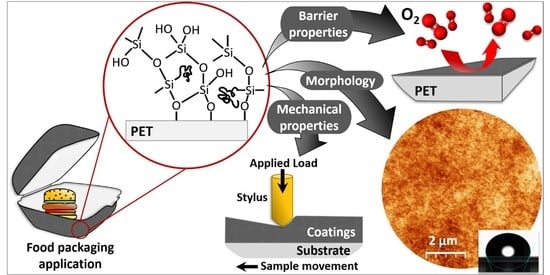
Structural and Functional Properties of Fluorinated Silica Hybrid Barrier Layers on Flexible Polymeric Foil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sol Preparation
2.3. Layer Fabrication
2.4. Methods of Layer Characterization
3. Results
3.1. Chemical Composition (FTIR Spectroscopy)
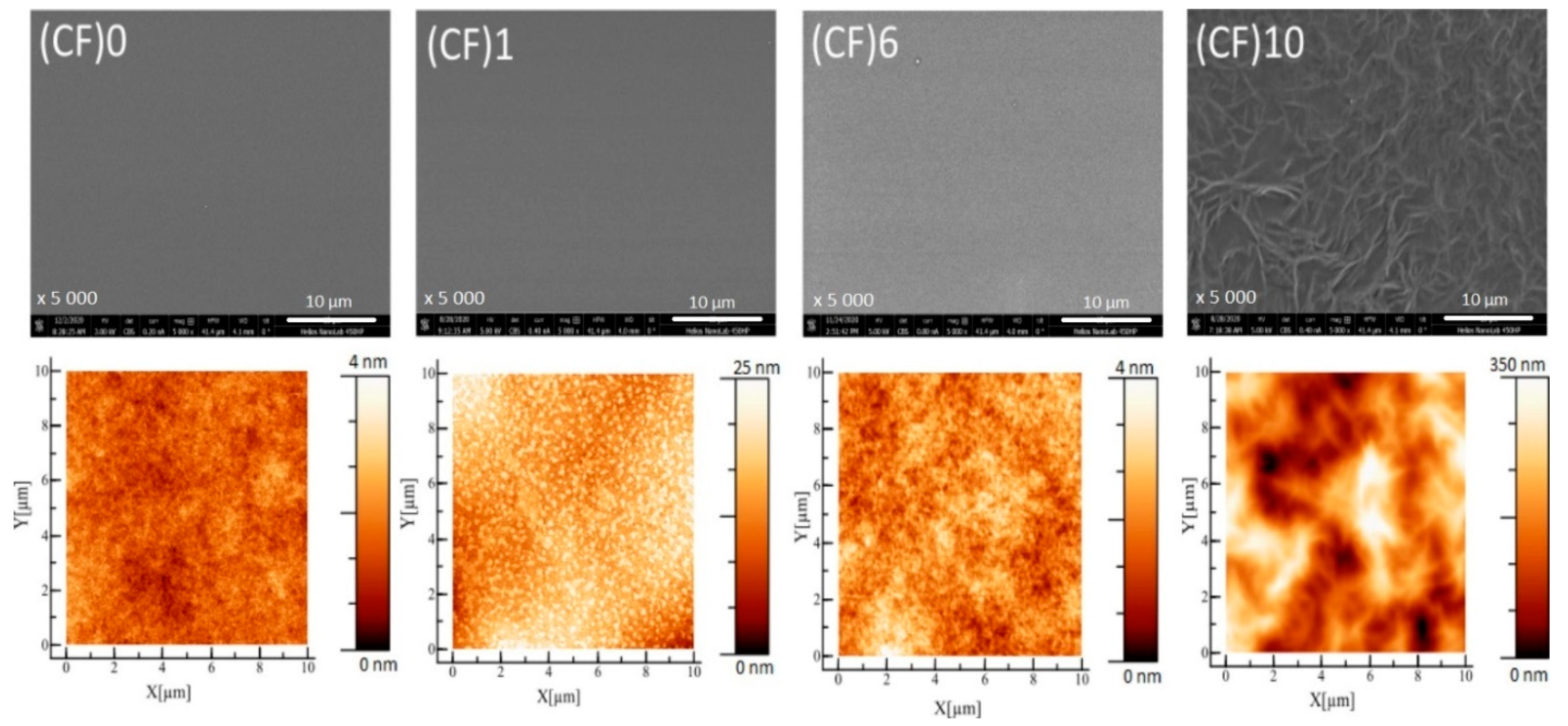
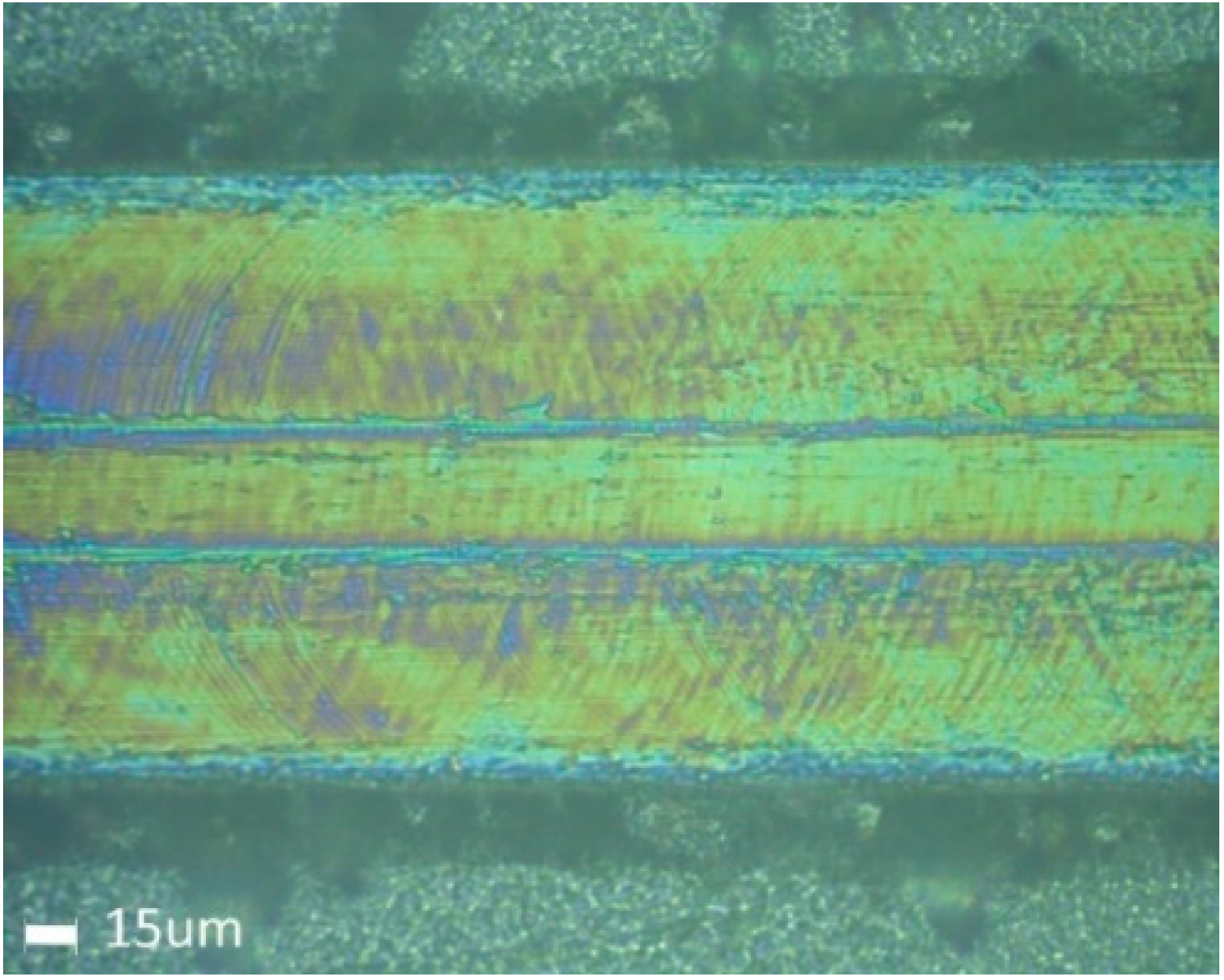
3.2. Microscopy Imaging
3.3. Wettability and Surface Energy Properties
3.4. TMA Results
3.5. Oxygen Permeability
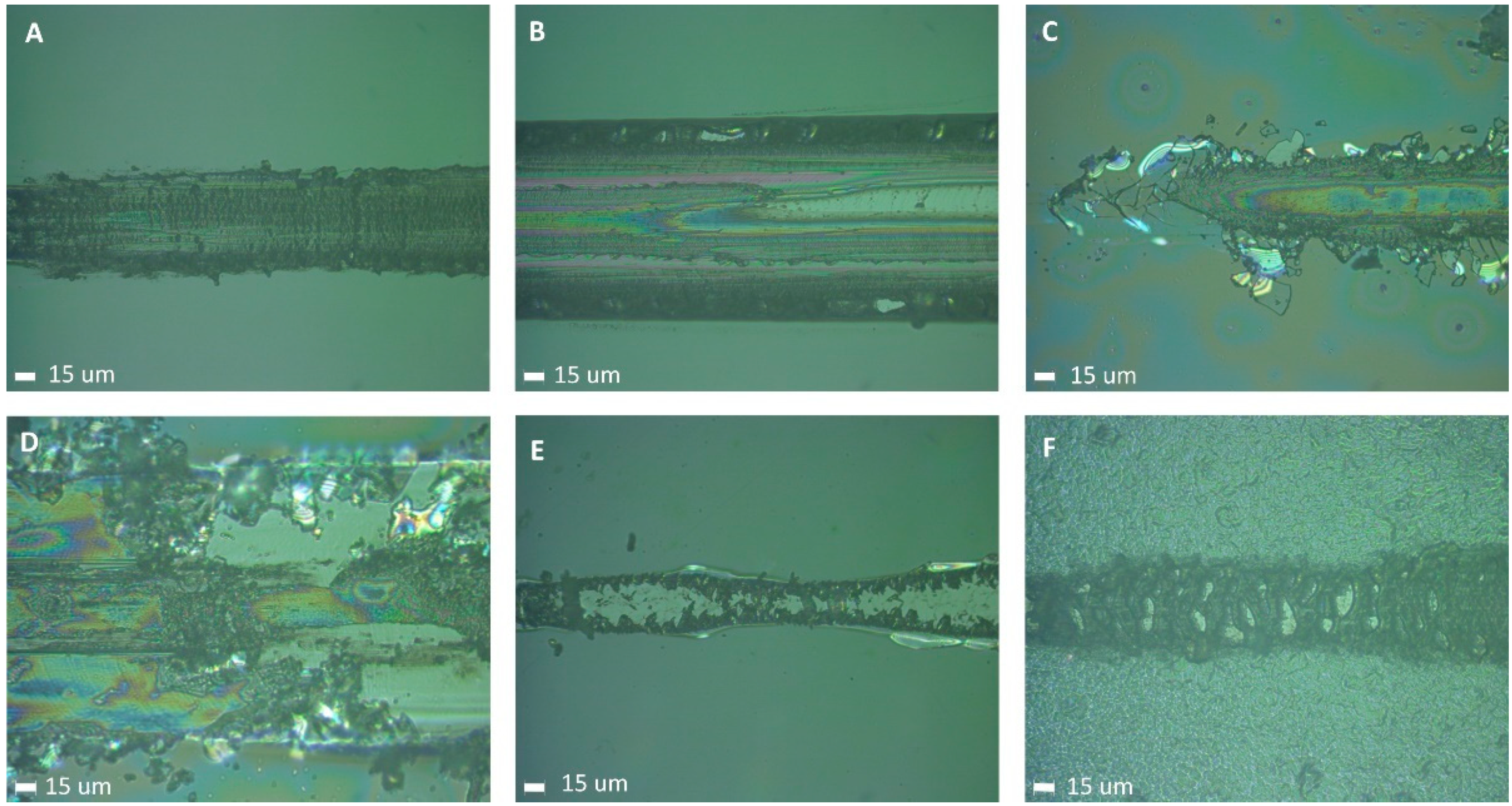
3.6. Adhesion and Scratch Resistance
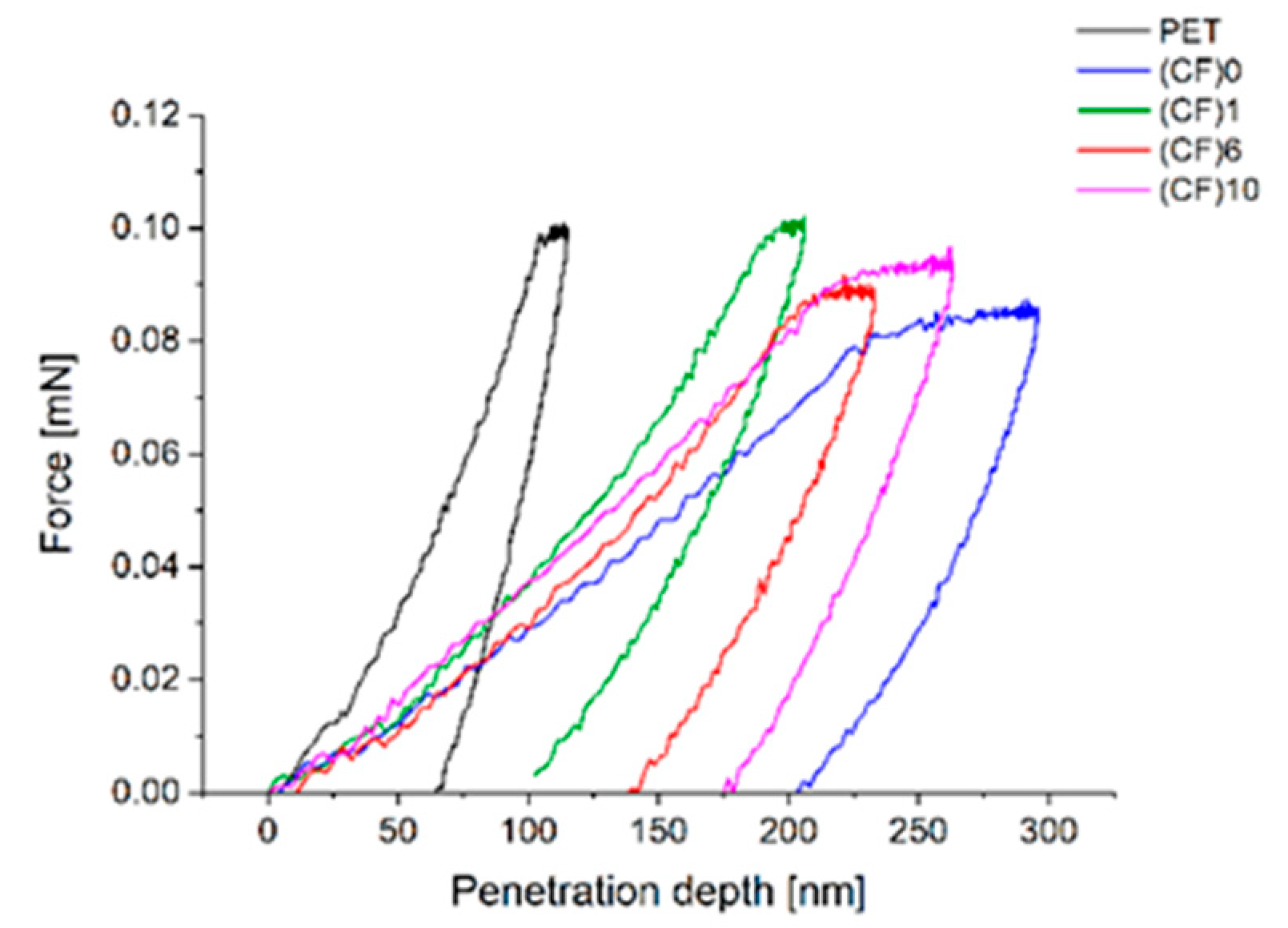
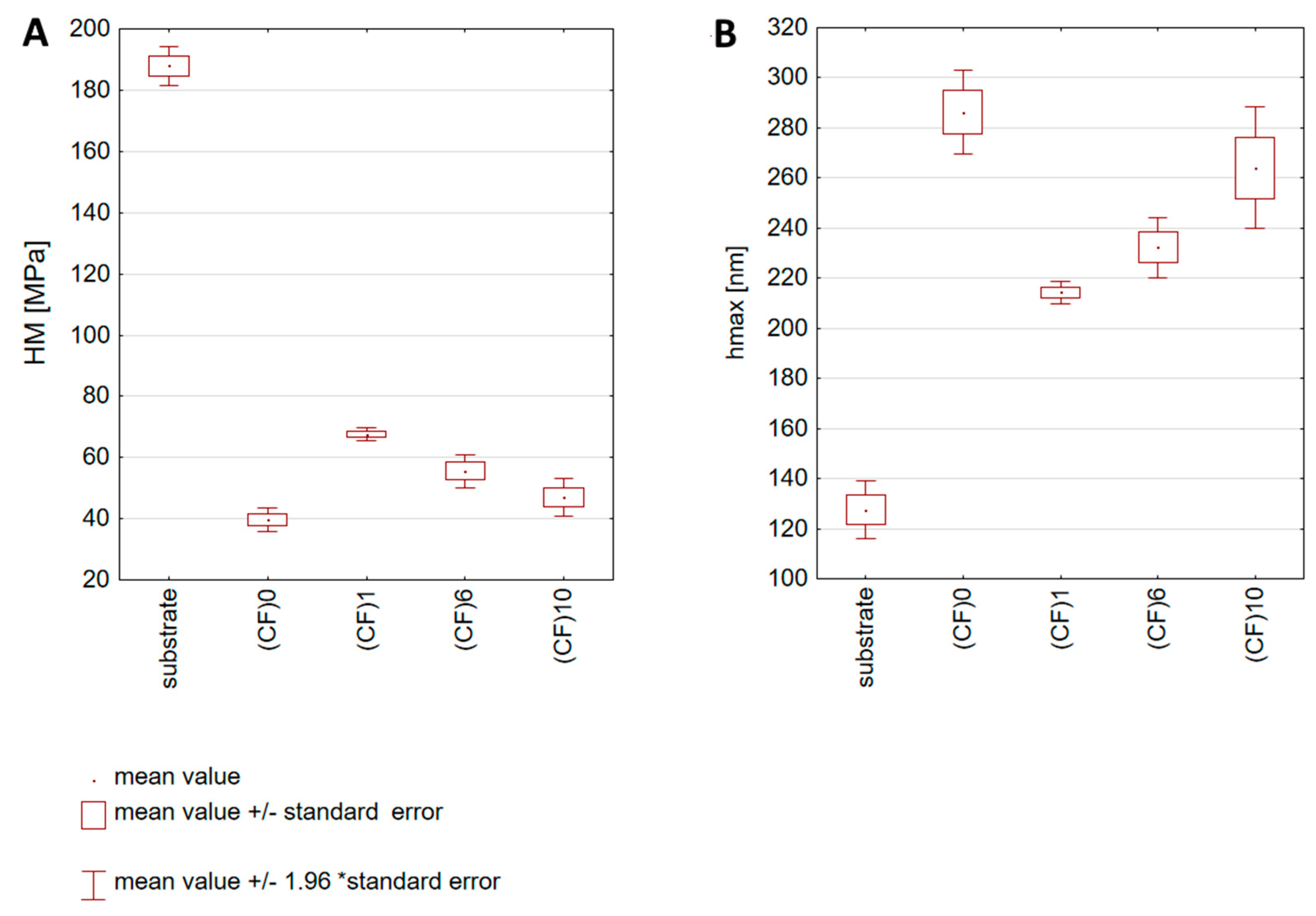
3.7. Nanoindentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Economic Forum; Ellen MacArthur Foundation; McKinsey Company. The New Plastics Economy: Rethinking the Future of Plastics. 2016. Available online: https://www.ellenmacarthurfoundation.org/publications/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 23 March 2021).
- Katiyar, V.; Gaur, S.S.; Pal, A.K.; Kumar, A. Properties of plastics for packaging applications. In Polymers for Packaging Applications; Alavi, S., Thomas, S., Sandeep, K.P., Kalarikkal, N., Varghese, J., Yaragalla, S., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2014; pp. 3–5. [Google Scholar]
- Kumar, R.; Münstedt, H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 2005, 26, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of titanium dioxide and zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef]
- Bautista, L.; Molina, L.; Niembro, S.; García, J.M.; López, J.; Vílchez, A. Coatings and inks for food packaging including nanomaterials. In Emerging Nanotechnologies in Food Science; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 149–173. [Google Scholar]
- Rovera, C.; Ghaani, M.; Farris, S. Nano-inspired oxygen barrier coatings for food packaging applications: An overview. Trends Food Sci. Technol. 2020, 97, 210–220. [Google Scholar] [CrossRef]
- Chatham, H. Oxygen diffusion barrier properties of transparent oxide coatings on polymeric substrates. Surf. Coat. Technol. 1996, 78, 1–9. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A. Free volume and mass transport in polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 132–140. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef] [PubMed]
- Massey, L.K. Permeability Properties of Plastics and Elastomers; Plastics Design Library/William Andrew Publishing: Norwich, CT, USA, 2003; pp. 1–18. [Google Scholar]
- Rezaei, M.; Mohseni, M.; Yahyaei, H. A study on water and oxygen permeability of BOPP coated with hybrid UV cured nanocoatings. Prog. Org. Coat. 2016, 99, 72–79. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An overview of paper and paper based food packaging materials: Health safety and environmental concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of polymer-based multilayer packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Morris, B.A. The Science and Technology of Flexible Packaging: Multilayer Films from Resin and Process to End Use, 1st ed.; Wiliam Andrew: Oxford, UK, 2017; pp. 104–115. [Google Scholar]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- NanoPack; NanoSealTM. Barrier Coating, OPP Barrier Coated Film, Polypropylene Film, Liquid Coating. Available online: http://www.nanopackinc.com/products.asp (accessed on 1 March 2021).
- Saes Coated Films. Trasparent Oxygen Barrier. Available online: https://www.saescoatedfilms.com/en/transparent-oxygen/ (accessed on 1 March 2021).
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Farris, S.; Introzzi, L.; Fuentes-Alventosa, J.M.; Santo, N.; Rocca, R.; Piergiovanni, L. Self-assembled pullulan–silica oxygen barrier hybrid coatings for food packaging applications. J. Agric. Food Chem. 2012, 60, 782–790. [Google Scholar] [CrossRef]
- Nicole, L.; Rozes, L.; Sanchez, C. Integrative approaches to hybrid multifunctional materials: From multidisciplinary research to applied technologies. Adv. Mater. 2010, 22, 3208–3214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Lin, T. Robust, Self-healing superamphiphobic fabrics prepared by two-step coating of fluoro-containing polymer, fluoroalkyl silane, and modified silica nanoparticles. Adv. Funct. Mater. 2013, 23, 1664–1670. [Google Scholar] [CrossRef]
- Wang, S.; Ye, X.; Zhang, H.; Qian, Z.; Li, Q.; Wu, Z.; Li, S. Superhydrophobic silane/fluorinated Attapulgite@SiO2 composite coatings on magnesium alloy for corrosion protection. ChemistrySelect 2020, 5, 10329–10338. [Google Scholar] [CrossRef]
- Oldani, V.; Sergi, G.; Pirola, C.; Sacchi, B.; Bianchi, C.L. Sol-gel hybrid coatings containing silica and a perfluoropolyether derivative with high resistance and anti-fouling properties in liquid media. J. Fluor. Chem. 2016, 188, 43–49. [Google Scholar] [CrossRef]
- Arianpour, F.; Farhadi, S.; Farzaneh, M. Effect of heterogeneity on hydro/ice-phobic properties of alkylsilane/fluoro-alkylsilane-based coatings on Al substrates. J. Coat. Technol. Res. 2017, 14, 267–275. [Google Scholar] [CrossRef]
- Ke, C.; Zhang, C.; Wu, X.; Jiang, Y. Highly transparent and robust superhydrophobic coatings fabricated via a facile sol-gel process. Thin Solid Films 2021, 723, 138583. [Google Scholar] [CrossRef]
- Hu, J.; Fang, Z.; Huang, Y.; Lu, J. Fabrication of superhydrophobic surfaces based on fluorosilane and TiO2/SiO2 nanocomposites. Surf. Eng. 2021, 37, 271–277. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Tieu, A.K.; Wan, S.; Zhu, H.; Pham, S.T.; Johnston, B. Surface characteristics and wettability of superhydrophobic silanized inorganic glass coating surfaces textured with a picosecond laser. Appl. Surf. Sci. 2021, 537, 147808. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Startek, K.; Marczak, J.; Lukowiak, A. Oxygen barrier enhancement of polymeric foil by sol–gel-derived hybrid silica layers. Polymer 2020, 195, 122437. [Google Scholar] [CrossRef]
- Babonneau, F.; Thorne, K.; MacKenzie, J.D. Dimethyldiethoxysilane/tetraethoxysilane copolymers: Precursors for the silicon-carbon-oxygen system. Chem. Mater. 1989, 1, 554–558. [Google Scholar] [CrossRef]
- Kim, M.T. Deposition behavior of hexamethydisiloxane films based on the FTIR analysis of Si–O–Si and Si–CH3 bonds. Thin Solid Films 1997, 311, 157–163. [Google Scholar] [CrossRef]
- Almeida, R.M.; Marques, A.C. Characterization of sol–gel materials by infrared spectroscopy. In Handbook of Sol-Gel Science and Technology; J.B. Metzler: Stuttgart, Germany, 2018; pp. 1121–1151. [Google Scholar]
- Li, Y.; Men, X.; Zhu, X.; Ge, B.; Chu, F.; Zhang, Z. One-step spraying to fabricate nonfluorinated superhydrophobic coatings with high transparency. J. Mater. Sci. 2016, 51, 2411–2419. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Sanz, J. Polymerization of hybrid organic–inorganic materials from several silicon compounds followed by TGA/DTA, FTIR and NMR techniques. Prog. Org. Coat. 2014, 77, 880–891. [Google Scholar] [CrossRef]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F. Synthesis and characterization of micro-mesoporous MCM-41 using various ionic liquids as co-templates. Microporous Mesoporous Mater. 2015, 217, 219–224. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. The defect structure of sol–gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non-Cryst. Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Chen, X.; Wen, S.; Feng, T.; Yuan, X. High solids organic-inorganic hybrid coatings based on silicone-epoxy-silica coating with improved anticorrosion performance for AA2024 protection. Prog. Org. Coat. 2020, 139, 105374. [Google Scholar] [CrossRef]
- Latthe, S.S.; Imai, H.; Ganesan, V.; Rao, A.V. Superhydrophobic silica films by sol–gel co-precursor method. Appl. Surf. Sci. 2009, 256, 217–222. [Google Scholar] [CrossRef]
- Purcar, V.; Rădițoiu, V.; Rădițoiu, A.; Manea, R.; Raduly, F.M.; Ispas, G.C.; Frone, A.N.; Nicolae, C.A.; Gabor, R.A.; Anastasescu, M.; et al. Preparation and characterization of some sol-gel modified silica coatings deposited on polyvinyl chloride (PVC) substrates. Coatings 2020, 11, 11. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X. Fabrication and characterization of stable superhydrophobic fluorinated-polyacrylate/silica hybrid coating. Appl. Surf. Sci. 2014, 298, 214–220. [Google Scholar] [CrossRef]
- Jeong, K.-M.; Park, S.S.; Nagappan, S.; Min, G.; Zhang, Y.; Qu, M.; Zhang, Y.; Ha, C.-S. Highly transparent, organic-inorganic hybrid UV-curable coating materials with amphiphobic characteristics. Prog. Org. Coat. 2019, 134, 323–332. [Google Scholar] [CrossRef]
- Brassard, J.-D.; Sarkar, D.K.; Perron, J. Synthesis of monodisperse fluorinated silica nanoparticles and their superhydrophobic thin films. ACS Appl. Mater. Interfaces 2011, 3, 3583–3588. [Google Scholar] [CrossRef]
- Kazancioglu, M.; Lehman, R.; Hara, M. Inorganic silicate polymers made directly from silica using ionic liquid as ionic plasticizer: Monovalent-only system. Mater. Today Commun. 2020, 24, 101058. [Google Scholar] [CrossRef]
- Bley, O.; Siepmann, J.; Bodmeier, R. Importance of glassy-to-rubbery state transitions in moisture-protective polymer coatings. Eur. J. Pharm. Biopharm. 2009, 73, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Carja, I.-D.; Serbezeanu, D.; Bruma, M.; Ronova, I.A.; Belomoina, N.M. Influence of conformational parameters on physical properties of copolyimides containing pendant diphenylphosphine oxide units. Struct. Chem. 2016, 27, 1465–1477. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Ghazzal, M.N.; Kebaili, H.; Joseph, M.; De Coninck, J.; Gaigneaux, E.M. Tuning the selectivity and sensitivity of mesoporous dielectric multilayers by modifiying the hydrophobic–hydrophilic balance of the silica layer. J. Mater. Chem. 2012, 22, 22526–22532. [Google Scholar] [CrossRef]
- Li, Y.-S.; Vecchio, N.E.; Lu, W. Infrared and Raman spectra of (3,3,3-trifluoropropyl)trimethoxysilane, its sol and xerogel. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 213–217. [Google Scholar] [CrossRef]
- Fukumi, A.; Shimoaka, T.; Shioya, N.; Nagai, N.; Hasegawa, T. Infrared active surface modes found in thin films of perfluoroalkanes reveal the dipole–dipole interaction and surface morphology. J. Chem. Phys. 2020, 153, 044703. [Google Scholar] [CrossRef] [PubMed]
- Surface Energy Data for PTFE. Polytetrafluoroethylene, CAS # 9002-84-0 Source. Direct, 2005, 1950 (9). Available online: https://www.accudynetest.com (accessed on 1 March 2021).
- Agustín-Sáenz, C.; Machado, M.; Tercjak, A. Polyfluoroalkyl-silica porous coatings with high antireflection properties and low surface free energy for glass in solar energy application. Appl. Surf. Sci. 2020, 509, 144864. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, A.M.; Ueda, Y. The lowest surface free energy based on –CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Basu, B.J.; Kumar, V.D.; Anandan, C. Surface studies on superhydrophobic and oleophobic polydimethylsiloxane–silica nanocomposite coating system. Appl. Surf. Sci. 2012, 261, 807–814. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, Y.; Zhang, H.; Yan, H.; Lv, H.; Jiang, B. Sol–gel preparation of hydrophobic silica antireflective coatings with low refractive index by base/acid two-step catalysis. ACS Appl. Mater. Interfaces 2014, 6, 11470–11475. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Cao, Y.; Su, Y.; Hui, H.; Shen, J. Surface free energy and microstructure dependent environmental stability of sol–gel SiO2 antireflective coatings: Effect of combined vapor phase surface treatment. J. Colloid Interface Sci. 2019, 555, 124–131. [Google Scholar] [CrossRef]
- Miller, J.D.; Veeramasuneni, S.; Drelich, J.; Yalamanchili, M.R.; Yamauchi, G. Effect of roughness as determined by atomic force microscopy on the wetting properties of PTFE thin films. Polym. Eng. Sci. 1996, 36, 1849–1855. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Roughness optimization for biomimetic superhydrophobic surfaces. Microsyst. Technol. 2005, 11, 535–549. [Google Scholar] [CrossRef]
- Szczurek, A.; Babiarczuk, B.; Kubacki, J.; Papin, P.; Renault, P.; Żak, A.; Kaleta, J.; Krzak, J. Sol-gel multilayered coatings for reduction of H2 permeation. Appl. Surf. Sci. 2019, 497, 143691. [Google Scholar] [CrossRef]
- Adak, D.; Bhattacharyya, R.; Saha, H.; Maiti, P.S. Sol-gel processed silica based highly transparent self-cleaning coatings for solar glass covers. Mater. Today Proc. 2020, 33, 2429–2433. [Google Scholar] [CrossRef]
- Bartosik, M.; Holec, D.; Apel, D.; Klaus, M.; Genzel, C.; Keckes, J.; Arndt, M.; Polcik, P.; Koller, C.; Mayrhofer, P. Thermal expansion of Ti-Al-N and Cr-Al-N coatings. Scr. Mater. 2017, 127, 182–185. [Google Scholar] [CrossRef]
- Bragaglia, M.; Cacciotti, I.; Cherubini, V.; Nanni, F. Influence of organic modified silica coatings on the tribological properties of elastomeric compounds. Wear 2019, 434–435, 202987. [Google Scholar] [CrossRef]
- Da Silva, L. Improving bonding at high and low temperatures. In Advances in Structural Adhesive Bonding; Elsevier: Amsterdam, The Netherlands, 2010; pp. 516–546. [Google Scholar]
- Li, C.; Yang, W.; Wang, M.; Yu, X.; Fan, J.; Xiong, Y.; Yang, Y.; Li, L. A review of coating materials used to improve the performance of optical fiber sensors. Sensors 2020, 20, 4215. [Google Scholar] [CrossRef] [PubMed]
- Consolati, G. On the thermal expansion of nanohole free volume in perfluoropolyethers. J. Phys. Chem. B 2005, 109, 10096–10099. [Google Scholar] [CrossRef] [PubMed]
- Recio, R.; Lozano, Á.E.; Prádanos, P.; Marcos, Á.; Tejerina, F.; Hernández, A. Effect of fractional free volume and Tg on gas separation through membranes made with different glassy polymers. J. Appl. Polym. Sci. 2007, 107, 1039–1046. [Google Scholar] [CrossRef]
- Goswami, S.; Ghosh, R.; Neog, A.; Das, B. Deep learning based approach for prediction of glass transition temperature in polymers. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Debot, A.; White, R.P.; Lipson, J.E.G.; Napolitano, S. Experimental test of the cooperative free volume rate model under 1D confinement: The interplay of free volume, temperature, and polymer film thickness in driving segmental mobility. ACS Macro Lett. 2018, 8, 41–45. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorova, L.M.; Yakushev, P.N.; Pissis, P.; Sysel, P.; Brozova, L. Molecular dynamics in nanostructured polyimide-silica hybrid materials and their thermal stability. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1056–1069. [Google Scholar] [CrossRef]
- Afzal, A.; Siddiqi, H.M. A comprehensive study of the bicontinuous epoxy-silica hybrid polymers: I. Synthesis, characterization and glass transition. Polymer 2011, 52, 1345–1355. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Guida, P.; Savio, R.L.; Repetto, L.; Valbusa, U. Gas permeation through rubbery polymer nano-corrugated membranes. Sci. Rep. 2018, 8, 6345. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, T.; Zhang, J.; Xu, X.; Yin, Q.; Han, J.; Wei, M. Hybrid films with excellent oxygen and water vapor barrier properties as efficient anticorrosive coatings. RSC Adv. 2018, 8, 21651–21657. [Google Scholar] [CrossRef]
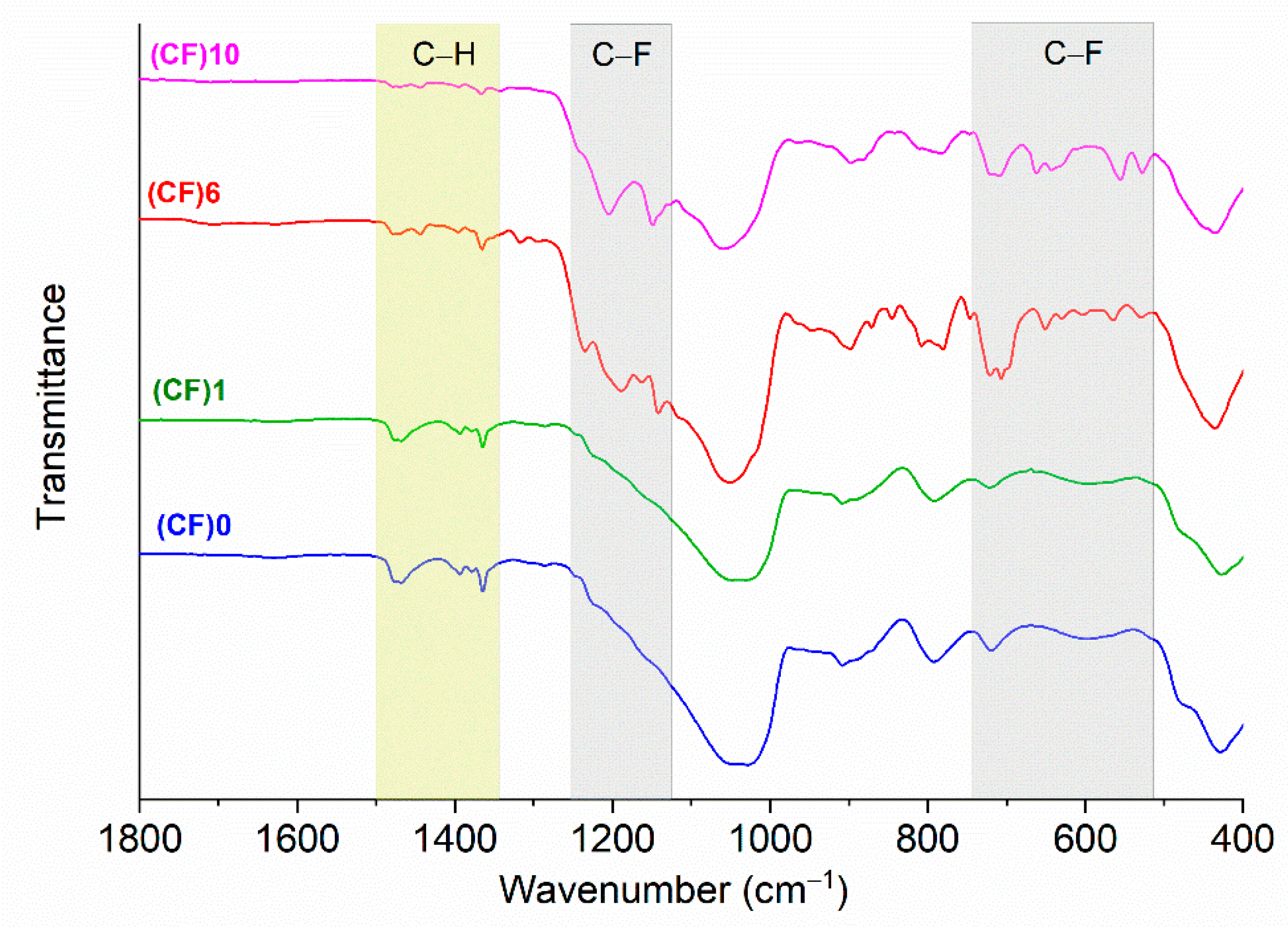
| Wavenumber (cm−1) | Bonds and Vibrations |
|---|---|
| 400–490 | Si–O–Si rocking |
| 520–730 | –CF2, –CF3 rocking –CF2 wagging |
| 800; 980–1200 | Si–O–Si stretching |
| 900 | Si–O− stretching |
| 1120–1250 | C–F stretching |
| 1350–1490 | C–H stretching C–H bending |
| 2850–2980 | C–H stretching |
| 3400 | Si–OH stretching |
| Sample | WCA (°) | SFE (mN/m) | Ra (nm) | Rq (nm) | Thickness (µm) |
|---|---|---|---|---|---|
| PET | 80 ± 0.5 | 31.50 ± 0.86 | 1.4 | 1.8 | 175 |
| (CF)0 | 103 ± 0.6 | 26.71 ± 0.95 | 0.9 | 0.7 | 1.50 ± 0.06 |
| (CF)1 | 102 ± 0.5 | 23.25 ± 0.76 | 5.5 | 4.3 | 1.50 ± 0.03 |
| (CF)6 | 108 ± 0.6 | 10.81 ± 0.77 | 0.4 | 0.5 | 1.70 ± 0.04 |
| (CF)10 | 113 ± 0.9 | 10.78 ± 0.69 | 78.3 | 63.1 | 1.50 ± 0.04 |
| Sample | Glass Transition Temperature, Tg (°C) | Mean Coefficient of Thermal Expansion 30–80 °C (ppm/K) | Elongation 30–140 °C (%) | Oxygen Permeability Coefficient (cm3/(m2·24 h·atm)) |
|---|---|---|---|---|
| PET | 73.72 ± 1.52 | 115.34 ± 1.23 | 1.71 ± 0.09 | 8.48 ± 0.48 |
| (CF)0 | 83.55 ± 1.33 | 41.79 ± 2.45 | 0.94 ± 0.04 | 5.92 ± 0.70 |
| (CF)1 | 84.7 ± 0.84 | 31.48 ± 1.34 | 0.81 ± 0.03 | 6.80 ± 0.80 |
| (CF)6 | 86.82 ± 1.06 | 45.72 ± 3.50 | 1.72 ± 0.08 | 4.26 ± 0.90 |
| (CF)10 | 84.14 ± 1.51 | 51.64 ± 2.38 | 1.02 ± 0.27 | 2.05 ± 0.01 |
| Sample | Lc1 | Lc2 | Lc3 | |||
|---|---|---|---|---|---|---|
| Mean Value (N) | Standard Error (N) | Mean Value (N) | Standard Error (N) | Mean Value (N) | Standard Error (N) | |
| (CF)0 | observed from the beginning of the scratch track | does not occur | 1.58 | 0.09 | ||
| (CF)1 | 0.12 | 0.06 | 0.17 | 0.09 | 5.96 | 0.41 |
| (CF)6 | - | - | observed from the beginning of the scratch track | |||
| (CF)10 | observed from the beginning of the scratch track | observed from the beginning of the scratch track | does not occur | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Startek, K.; Szczurek, A.; Tran, T.N.L.; Krzak, J.; Bachmatiuk, A.; Lukowiak, A. Structural and Functional Properties of Fluorinated Silica Hybrid Barrier Layers on Flexible Polymeric Foil. Coatings 2021, 11, 573. https://doi.org/10.3390/coatings11050573
Startek K, Szczurek A, Tran TNL, Krzak J, Bachmatiuk A, Lukowiak A. Structural and Functional Properties of Fluorinated Silica Hybrid Barrier Layers on Flexible Polymeric Foil. Coatings. 2021; 11(5):573. https://doi.org/10.3390/coatings11050573
Chicago/Turabian StyleStartek, Kamila, Anna Szczurek, Thi Ngoc Lam Tran, Justyna Krzak, Alicja Bachmatiuk, and Anna Lukowiak. 2021. "Structural and Functional Properties of Fluorinated Silica Hybrid Barrier Layers on Flexible Polymeric Foil" Coatings 11, no. 5: 573. https://doi.org/10.3390/coatings11050573
APA StyleStartek, K., Szczurek, A., Tran, T. N. L., Krzak, J., Bachmatiuk, A., & Lukowiak, A. (2021). Structural and Functional Properties of Fluorinated Silica Hybrid Barrier Layers on Flexible Polymeric Foil. Coatings, 11(5), 573. https://doi.org/10.3390/coatings11050573