 | 0.851 | 4640 | 190.9 | 2.409 × 10−4 | 0.746 | 1467 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wei, B.; Xu, X. Effect of Sulfate-Reducing Bacteria (SRB) on the Corrosion of Buried Pipe Steel in Acidic Soil Solution. Coatings 2021, 11, 625. https://doi.org/10.3390/coatings11060625
Chen L, Wei B, Xu X. Effect of Sulfate-Reducing Bacteria (SRB) on the Corrosion of Buried Pipe Steel in Acidic Soil Solution. Coatings. 2021; 11(6):625. https://doi.org/10.3390/coatings11060625
Chicago/Turabian StyleChen, Lijuan, Bo Wei, and Xianghong Xu. 2021. "Effect of Sulfate-Reducing Bacteria (SRB) on the Corrosion of Buried Pipe Steel in Acidic Soil Solution" Coatings 11, no. 6: 625. https://doi.org/10.3390/coatings11060625
APA StyleChen, L., Wei, B., & Xu, X. (2021). Effect of Sulfate-Reducing Bacteria (SRB) on the Corrosion of Buried Pipe Steel in Acidic Soil Solution. Coatings, 11(6), 625. https://doi.org/10.3390/coatings11060625






