Recent Advances in Protective Coatings for Accident Tolerant Zr-Based Fuel Claddings
Abstract
1. Introduction
2. Zirconium-Based Alloys
2.1. Zr-Sn and Zr-Nb Alloys
2.2. HT Oxidation Behavior of Zr-Based Alloys
3. Protective Coatings for ATF Claddings
3.1. Metallic Coatings on Zr-Based Alloys
3.1.1. Fe-Based Coatings
3.1.2. Chromium-Based Coatings
3.1.3. Yttrium-Based Coatings
3.1.4. HEAs Coatings
3.2. Non-Metallic Coatings on Zr-Based Alloys
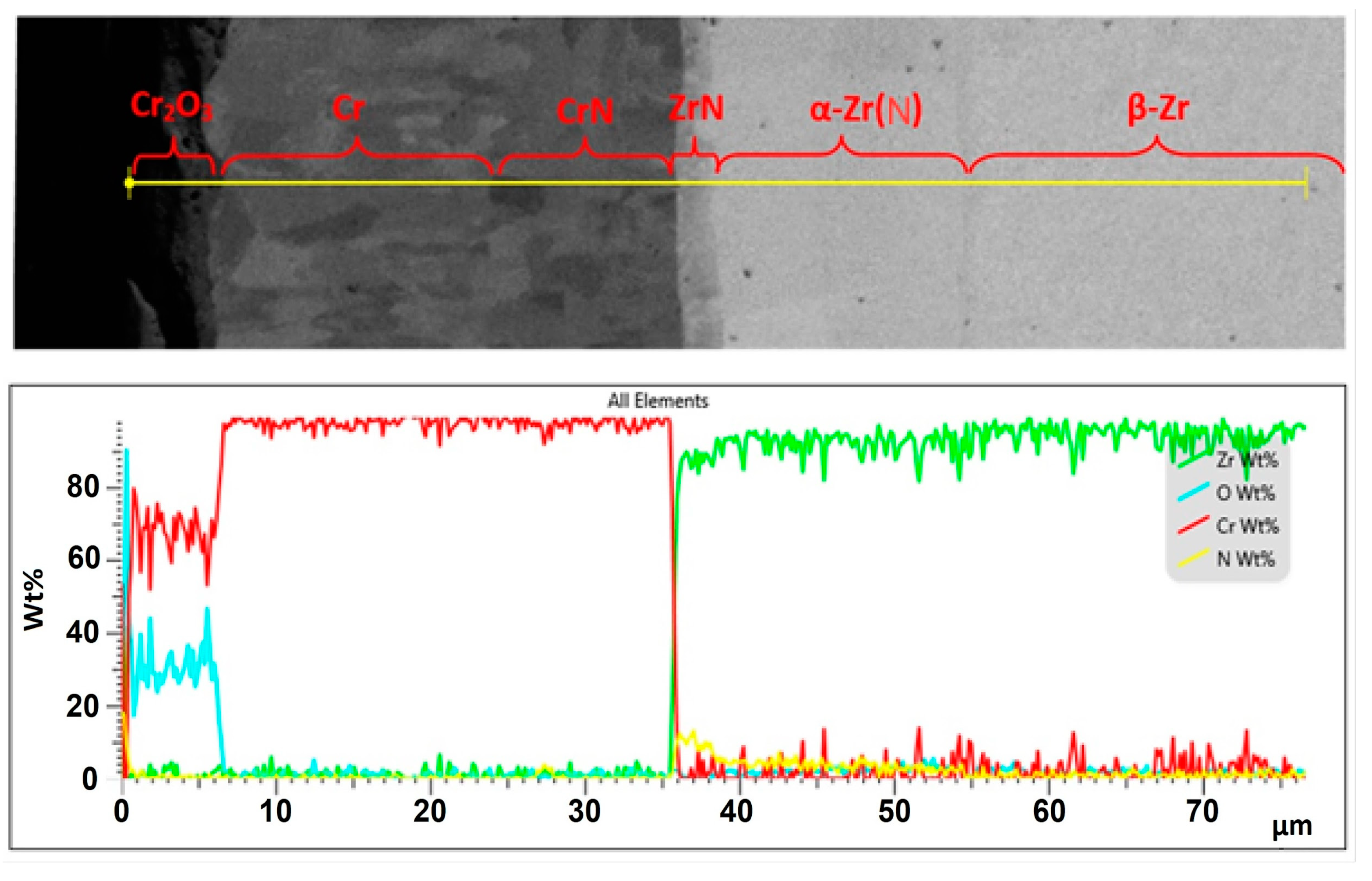
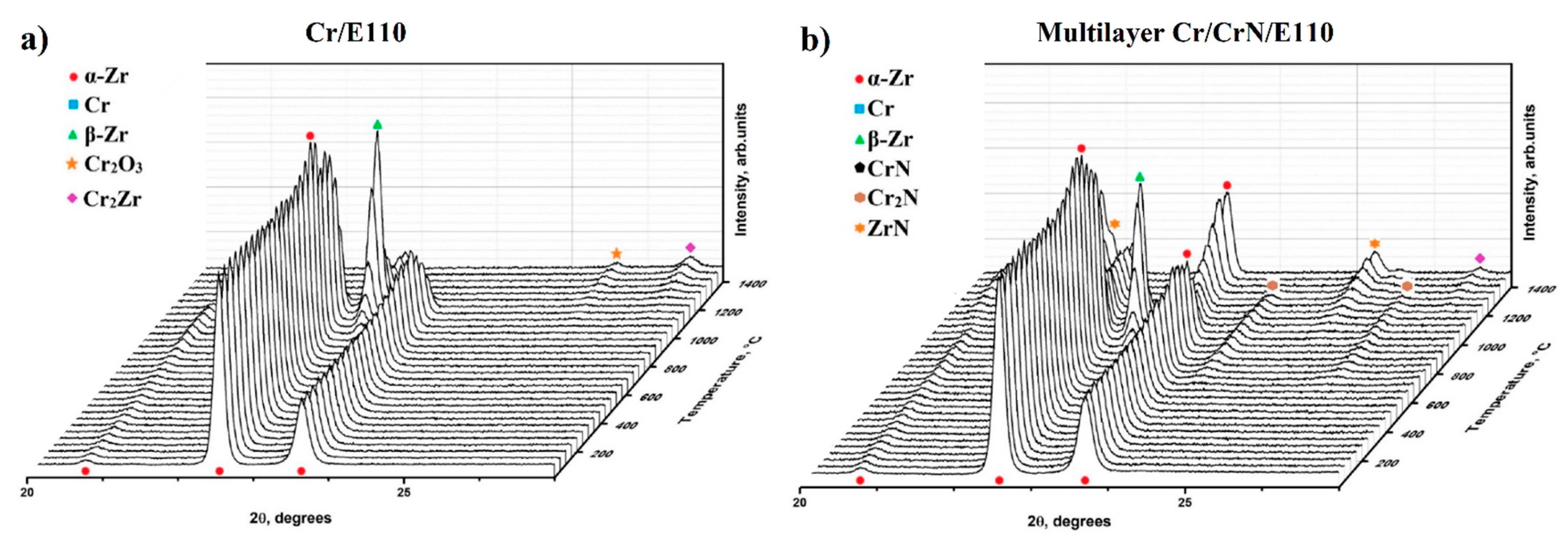
3.2.1. Nitride Coatings
3.2.2. Zirconium Silicide Coatings
3.2.3. Carbide Coatings
3.2.4. MAX-Phase Coatings
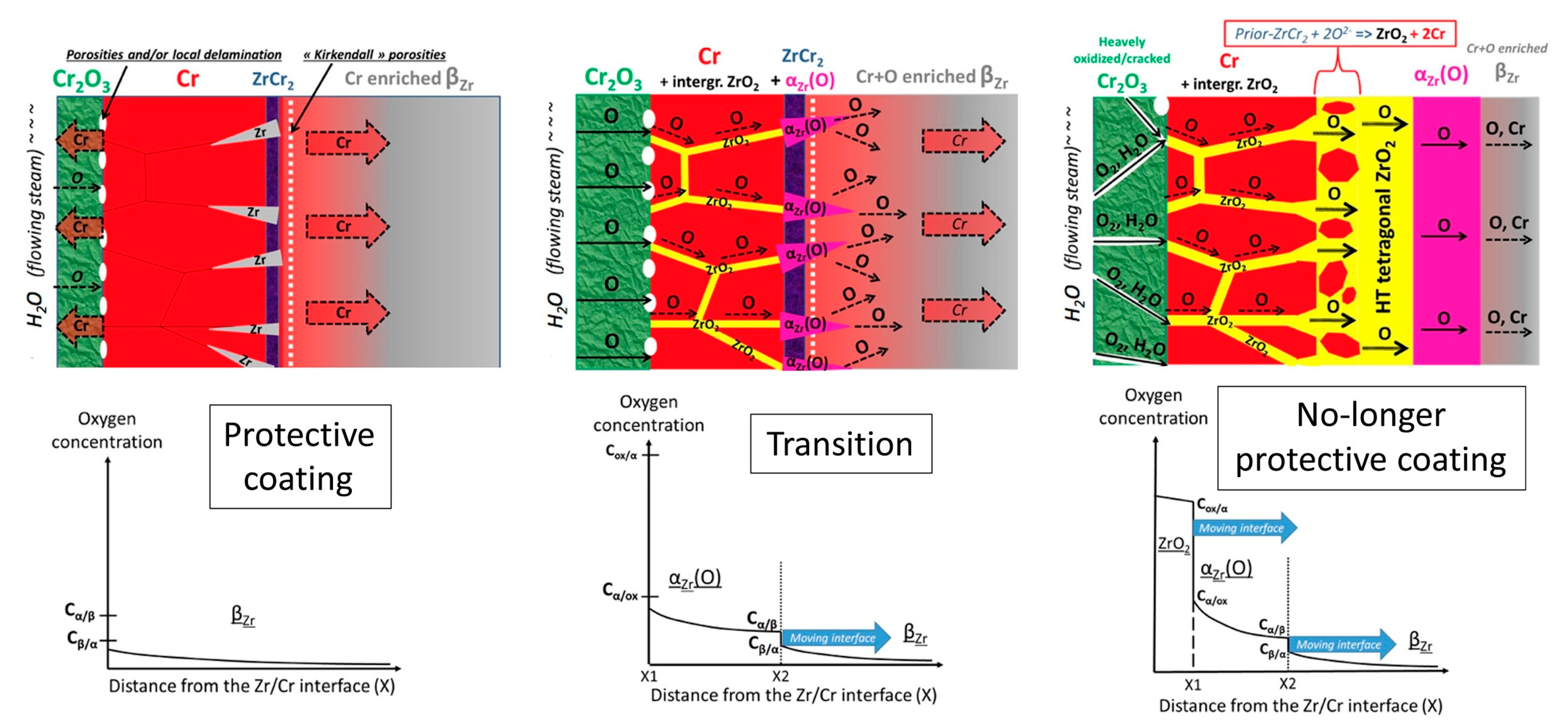
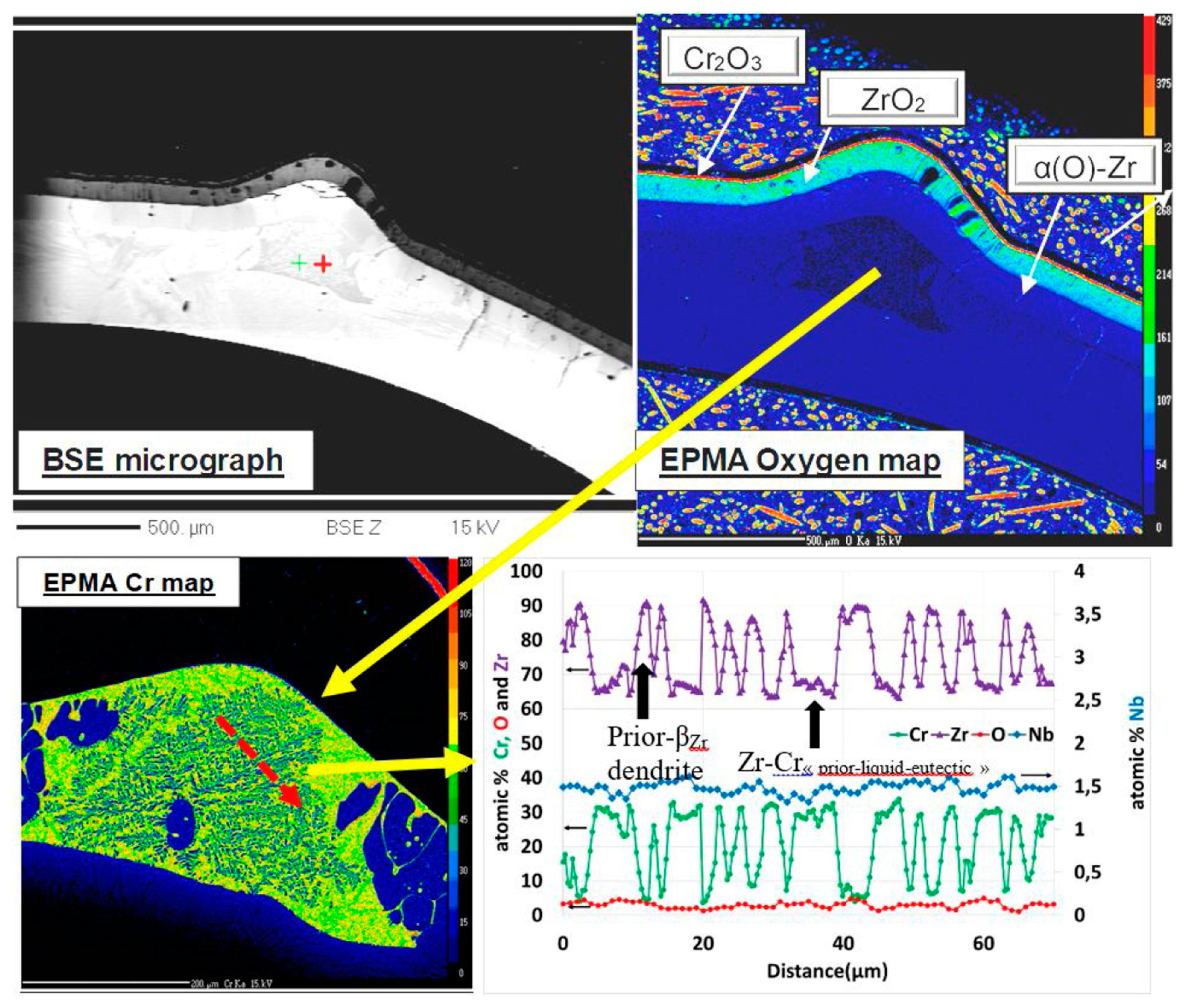
4. Mechanism of Protection
4.1. Microstructure and Thickness of the Coatings
| Coating/Substrate | Deposition Method | Microstructure | Thickness, μm | Oxidation Conditions | WG, mg/cm2 | Comments | Refs. |
|---|---|---|---|---|---|---|---|
| Cr/Zry-4 | PVD | Dense | 15–20 | PWR medium, 360 °C, 415 °C, 10 MPa, 200 days | 0.05 |
| [92] |
| Steam 1200 °C, 1500 s | 2.60 | ||||||
| 3. Steam, 1300 °C, 5600 s | 21.20 | ||||||
| Cr/E110 | Dual MS | Dense voids-free | 3.1 | Air, 1100 °C, 20 min | 2.86 |
| [72], [165] |
| 4.5 | Steam, 10 min: 900 °C 1050 °C 1200 °C | 0.33 1.40 22.90 | |||||
| Hot target MS | Columnar | 6.0 | Steam, 10 min: 900 °C 1050 °C 1200 °C | 0.52 1.60 12.6 | |||
| 9.0 | Steam, 10 min 900 °C 1050 °C 1200 °C | 0.45 1.00 4.10 | |||||
| Cr/Zry-4 | CAPVD | Columnar | 20 | PWR medium, 360 °C, 18.6 MPa, 2000 h | 0.048 |
| [67] |
| BWR medium, 360 °C, 18.6 MPa, 3000 h | 0.035 | ||||||
| Air, 800–1200 °C, 1 h | – | ||||||
| Cr/Zry-4 | PVD/HiPIMS | Columnar | 1 | Steam, 1100 °C, 850 s | 6.5 |
| [87] |
| Columnar | 5 | 3.5 | |||||
| Dense/Multilayered | 7 | 1.0 | |||||
| Dense | 5–12 | Steam, 1200 °C, 5 min | <1 | ||||
| NiCr/E110 | MS | Columnar/Dense | 2 | Air, 1100 °C, 20 min | >20 |
| [106] |
| Cr3C2-NiCr/Zry-4 | HVOF | Dense with closed pores | 120 | Steam, 1200 °C, 1 h | 29.8 |
| [149] |
| Ni-Cr/Zry-4 | 64.4 | ||||||
| FeCrAl/Zry-4 (Zirlo) FeCrAl/Mo/Zirlo | CS | Dense | 100 | Air, 1200 °C, 20 min | – |
| [84] |
| FeCrAl/Mo/Zry-4 | dcMS | Dense | 6.6/10.6 | Steam, 1000–1200 °C, 1 h | – |
| [177] |
| Cr/SiC | HiPIMS | Dense | 5–15 | Steam, 1200 °C, 4 h | 0.9 |
| [34] |
| CAPVD | Dense/cracks | 1.5 | |||||
| HiPIMS + CAPVD | Dense | 1.2 | |||||
| SiC/Zry-4 | PECVD | – | 1 | Autoclave, 350 °C, 20 MPa, 24 h | Weight loss |
| [144] |
| ZrSi2/Zry-4 | dcMS | Columnar | 3.9 | Autoclave, 400 °C, 10.3 MPa, 72 h | Weight loss |
| [137] |
| Cr/Zry-4 | CAPVD | Dense | 10 | Steam, 1200 °C, 2000 s | 12 |
| [178] |
| Cr | CS | Dense with some closed pores | 50 | Steam, 1130–1310 °C for 10–90min | – |
| [179] |
| Cr2AlC/Zr702 | HiPIMS | Columnar | 3.2 | Air, 1200 °C, 2 h | 2–5 |
| [175] |
| Ti2AlC/TiC/Zry-4 | MS | Dense voids-free | 5.0/0.5 | Steam, 800 °C, 250 min | 2.6 |
| [157] |
| Steam, 1000 °C, 250 min | 42.5 | ||||||
| Ti2AlC/TiC/Zirlo | hybrid arc/MS | Dense | 12.0/1.5 | Steam, 1000 °C, 10 min | 0.13 |
| [155] |
| Steam, 1100 °C, 10 min | 1.94 | ||||||
| Steam, 1200 °C, 5 min | 1.17 | ||||||
| Steam, 1200 °C, 10 min | 16.65 | ||||||
| CrAlN/Zry-4 | CAPVD | Dense | 5.5 | Ar/steam, 1200 °C, up to 1 h | ~17.1 (15 min) |
| [176] |
| CrAlSiN/Zry-4 | 5.8 | ~14.8 (15 min) | |||||
| AlCrMoNbZr/N36 (Zr-1Sn-1Nb-0.3Fe) | MS | Dense | 3 | Autoclave, 360 °C, 18.6 MPa, 30 days | 0.09 |
| [115] |
4.2. Interdiffusion and Eutectics under B-DBA Conditions
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Azhazha, V.; Borts, B.; Butenko, I.; Voevodin, V.; V’yugov, P.; Gritsina, V.; Krasnorutskij, V.; Lavrinenko, S.; Levenets, V.; Neklyudov, I. Zirconium-Niobium Alloys for NPP; Splav Tsirkonij-Niobij Dlya AehS: Alushta, Ukraine, 2012.
- Zieliński, A.; Sobieszczyk, S. Hydrogen-enhanced degradation and oxide effects in zirconium alloys for nuclear applications. Int. J. Hydrog. Energy 2011, 36, 8619–8629. [Google Scholar] [CrossRef]
- Charit, I. Accident tolerant nuclear fuels and cladding materials. JOM 2018, 70, 173–175. [Google Scholar] [CrossRef]
- Motta, A.T.; Capolungo, L.; Chen, L.Q.; Cinbiz, M.N.; Daymond, M.R.; Koss, D.A.; Lacroix, E.; Pastore, G.; Simon, P.-C.A.; Tonks, M.R.; et al. Hydrogen in zirconium alloys: A review. J. Nucl. Mater. 2019, 518, 440–460. [Google Scholar] [CrossRef]
- Baba, M. Fukushima accident: What happened? Radiat. Meas. 2013, 55, 17–21. [Google Scholar] [CrossRef]
- National Research Council. Lessons learned from the Fukushima nuclear accident for improving safety of US nuclear plants. Natl. Acad. Sci. 2014, 394. [Google Scholar] [CrossRef]
- Shebaldov, P.; Peregud, M.M.; Nikulina, A.; Bibilashvili, Y.K.; Lositski, A.; Kuz’menko, N.; Belov, V.; Novoselov, A. E110 alloy cladding tube properties and their interrelation with alloy structure-phase condition and impurity content. In Proceedings of the Zirconium in the Nuclear Industry: Twelfth International Symposium, West Conshohocken, PA, USA, 15–18 June 1998. [Google Scholar]
- Pellegrini, M.; Herranz, L.; Sonnenkalb, M.; Lind, T.; Maruyama, Y.; Gauntt, R.; Bixler, N.; Morreale, A.; Dolganov, K.; Sevon, T. Main findings, remaining uncertainties and lessons learned from the OECD/NEA BSAF project. Nucl. Technol. 2020, 206, 1449–1463. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Terrani, K.A.; Zinkle, S.J.; Snead, L.L. Advanced oxidation-resistant iron-based alloys for LWR fuel cladding. J. Nucl. Mater. 2014, 448, 420–435. [Google Scholar] [CrossRef]
- Karoutas, Z.; Brown, J.; Atwood, A.; Hallstadius, L.; Lahoda, E.; Ray, S.; Bradfute, J. The maturing of nuclear fuel: Past to Accident Tolerant Fuel. Prog. Nucl. Energy 2018, 102, 68–78. [Google Scholar] [CrossRef]
- Carmack, J.; Goldner, F.; Bragg-Sitton, S.M.; Snead, L.L. Overview of the US DOE Accident Tolerant Fuel Development Program; Idaho National Laboratory (INL): Idaho, ID, USA, 2013.
- Kim, D.; Lee, H.-G.; Park, J.Y.; Kim, W.-J. Fabrication and measurement of hoop strength of SiC triplex tube for nuclear fuel cladding applications. J. Nucl. Mater. 2015, 458, 29–36. [Google Scholar] [CrossRef]
- Katoh, Y.; Snead, L.L.; Henager Jr, C.H.; Nozawa, T.; Hinoki, T.; Iveković, A.; Novak, S.; De Vicente, S.G. Current status and recent research achievements in SiC/SiC composites. J. Nucl. Mater. 2014, 455, 387–397. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.; Zhao, S. Progress of SiCf/SiC composites for nuclear application. Adv. Ceram. 2016, 37, 151–167. [Google Scholar]
- Qiu, B.; Wang, J.; Deng, Y.; Wang, M.; Wu, Y.; Qiu, S. A review on thermohydraulic and mechanical-physical properties of SiC, FeCrAl and Ti3SiC2 for ATF cladding. Nucl. Eng. Technol. 2020, 52, 1–13. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Yun, D. A complete review and a prospect on the candidate materials for accident-tolerant fuel claddings. Mater. Rev. 2018, 32, 1757–1778. [Google Scholar]
- Bragg-Sitton, S.M.; Todosow, M.; Montgomery, R.; Stanek, C.R.; Montgomery, R.; Carmack, W.J. Metrics for the technical performance evaluation of light water reactor accident-tolerant fuel. Nucl. Technol. 2016, 195, 111–123. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and development progress of Cr-based surface coatings in nuclear fuel element: I. selection, preparation, and characteristics of coating materials. Coatings 2020, 10, 808. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and Development Progress of Cr-Based Surface Coating in Nuclear Fuel Elements: II. Current Status and Shortcomings of Performance Studies. Coatings 2020, 10, 835. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Cheng, B. Fuel behavior in severe accidents and Mo-alloy based cladding designs to improve accident tolerance. Atw. Int. Z. Fuer Kernenerg. 2013, 59, 158–160. [Google Scholar]
- Nelson, A.; Sooby, E.; Kim, Y.-J.; Cheng, B.; Maloy, S. High temperature oxidation of molybdenum in water vapor environments. J. Nucl. Mater. 2014, 448, 441–447. [Google Scholar] [CrossRef]
- Cheng, B.; Chou, P.; Kim, Y.-J. Evaluations of Mo-alloy for light water reactor fuel cladding to enhance accident tolerance. EPJ Nucl. Sci. Technol. 2016, 2, 5. [Google Scholar] [CrossRef]
- Cheng, B.; Chou, P.; Kim, Y.-J. Development of Mo-Based Accident Tolerant LWR Fuel Cladding; International Atomic Energy Agency: Vienna, Austria, 2016. [Google Scholar]
- Karpyuk, L.; Savchenko, A.; Leont’eva-Smirnova, M.; Kulakov, G.; Konovalov, Y.V. Steel Cladding for VVER Fuel Pins in the Context of Accident-Tolerant Fuel: Prospects. At. Energy 2020, 128, 218–222. [Google Scholar] [CrossRef]
- Guanghai, B.; Zhilin, C.; Yanwei, Z.; Erwei, L.; Jiaxiang, X.; Weiwei, Y.; Rongshan, W.; Rui, L.; Tong, L. Research progress of coating on zirconium alloy for nuclear fuel cladding. Rare Met. Mater. Eng. 2017, 46, 2035–2040. [Google Scholar]
- Maier, B.; Yeom, H.; Johnson, G.; Dabney, T.; Walters, J.; Romero, J.; Shah, H.; Xu, P.; Sridharan, K. Development of cold spray coatings for accident-tolerant fuel cladding in light water reactors. JOM 2018, 70, 198–202. [Google Scholar] [CrossRef]
- Lustman, B. Zirconium technology—twenty years of evolution. In Zirconium in the Nuclear Industry; ASTM International: West Conshohocken, PA, USA, 1979. [Google Scholar]
- Baczynski, J. High Temperature Steam Oxidation of Titanium-Coated Zircaloy-2 and Titanium-Zirconium Alloys. Master’s Thesis, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2014. [Google Scholar]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Yang, J.-H.; Koo, Y.-H. Development of surface modified Zr cladding by coating technology for ATF. Proc. Top Fuel 2016, 1157–1163. [Google Scholar]
- Sagiroun, M.I.; Xinrong, C. Zirconium-Based Cladding Coating Technique for Oxidation, Corrosion and Embrittlement Reduction at High-Temperature: An Overview. IOP Conf. Ser. Mater. Sci. Eng. 2019, 649, 012008. [Google Scholar] [CrossRef]
- Barrett, F.; Huang, X.; Guzonas, D. Characterization of TiO2-doped yttria-stabilized zirconia (YSZ) for supercritical water-cooled reactor insulator application. J. Therm. Spray Technol. 2013, 22, 734–743. [Google Scholar] [CrossRef]
- Kane, K.A.; Stack, P.; Mouche, P.A.; Pillai, R.R.; Pint, B.A. Steam oxidation of chromium corrosion barrier coatings for sic-based accident tolerant fuel cladding. J. Nucl. Mater. 2021, 543, 152561. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Zhang, R.; Yang, H.; Wei, T.; Wang, Y.; Li, Z.; He, L.; Wang, J.; Wang, L. Microstructure and high-temperature steam oxidation properties of thick Cr coatings prepared by magnetron sputtering for accident tolerant fuel claddings: The role of bias in the deposition process. Corros. Sci. 2020, 165, 108378. [Google Scholar] [CrossRef]
- Rickover, H.G.; Geiger, L.D.; Lustman, B. History of the Development of Zirconium Alloys for Use in Nuclear Reactors; Energy Research and Development Administration: Washington, DC, USA, 1975.
- Gadiyar, H. Corrosion of zirconium base alloys-an overview. In Proceedings of Symposium on Zirconium Alloys for Reactor Components; Bhabha Atomic Research Centre: Bombay, India, 1992. [Google Scholar]
- Kass, S. The development of the zircaloys. In Corrosion of Zirconium Alloys; ASTM International: West Conshohocken, PA, USA, 1964. [Google Scholar]
- Motta, A.T.; Couet, A.; Comstock, R.J. Corrosion of zirconium alloys used for nuclear fuel cladding. Annu. Rev. Mater. Res. 2015, 45, 311–343. [Google Scholar] [CrossRef]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Alam, T.; Khan, M.K.; Pathak, M.; Ravi, K.; Singh, R.; Gupta, S. A review on the clad failure studies. Nucl. Eng. Des. 2011, 241, 3658–3677. [Google Scholar] [CrossRef]
- Moorthy, K.B. Current trends in the Use of Zirconium Alloys; National Metallurgical Laboratory: Jamshedpur, India, 1969. [Google Scholar]
- Shimada, S.; Cheng, B.; Lutz, D.; Kubota, O.; Ichikawa, N.; Ibe, H. In-core tests of effects of BWR water chemistry impurities on zircaloy corrosion. In Zirconium in the Nuclear Industry: Fourteenth International Symposium; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Krishnan, R.; Asundi, M. Zirconium alloys in nuclear technology. Proc. Indian Acad. Sci. Sect. C Eng. Sci. 1981, 4, 41–56. [Google Scholar]
- The Fukushima Daiichi Nuclear Power Plant Accident; OECD: Paris, France, 2013.
- Nikulina, A. Zirconium alloys in nuclear power engineering. Met. Sci. Heat Treat. 2004, 46, 458–462. [Google Scholar] [CrossRef]
- Nikulina, A.V.; Markelov, V.; Peregud, M.; Bibilashvili, Y.K.; Kotrekhov, V.; Lositsky, A.; Kuzmenko, N.; Shevnin, Y.P.; Shamardin, V.; Kobylyansky, G. Zirconium alloy E635 as a material for fuel rod cladding and other components of VVER and RBMK cores. In Zirconium in the Nuclear Industry: Eleventh International Symposium; ASTM International: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Markelov, V.; Novikov, V.; Nikulina, A.; Konkov, V.; Sablin, M.; Novoselov, A.; Kobylyansky, J. Application of E635 alloy as structural components of WWER-1000 fuel assemblies. In Proceedings of the International Conference on WWER fuel Performance, Modelling and Experimental Support, Albena Bulgaria, 19–23 September 2005. [Google Scholar]
- Hózer, Z.; Győri, C.; Matus, L.; Horváth, M. Ductile-to-brittle transition of oxidised Zircaloy-4 and E110 claddings. J. Nucl. Mater. 2008, 373, 415–423. [Google Scholar] [CrossRef]
- Novikov, V.; Nesterov, B.; Troyanov, V.; Izhutov, A.; Burukin, A.; Shahmut, E. Out of reactor investigation corrosive characteristics cladding of new Zirconium alloys as applied to conditions reactor-plant WWER-1200 (AES-2006) and program irradiation tests of this alloys. In Proceedings of the International Conference on WWER Fuel Performance, Modelling and Experimental Support, Albena, Bulgaria, 17–24 September 2011. [Google Scholar]
- Novikov, V.; Markelov, V.; Gusev, A.; Malgin, A.; Kabanov, A.; Pimenov, Y. Some results on the properties in-vestigations of zirconium alloys for WWER-1000 fuel cladding. In Proceedings of the International Conference on WWER Fuel Performance, Modelling and Experimental Support, Albena, Bulgaria, 17–24 September 2011. [Google Scholar]
- Foster, J.P.; Yueh, H.K.; Comstock, R.J. ZIRLO TM cladding improvement. J. Astm Int. 2008, 5, 1–13. [Google Scholar] [CrossRef]
- Le Saux, M.; Vandenberghe, V.; Crébier, P.; Brachet, J.; Gilbon, D.; Mardon, J.; Jacques, P.; Cabrera, A. Influence of steam pressure on the high temperature oxidation and post-cooling mechanical properties of Zircaloy-4 and M5 cladding (LOCA conditions). In Zirconium in the Nuclear Industry: 17th Volume; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Brachet, J.-C.; Portier, L.; Forgeron, T.; Hivroz, J.; Hamon, D.; Guilbert, T.; Bredel, T.; Yvon, P.; Mardon, J.-P.; Jacques, P. Influence of hydrogen content on the α/β phase transformation temperatures and on the thermal-mechanical behavior of Zy-4, M4 (ZrSnFeV), and M5™(ZrNbO) alloys during the first phase of LOCA transient. In Zirconium in the Nuclear Industry: Thirteenth International Symposium; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Wakamatsu, A.; Nunokawa, K.; Nakano, M.; Hamasaki, M.; Uno, Y.; Kawagoe, T. Development of advanced fuel and core for high reliability and high performance. Mitsubishi. Juko Giho. 2006, 43, 20–24. [Google Scholar]
- Malgin, A.G.; Markelov, V.A.; Novikov, V.V.; Shelepov, I.A.; Donnikov, V.E.; Latunin, V.I.; Krejci, J. Research of high-temperature oxidation behavior of E110 opt and E110М sponge based zirconium alloys. Proc. Top Fuel 2018, 239, 1–10. [Google Scholar]
- Pawel, R.E.; Cathcart, J.V.; McKee, R.A. The kinetics of oxidation of Zircaloy-4 in steam at high temperatures. J. Electrochem. Soc. 1979, 126, 1105. [Google Scholar] [CrossRef]
- Yan, Y.; Garrison, B.E.; Howell, M.; Bell, G.L. High-temperature oxidation kinetics of sponge-based E110 cladding alloy. J. Nucl. Mater. 2018, 499, 595–612. [Google Scholar] [CrossRef]
- NRC. 50.46 Acceptance Criteria for Emergency Core Cooling Systems for Light-Water Nuclear Power Reactors; US NRC: Washington, DC, USA, 2017.
- Le Saux, M.; Brachet, J.-C.; Vandenberghe, V.; Ambard, A.; Chosson, R. Breakaway oxidation of zirconium alloys exposed to steam around 1000 °C. Corros. Sci. 2020, 176, 108936. [Google Scholar] [CrossRef]
- Steinbrueck, M.; da Silva, F.O.; Grosse, M. Oxidation of Zircaloy-4 in steam-nitrogen mixtures at 600–1200 °C. J. Nucl. Mater. 2017, 490, 226–237. [Google Scholar] [CrossRef]
- Steinbrück, M. Prototypical experiments relating to air oxidation of Zircaloy-4 at high temperatures. J. Nucl. Mater. 2009, 392, 531–544. [Google Scholar] [CrossRef]
- Steinbrück, M.; Böttcher, M. Air oxidation of Zircaloy-4, M5® and ZIRLO™ cladding alloys at high temperatures. J. Nucl. Mater. 2011, 414, 276–285. [Google Scholar] [CrossRef]
- Tang, C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-tolerant fuel (ATF) claddings. Corros. Rev. 2017, 35, 141–165. [Google Scholar] [CrossRef]
- Bischoff, J.; Vauglin, C.; Delafoy, C.; Barberis, P.; Perche, D.; Guerin, B.; Vassault, J.; Brachet, J. Development of Cr-coated zirconium alloy cladding for enhanced accident tolerance. In Proceedings of the Top Fuel 2016, Boise, ID, USA, 11–16 September 2016; pp. 1165–1171. [Google Scholar]
- Maier, B.; Yeom, H.; Johnson, G.; Dabney, T.; Walters, J.; Xu, P.; Romero, J.; Shah, H.; Sridharan, K. Development of cold spray chromium coatings for improved accident tolerant zirconium-alloy cladding. J. Nucl. Mater. 2019, 519, 247–254. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Bryan, W.J.; Jones, D. Wear resistant coating for fuel cladding. Patent 5,268,946, 7 December 1993. [Google Scholar]
- Gray, D.M.; White, D.W.; Andresen, P.L.; Kim, Y.J.; Lin, Y.P.; Curtis, T.C.; Patterson, C.B. Fuel Rod with Wear-Inhibiting Coating. U.S. Patent 11/780,537, 22 January 2009. [Google Scholar]
- Kashkarov, E.B.; Nikitenkov, N.; Sutygina, A.; Obrosov, A.; Manakhov, A.; Polčak, J.; Weiß, S. Hydrogen absorption by Ti-implanted Zr-1Nb alloy. Int. J. Hydrogen Energy 2018, 43, 2484–2491. [Google Scholar] [CrossRef]
- Kashkarov, E.; Nikitenkov, N.; Sutygina, A.; Laptev, R.; Bordulev, Y.; Obrosov, A.; Liedke, M.O.; Wagner, A.; Zak, A.; Weiβ, S. Microstructure, defect structure and hydrogen trapping in zirconium alloy Zr-1Nb treated by plasma immersion Ti ion implantation and deposition. J. Alloys Compd. 2018, 732, 80–87. [Google Scholar] [CrossRef]
- Kashkarov, E.; Sidelev, D.; Rombaeva, M.; Syrtanov, M.; Bleykher, G. Chromium coatings deposited by cooled and hot target magnetron sputtering for accident tolerant nuclear fuel claddings. Surf. Coat. Technol. 2020, 389, 125618. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.I.; No, H.C. Mechanical analysis of surface-coated zircaloy cladding. Nucl. Eng. Technol. 2017, 49, 1031–1043. [Google Scholar] [CrossRef]
- Younker, I.; Fratoni, M. Neutronic evaluation of coating and cladding materials for accident tolerant fuels. Prog. Nucl. Energy 2016, 88, 10–18. [Google Scholar] [CrossRef]
- Kam, D.H.; Lee, J.H.; Lee, T.; Jeong, Y.H. Critical heat flux for SiC-and Cr-coated plates under atmospheric condition. Ann. Nucl. Energy 2015, 76, 335–342. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science Publishers Ltd.: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Sarrazin, P.; Galerie, A.; Fouletier, J. Mechanisms of High Temperature Corrosion; Trans Tech Publications Ltd.: Bäch, Switzerland, 2008. [Google Scholar]
- Pasamehmetoglu, K.; Massara, S.; Costa, D.; Bragg-Sitton, S.; Moatti, M.; Kurata, M.; Iracane, D.; Ivanova, T.; Bischoff, J.; Delafoy, C. State-of-the-Art Report on Light Water Reactor Accident-Tolerant Fuels; Organisation for Economic Co-Operation and Development: Paris, France, 2018. [Google Scholar]
- Zhong, W.; Mouche, P.A.; Han, X.; Heuser, B.J.; Mandapaka, K.K.; Was, G.S. Performance of iron–chromium–aluminum alloy surface coatings on Zircaloy 2 under high-temperature steam and normal BWR operating conditions. J. Nucl. Mater. 2016, 470, 327–338. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-H.; Yang, J.-H.; Koo, Y.-H. Progress of surface modified Zr cladding development for ATF at KAERI. In Proceedings of the 2017 Water Reactor Fuel Performance Meeting, Ramada Plaza Jeju, Jeju Island, Korea, 10–14 September 2017; pp. 10–14. [Google Scholar]
- Aydogan, E.; Weaver, J.S.; Maloy, S.A.; El-Atwani, O.; Wang, Y.Q.; Mara, N.A. Microstructure and mechanical properties of FeCrAl alloys under heavy ion irradiations. J. Nucl. Mater. 2018, 503, 250–262. [Google Scholar] [CrossRef]
- Dabney, T.; Johnson, G.; Yeom, H.; Maier, B.; Walters, J.; Sridharan, K. Experimental evaluation of cold spray FeCrAl alloys coated zirconium-alloy for potential accident tolerant fuel cladding. Nucl. Mater. Energy 2019, 21, 100715. [Google Scholar] [CrossRef]
- Gigax, J.G.; Kennas, M.; Kim, H.; Maier, B.R.; Yeom, H.; Johnson, G.O.; Sridharan, K.; Shao, L. Interface reactions and mechanical properties of FeCrAl-coated Zircaloy-4. J. Nucl. Mater. 2019, 519, 57–63. [Google Scholar] [CrossRef]
- Park, D.J.; Kim, H.G.; Jung, Y.I.; Park, J.H.; Yang, J.H.; Koo, Y.H. Behavior of an improved Zr fuel cladding with oxidation resistant coating under loss-of-coolant accident conditions. J. Nucl. Mater. 2016, 482, 75–82. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Idarraga-Trujillo, I.; Le Flem, M.; Le Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, C.; Tupin, M. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Wang, X.; Guan, H.; Liao, Y.; Zhu, M.; Xu, C.; Jin, X.; Liao, B.; Xue, W.; Zhang, Y.; Bai, G.; et al. Enhancement of high temperature steam oxidation resistance of Zr–1Nb alloy with ZrO2/Cr bilayer coating. Corr. Sci. 2021, 187, 109494. [Google Scholar] [CrossRef]
- Yeom, H.; Sridharan, K. Cold spray technology in nuclear energy applications: A review of recent advances. Ann. Nucl. Energy 2021, 150, 107835. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-Y.; Koo, Y.-H. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Ran, G.; Yang, Z.; Wang, P. Cr-coated Zr-4 alloy prepared by electroplating and its in situ He+ irradiation behavior. J. Nucl. Mater. 2020, 538, 152240. [Google Scholar] [CrossRef]
- Brachet, J.; Le Saux, M.; Le Flem, M.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Cobac, C.; Lahogue, F.; Rousselot, J.; Tupin, M. On-going studies at CEA on chromium coated zirconium based nuclear fuel claddings for enhanced accident tolerant LWRs fuel. In Proceedings of the TopFuel, Zurich, Switzerlan, 13–19 September 2015; pp. 13–19. [Google Scholar]
- Wagih, M.; Spencer, B.; Hales, J.; Shirvan, K. Fuel performance of chromium-coated zirconium alloy and silicon carbide accident tolerant fuel claddings. Ann. Nucl. Energy 2018, 120, 304–318. [Google Scholar] [CrossRef]
- Holzwarth, U.; Stamm, H. Mechanical and thermomechanical properties of commercially pure chromium and chromium alloys. J. Nucl. Mater. 2002, 300, 161–177. [Google Scholar] [CrossRef]
- Ma, H.-B.; Yan, J.; Zhao, Y.-H.; Liu, T.; Ren, Q.-S.; Liao, Y.-H.; Zuo, J.-D.; Liu, G.; Yao, M.-Y. Oxidation behavior of Cr-coated zirconium alloy cladding in high-temperature steam above 1200 °C. Npj Mater. Degrad. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Han, X.; Xue, J.; Peng, S.; Zhang, H. An interesting oxidation phenomenon of Cr coatings on Zry-4 substrates in high temperature steam environment. Corros. Sci. 2019, 156, 117–124. [Google Scholar] [CrossRef]
- Le Saux, M.; Brachet, J.; Vandenberghe, V.; Gilbon, D.; Mardon, J.; Sebbari, B. Influence of pre-transient oxide on LOCA high temperature steam oxidation and post-quench mechanical properties of zircaloy-4 and M5™ cladding. In Proceedings of the Water Reactor Fuel Performance Meeting, paper T3-040, Chengdu, China, 11–14 September 2011. [Google Scholar]
- Ribis, J.; Wu, A.; Brachet, J.-C.; Barcelo, F.; Arnal, B. Atomic-scale interface structure of a Cr-coated Zircaloy-4 material. J. Mater. Sci. 2018, 53, 9879–9895. [Google Scholar] [CrossRef]
- Jiang, J.; Zhan, D.; Lv, J.; Ma, X.; He, X.; Wang, D.; Hu, Y.; Zhai, H.; Tu, J.; Zhang, W. Comparative study on the tensile cracking behavior of CrN and Cr coatings for accident-tolerant fuel claddings. Surf. Coat. Technol. 2021, 409, 126812. [Google Scholar] [CrossRef]
- Wu, A.; Ribis, J.; Brachet, J.C.; Clouet, E.; Leprêtre, F.; Bordas, E.; Arnal, B. HRTEM and chemical study of an ion-irradiated chromium/zircaloy-4 interface. J. Nucl. Mater. 2018, 504, 289–299. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Vasilenko, R.L.; Ovcharenko, V.D.; Tolstolutskaya, G.D.; Kopanets, I.E.; Kolodiy, I.V. Irradiation resistance of vacuum arc chromium coatings for zirconium alloy fuel claddings. J. Nucl. Mater. 2018, 510, 163–167. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Vasilenko, R.L.; Tolstolutskaya, G.D.; Voyevodin, V.N.; Belous, V.A.; Ovcharenko, V.D.; Kopanets, I.E. Irradiation resistance of chromium coatings for ATFC in the temperature range 300–550 °C. J. Nucl. Mater. 2021, 549, 152908. [Google Scholar] [CrossRef]
- Chen, S.-L.; He, X.-J.; Yuan, C.-X. Recent studies on potential accident-tolerant fuel-cladding systems in light water reactors. Nucl. Sci. Tech. 2020, 31, 1–30. [Google Scholar] [CrossRef]
- Kim, J.-M.; Ha, T.-H.; Kim, I.-H.; Kim, H.-G. Microstructure and oxidation behavior of CrAl laser-coated Zircaloy-4 alloy. Metals 2017, 7, 59. [Google Scholar] [CrossRef]
- Park, D.J.; Jung, Y.I.; Park, J.H.; Lee, Y.H.; Choi, B.K.; Kim, H.G. Microstructural characterization of accident tolerant fuel cladding with Cr–Al alloy coating layer after oxidation at 1200° C in a steam environment. Nucl. Eng. Technol. 2020, 52, 2299–2305. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Kashkarov, E.B.; Syrtanov, M.S.; Krivobokov, V.P. Nickel-chromium (Ni-Cr) coatings deposited by magnetron sputtering for accident tolerant nuclear fuel claddings. Surf. Coat. Technol. 2019, 369, 69–78. [Google Scholar] [CrossRef]
- Jin, D.; Yang, F.; Zou, Z.; Gu, L.; Zhao, X.; Guo, F.; Xiao, P. A study of the zirconium alloy protection by Cr3C2–NiCr coating for nuclear reactor application. Surf. Coat. Technol. 2016, 287, 55–60. [Google Scholar] [CrossRef]
- Sridharan, K.; Harrington, S.; Johnson, A.; Licht, J.; Anderson, M.; Allen, T. Oxidation of plasma surface modified zirconium alloy in pressurized high temperature water. Mater. Des. 2007, 28, 1177–1185. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-Y.; Koo, Y.-H. Microstructure and mechanical strength of surface ODS treated Zircaloy-4 sheet using laser beam scanning. Nucl. Eng. Technol. 2014, 46, 521–528. [Google Scholar] [CrossRef]
- Kim, H.-G.; Yang, J.-H.; Kim, W.-J.; Koo, Y.-H. Development status of accident-tolerant fuel for light water reactors in Korea. Nucl. Eng. Technol. 2016, 48, 1–15. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Fereiduni, E.; Ghasemi, A.; Elbestawi, M. Characterization of composite powder feedstock from powder bed fusion additive manufacturing perspective. Materials 2019, 12, 3673. [Google Scholar] [CrossRef]
- Do, H.-S.; Lee, B.-J. Origin of radiation resistance in multi-principal element alloys. Sci. Rep. 2018, 8, 1–9. [Google Scholar]
- Gao, L.; Liao, W.; Zhang, H.; Surjadi, J.U.; Sun, D.; Lu, Y. Microstructure, mechanical and corrosion behaviors of CoCrFeNiAl0. 3 high entropy alloy (HEA) films. Coatings 2017, 7, 156. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.; Liu, C.; Chang, H.; Yang, J.; Liao, J.; Yang, Y.; Liu, N. Preparation, structure, and properties of an AlCrMoNbZr high-entropy alloy coating for accident-tolerant fuel cladding. Surf. Coat. Technol. 2018, 347, 13–19. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Wang, L.; Liu, C.; Chang, H.; Yang, J.; Liao, J.; Yang, Y.; Liu, N. Interface stability, mechanical and corrosion properties of AlCrMoNbZr/(AlCrMoNbZr) N high-entropy alloy multilayer coatings under helium ion irradiation. Appl. Surf. Sci. 2019, 485, 108–118. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.; Liu, C.; Chang, H.; Yang, J.; Liao, J.; Yang, Y.; Liu, N. Preparation, structure, and properties of high-entropy alloy multilayer coatings for nuclear fuel cladding: A case study of AlCrMoNbZr/(AlCrMoNbZr) N. J. Nucl. Mater. 2018, 512, 15–24. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, P.; Wang, C.; Ma, Z.; Zhang, Y.; Xue, F.; Bai, G.; Yuan, Y.; Lan, R. Design and characterisation of AlCrFeCuNbx alloys for accident-tolerant fuel cladding. J. Alloys Compd. 2021, 859, 157805. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, E.-H.; Xiang, C. Irradiation behaviors of two novel single-phase bcc-structure high-entropy alloys for accident-tolerant fuel cladding. J. Mater. Sci. Technol. 2021, 84, 230–238. [Google Scholar] [CrossRef]
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburgh, S.C.; Armstrong, D.E.; Gandy, A.S. High-Entropy Alloys for Advanced Nuclear Applications. Entropy 2021, 23, 98. [Google Scholar] [CrossRef]
- Yun, D.; Lu, C.; Zhou, Z.; Wu, Y.; Liu, W.; Guo, S.; Shi, T.; Stubbins, J.F. Current state and prospect on the development of advanced nuclear fuel system materials: A review. Mater. Rep. Energy 2021. [Google Scholar] [CrossRef]
- Khatkhatay, F.; Jiao, L.; Jian, J.; Zhang, W.; Jiao, Z.; Gan, J.; Zhang, H.; Zhang, X.; Wang, H. Superior corrosion resistance properties of TiN-based coatings on Zircaloy tubes in supercritical water. J. Nucl. Mater. 2014, 451, 346–351. [Google Scholar] [CrossRef]
- Kashkarov, E.; Nikitenkov, N.; Sutygina, A.; Bezmaternykh, A.; Kudiiarov, V.; Syrtanov, M.; Pryamushko, T. Hydrogenation behavior of Ti-implanted Zr-1Nb alloy with TiN films deposited using filtered vacuum arc and magnetron sputtering. Appl. Surf. Sci. 2018, 432, 207–213. [Google Scholar] [CrossRef]
- Tunes, M.A.; Da Silva, F.C.; Camara, O.; Schön, C.G.; Sagás, J.C.; Fontana, L.C.; Donnelly, S.E.; Greaves, G.; Edmondson, P.D. Energetic particle irradiation study of TiN coatings: Are these films appropriate for accident tolerant fuels? J. Nucl. Mater. 2018, 512, 239–245. [Google Scholar] [CrossRef]
- Khatkhatay, F.; Jian, J.; Jiao, L.; Su, Q.; Gan, J.; Cole, J.I.; Wang, H. Diffusion barrier properties of nitride-based coatings on fuel cladding. J. Alloys Compd. 2013, 580, 442–448. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, H.; Liu, X.; Tang, D.; Deng, H.; Zou, S.; Ren, Y.; Zhou, X.; Lei, M. Thermal shock resistance of TiN-, Cr-, and TiN/Cr-coated zirconium alloy. J. Nucl. Mater. 2019, 526, 151777. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Ceramic coating for corrosion (c3) resistance of nuclear fuel cladding. Surf. Coat. Technol. 2015, 281, 133–143. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Alat, E.; Brova, M.; Younker, I.; Motta, A.; Fratoni, M.; Wolfe, D. Neutronic and mechanical evaluation of rare earth doped and undoped nitride-based coatings for accident tolerant fuels. J. Nucl. Mater. 2019, 518, 419–430. [Google Scholar] [CrossRef]
- Daub, K.; Van Nieuwenhove, R.; Nordin, H. Investigation of the impact of coatings on corrosion and hydrogen uptake of Zircaloy-4. J. Nucl. Mater. 2015, 467, 260–270. [Google Scholar] [CrossRef]
- Van Nieuwenhove, R.; Andersson, V.; Balak, J.; Oberländer, B. In-Pile testing of CrN, TiAlN, and AlCrN coatings on zircaloy cladding in the Halden reactor. ASTM Int. 2018, 965–982. [Google Scholar] [CrossRef]
- Meng, C.; Yang, L.; Wu, Y.; Tan, J.; Dang, W.; He, X.; Ma, X. Study of the oxidation behavior of CrN coating on Zr alloy in air. J. Nucl. Mater. 2019, 515, 354–369. [Google Scholar] [CrossRef]
- Krejčí, J.; Ševeček, M.; Cvrcek, L.; Kabátová, J.; Manoch, F. Chromium and chromium nitride coated cladding for nuclear reactor fuel. In Proceedings of the 20th International Corrosion Congress, EUROCORR, Prague, Czech Republic, 3–7 September 2017. [Google Scholar]
- Krejčí, J.; Ševeček, M.; Kabátová, J.; Manoch, F.; Kočí, J.; Cvrček, L.; Málek, J.; Krum, S.; Šutta, P.; Bublíková, P. Experimental behavior of chromium-based coatings. In Proceedings of the TopFuel, Prague, Czech Republic, 30 September–4 October 2018; pp. 1–13. [Google Scholar]
- Song, J.; Huang, Z.; Qin, Y.; Wang, H.; Shi, M. Effects of Zirconium Silicide on the Vulcanization, Mechanical and Ablation Resistance Properties of Ceramifiable Silicone Rubber Composites. Polymers 2020, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.; Maier, B.; Mariani, R.; Bai, D.; Fronek, S.; Xu, P.; Sridharan, K. Magnetron sputter deposition of zirconium-silicide coating for mitigating high temperature oxidation of zirconium-alloy. Surf. Coat. Technol. 2017, 316, 30–38. [Google Scholar] [CrossRef]
- Yeom, H.; Lockhart, C.; Mariani, R.; Xu, P.; Corradini, M.; Sridharan, K. Evaluation of steam corrosion and water quenching behavior of zirconium-silicide coated LWR fuel claddings. J. Nucl. Mater. 2018, 499, 256–267. [Google Scholar] [CrossRef]
- Terrani, K.A.; Pint, B.A.; Parish, C.M.; Silva, C.M.; Snead, L.L.; Katoh, Y. Silicon carbide oxidation in steam up to 2 MPa. J. Am. Ceram. Soc. 2014, 97, 2331–2352. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.; Yoon, Y.S. SiC/Si thin film deposited on zircaloy to improved accident tolerant fuel cladding. Thin Solid Film. 2018, 660, 221–230. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Syrtanov, M.S.; Murashkina, T.L.; Kurochkin, A.V.; Shanenkova, Y.; Obrosov, A. Hydrogen sorption kinetics of SiC-coated Zr-1Nb alloy. Coatings 2019, 9, 31. [Google Scholar] [CrossRef]
- Usui, T.; Sawada, A.; Amaya, M.; Suzuki, A.; Chikada, T.; Terai, T. SiC coating as hydrogen permeation reduction and oxidation resistance for nuclear fuel cladding. J. Nucl. Sci. Technol. 2015, 52, 1318–1322. [Google Scholar] [CrossRef]
- Bao, W.; Xue, J.; Liu, J.-X.; Wang, X.; Gu, Y.; Xu, F.; Zhang, G.-J. Coating SiC on Zircaloy-4 by magnetron sputtering at room temperature. J. Alloys Compd. 2018, 730, 81–87. [Google Scholar] [CrossRef]
- Al-Olayyan, Y.; Fuchs, G.; Baney, R.; Tulenko, J. The effect of Zircaloy-4 substrate surface condition on the adhesion strength and corrosion of SiC coatings. J. Nucl. Mater. 2005, 346, 109–119. [Google Scholar] [CrossRef]
- Baney, R.H.; Butt, D.; Demkowicz, P.; Tulenko, J.S. An Innovative Ceramic Corrosion Protection System for Zircaloy Cladding; University of Florida (US): Gainesville, FL, USA, 2003. [Google Scholar]
- Tang, C.; Steinbrueck, M.; Grosse, M.; Ulrich, S.; Stueber, M.; Seifert, H.J. Evaluation of magnetron sputtered protective Zr-C-Al coatings for accident tolerant Zircaloy claddings. In Proceedings of the Water Reactor Fuel Performance Meeting, Jeju Island, Korea, 10–14 September 2017. [Google Scholar]
- Michau, A.; Maury, F.; Schuster, F.; Lomello, F.; Brachet, J.-C.; Rouesne, E.; Le Saux, M.; Boichot, R.; Pons, M. High-temperature oxidation resistance of chromium-based coatings deposited by DLI-MOCVD for enhanced protection of the inner surface of long tubes. Surf. Coat. Technol. 2018, 349, 1048–1057. [Google Scholar] [CrossRef]
- Michau, A.; Maury, F.; Schuster, F.; Nuta, I.; Gazal, Y.; Boichot, R.; Pons, M. Chromium carbide growth by direct liquid injection chemical vapor deposition in long and narrow tubes, experiments, modeling and simulation. Coatings 2018, 8, 220. [Google Scholar] [CrossRef]
- Michau, A.; Gazal, Y.; Addou, F.; Maury, F.; Duguet, T.; Boichot, R.; Pons, M.; Monsifrot, E.; Maskrot, H.; Schuster, F. Scale up of a DLI-MOCVD process for the internal treatment of a batch of 16 nuclear fuel cladding segments with a CrCx protective coating. Surf. Coat. Technol. 2019, 375, 894–902. [Google Scholar] [CrossRef]
- Yang, Z.; Niu, Y.; Xue, J.; Liu, T.; Chang, C.; Zheng, X. Steam oxidation resistance of plasma sprayed chromium-containing coatings at 1200 °C. Mater. Corros. 2019, 70, 37–47. [Google Scholar] [CrossRef]
- Lyu, J.; Kashkarov, E.; Travitzky, N.; Syrtanov, M.; Lider, A. Sintering of MAX-phase materials by spark plasma and other methods. J. Mater. Sci. 2020, 56, 1–36. [Google Scholar]
- Barsoum, M.W. The MN+ 1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T. The MAX phases: Unique new carbide and nitride materials: Ternary ceramics turn out to be surprisingly soft and machinable, yet also heat-tolerant, strong and lightweight. Am. Sci. 2001, 89, 334–343. [Google Scholar] [CrossRef]
- Garcia-Diaz, B.; Olson, L.; Verst, C.; Sindelar, R.; Hoffman, E.; Hauch, B.; Maier, B.; Sridharan, K. MAX phase coatings for accident tolerant nuclear fuel. Trans. Am. Nucl. Soc 2014, 110, 994–996. [Google Scholar]
- Tallman, D.J.; Anasori, B.; Barsoum, M.W. A critical review of the oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in air. Mater. Res. Lett. 2013, 1, 115–125. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Shuai, J.; Xu, B.; Wang, A.; Ke, P. A high oxidation resistance Ti2AlC coating on Zirlo substrates for loss-of-coolant accident conditions. Ceram. Int. 2019, 45, 13912–13922. [Google Scholar] [CrossRef]
- Maier, B.R.; Garcia-Diaz, B.L.; Hauch, B.; Olson, L.C.; Sindelar, R.L.; Sridharan, K. Cold spray deposition of Ti2AlC coatings for improved nuclear fuel cladding. J. Nucl. Mater. 2015, 466, 712–717. [Google Scholar] [CrossRef]
- Tang, C.; Steinbrueck, M.; Stueber, M.; Grosse, M.; Yu, X.; Ulrich, S.; Seifert, H.J. Deposition, characterization and high-temperature steam oxidation behavior of single-phase Ti2AlC-coated Zircaloy-4. Corros. Sci. 2018, 135, 87–98. [Google Scholar] [CrossRef]
- Roberts, D.A. Magnetron Sputtering and Corrosion of Ti-Al-C and Cr-Al-C Coatings for Zr-Alloy Nuclear Fuel Cladding. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2016. [Google Scholar]
- Tang, C.; Steinbrueck, M.; Grosse, M.; Ulrich, S.; Stueber, M.; Seifert, H.J. Improvement of the high-temperature oxidation resistance of Zr alloy cladding by surface modification with aluminum-containing ternary carbide coatings. In Proceedings of the 2018 International Congress on Advances in Nuclear Power Plants, Charlotte, NC, USA, 8–11 April 2018. [Google Scholar]
- Imtyazuddin, M.; Mir, A.H.; Tunes, M.A.; Vishnyakov, V.M. Radiation resistance and mechanical properties of magnetron-sputtered Cr2AlC thin films. J. Nucl. Mater. 2019, 526, 151742. [Google Scholar] [CrossRef]
- Tang, C.; Grosse Karl, M.; Trtik, P.; Steinbrück, M.; Stüber, M.; Seifert, H.J. H2 Permeation Behavior of Cr2AlC and Ti2AlC MAX Phase Coated Zircaloy4 by Neutron Radiography. Acta Polytech. 2018, 58, 69–76. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, G.; Liu, L.; Wang, L.; Ke, P.; Xue, Q.; Wang, A. High-performance Cr2AlC MAX phase coatings: Oxidation mechanisms in the 900–1100 C temperature range. Corros. Sci. 2020, 167, 108492. [Google Scholar] [CrossRef]
- Opila, E.J.; Jacobson, N.S.; Myers, D.L.; Copland, E.H. Predicting oxide stability in high-temperature water vapor. JOM 2006, 58, 22–28. [Google Scholar] [CrossRef]
- Doyle, P.J.; Raiman, S.S.; Rebak, R.; Terrani, K.A. Characterization of the hydrothermal corrosion behavior of ceramics for accident tolerant fuel cladding. In Proceedings of the 18th International Conference on Environmental Degradation of Materials in Nuclear Power Systems–Water Reactors, Portland, OR, USA, 13–17 August 2017. [Google Scholar]
- Kashkarov, E.; Sidelev, D.; Syrtanov, M.; Tang, C.; Steinbrück, M. Oxidation kinetics of Cr-coated zirconium alloy: Effect of coating thickness and microstructure. Corros. Sci. 2020, 175, 108883. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Rouesne, E.; Ribis, J.; Guilbert, T.; Urvoy, S.; Nony, G.; Toffolon-Masclet, C.; Le Saux, M.; Chaabane, N.; Palancher, H. High temperature steam oxidation of chromium-coated zirconium-based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
- Novák, O.; Ševeček, M. Neutronic analysis of the candidate multi-layer cladding materials with enhanced accident tolerance for wwer reactors. Acta Polytech. Ctu Proc. 2018, 14, 27–33. [Google Scholar] [CrossRef]
- Yang, J.; Stegmaier, U.; Tang, C.; Steinbrück, M.; Große, M.; Wang, S.; Seifert, H.J. High temperature Cr-Zr interaction of two types of Cr-coated Zr alloys in inert gas environment. J. Nucl. Mater. 2021, 547, 152806. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y.; Zhang, Y.; Li, H.; Wang, X.; Zheng, M.; Li, Y. High Temperature Anti-Oxidation Behavior and Mechanical Property of Radio Frequency Magnetron Sputtered Cr Coating. Metals 2020, 10, 1509. [Google Scholar] [CrossRef]
- Umretiya, R.V.; Elward, B.; Lee, D.; Anderson, M.; Rebak, R.B.; Rojas, J.V. Mechanical and chemical properties of PVD and cold spray Cr-coatings on Zircaloy-4. J. Nucl. Mater. 2020, 541, 152420. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Anders, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Film. 2010, 518, 4087–4090. [Google Scholar] [CrossRef]
- Efeoglu, I.; Arnell, R.; Tinston, S.; Teer, D. The mechanical and tribological properties of titanium aluminium nitride coatings formed in a four magnetron closed-field sputtering system. Surf. Coat. Technol. 1993, 57, 117–121. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High power pulsed magnetron sputtering: A review on scientific and engineering state of the art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Ougier, M.; Michau, A.; Lomello, F.; Schuster, F.; Maskrot, H.; Schlegel, M.L. High-temperature oxidation behavior of HiPIMS as-deposited Cr–Al–C and annealed Cr2AlC coatings on Zr-based alloy. J. Nucl. Mater. 2020, 528, 151855. [Google Scholar] [CrossRef]
- Liu, J.; Cui, Z.; Ma, D.; Lu, J.; Cui, Y.; Li, C.; Liu, W.; Hao, Z.; Hu, P.; Yao, M. Investigation of oxidation behaviors of coated Zircaloy as accident-tolerant fuel with CrAlN and CrAlSiN coatings in high-temperature steam. Corros. Sci. 2020, 175, 108896. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Peng, S.; Zhang, H. Oxidation behavior of FeCrAl coated Zry-4 under high temperature steam environment. Corros. Sci. 2019, 149, 45–53. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, H.-G.; Park, J.-y.; Jung, Y.-I.; Park, D.-J.; Koo, Y.-H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High temperature oxidation and microstructural evolution of cold spray chromium coatings on Zircaloy-4 in steam environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Wen, Q.; Ruan, X.; Luo, F.; Bai, G.; Qing, Y.; Zhu, D.; Huang, Z.; Zhang, Y. Behavior of plasma sprayed Cr coatings and FeCrAl coatings on Zr fuel cladding under loss-of-coolant accident conditions. Surf. Coat. Technol. 2018, 344, 141–148. [Google Scholar] [CrossRef]
- Bhanumurthy, K.; Kale, G.; Khera, S. Reaction diffusion in the zirconium-iron system. J. Nucl. Mater. 1991, 185, 208–213. [Google Scholar] [CrossRef]
- Wang, X.; Wei, K.; Guan, H.; Xu, C.; Xue, W.; Zhang, Y.; Wang, R. High temperature oxidation of Zr1Nb alloy with plasma electrolytic oxidation coating in 900–1200° C steam environment. Surf. Coat. Technol. 2021, 407, 126768. [Google Scholar] [CrossRef]
- Krejčí, J.; Kabátová, J.; Manoch, F.; Kočí, J.; Cvrček, L.; Málek, J.; Krum, S.; Šutta, P.; Bublíková, P.; Halodová, P. Development and testing of multicomponent fuel cladding with enhanced accidental performance. Nucl. Eng. Technol. 2020, 52, 597–609. [Google Scholar] [CrossRef]
- Azevedo, C. Selection of fuel cladding material for nuclear fission reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Fejt, F.; Sevecek, M.; Frybort, J.; Novak, O. Study on neutronics of VVER-1200 with accident tolerant fuel cladding. Ann. Nucl. Energy 2019, 124, 579–591. [Google Scholar] [CrossRef]
- Tiwari, G.; Sharma, B.; Raghunathan, V.; Patil, R. Self-and solute-diffusion in dilute zirconium-niobium alloys in β-phase. J. Nucl. Mater. 1973, 46, 35–40. [Google Scholar] [CrossRef]
- Patil, R.; Kale, G.; Garg, S. Chemical diffusion in Zr Nb system. J. Nucl. Mater. 1995, 223, 169–173. [Google Scholar] [CrossRef]
- Graedel, T.E.; Harper, E.; Nassar, N.T.; Nuss, P.; Reck, B.K. Criticality of metals and metalloids. Proc. Natl. Acad. Sci. USA 2015, 112, 4257–4262. [Google Scholar] [CrossRef]
- Chen, P.; Qiu, B.; Li, Y.; Wu, Y.; Hui, Y.; Deng, Y.; Zhang, K. An evaluation on in-pile behaviors of SiCf/SiC cladding under normal and accident conditions with updated FROBA-ATF code. Nucl. Eng. Technol. 2020, 53, 1236–1249. [Google Scholar] [CrossRef]
- Doyle, P.J.; Koyanagi, T.; Ang, C.; Snead, L.; Mouche, P.; Katoh, Y.; Raiman, S.S. Evaluation of the effects of neutron irradiation on first-generation corrosion mitigation coatings on SiC for accident-tolerant fuel cladding. J. Nucl. Mater. 2020, 536, 152203. [Google Scholar] [CrossRef]
- Cinbiz, M.N.; Brown, N.; Lowden, R.R.; Gussev, M.N.; Linton, K.D.; Terrani, K.A. Report on Design and Failure Limits of SiC/SiC and FeCrAl ATF Cladding Concepts under RIA; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2018.
- Musil, J.; Sklenka, J.; Prochazka, J. Protective over-layer coating preventing cracking of thin films deposited on flexible substrates. Surf. Coat. Technol. 2014, 240, 275–280. [Google Scholar] [CrossRef]
- Musil, J.; Sklenka, J.; Čerstvý, R. Protection of brittle film against cracking. Appl. Surf. Sci. 2016, 370, 306–311. [Google Scholar] [CrossRef]
- Musil, J. Flexible hard nanocomposite coatings. Rsc. Adv. 2015, 5, 60482–60495. [Google Scholar] [CrossRef]
- Sidelev, D.V.; Syrtanov, M.S.; Ruchkin, S.E.; Pirozhkov, A.V.; Kashkarov, E.B. Protection of Zr Alloy under High-Temperature Air Oxidation: A Multilayer Coating Approach. Coatings 2021, 11, 227. [Google Scholar] [CrossRef]
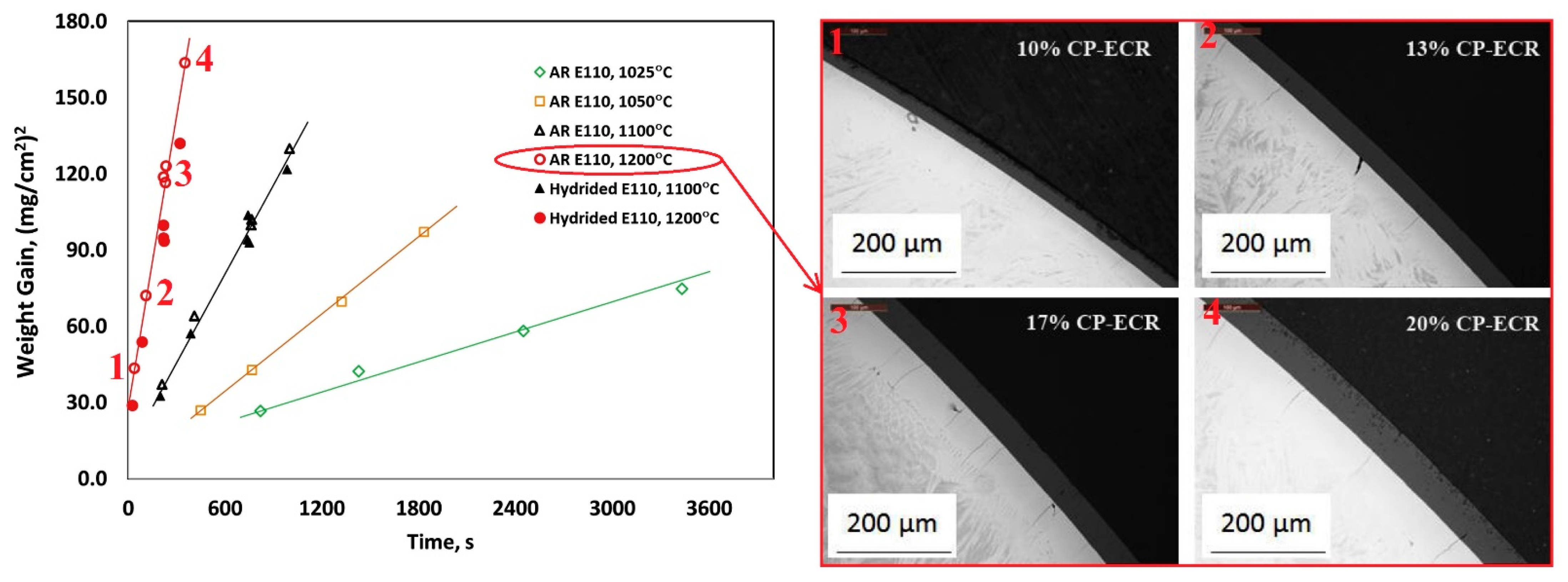
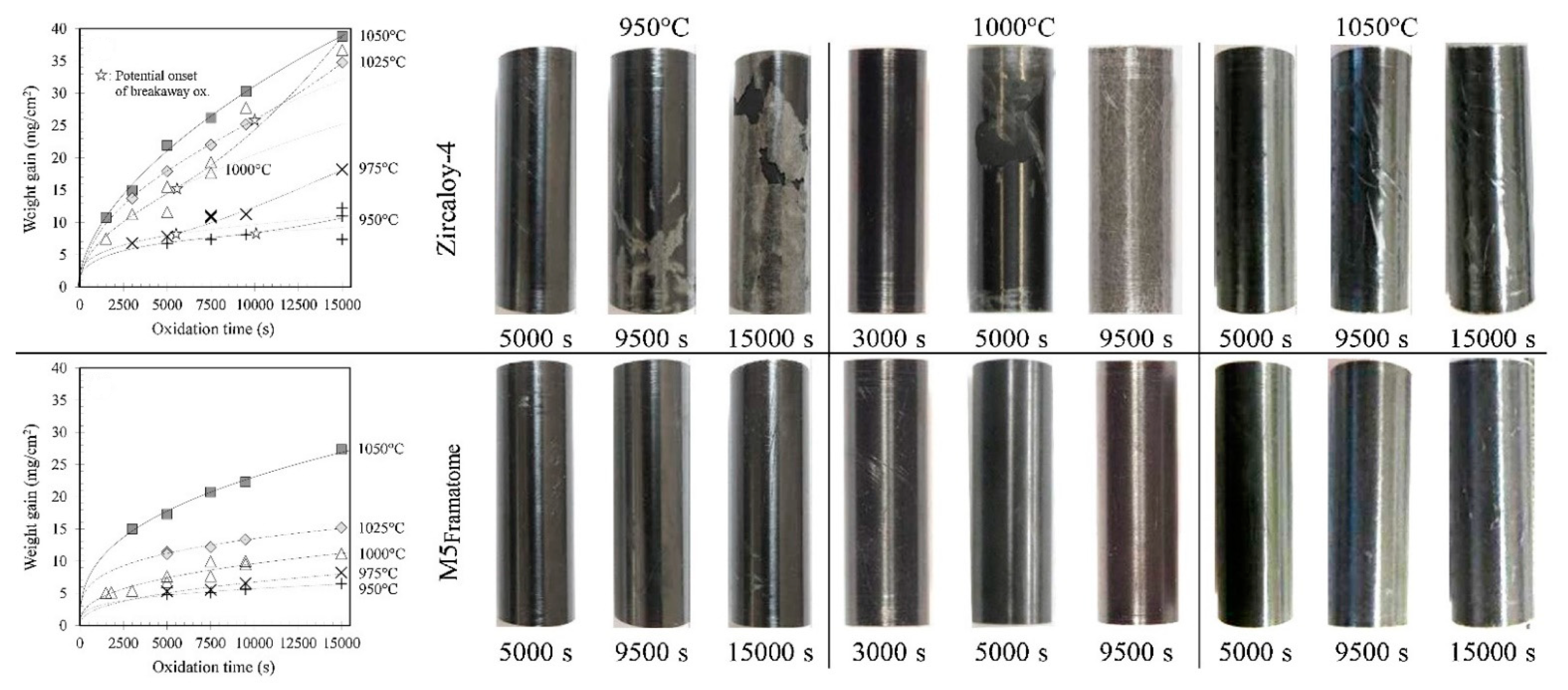
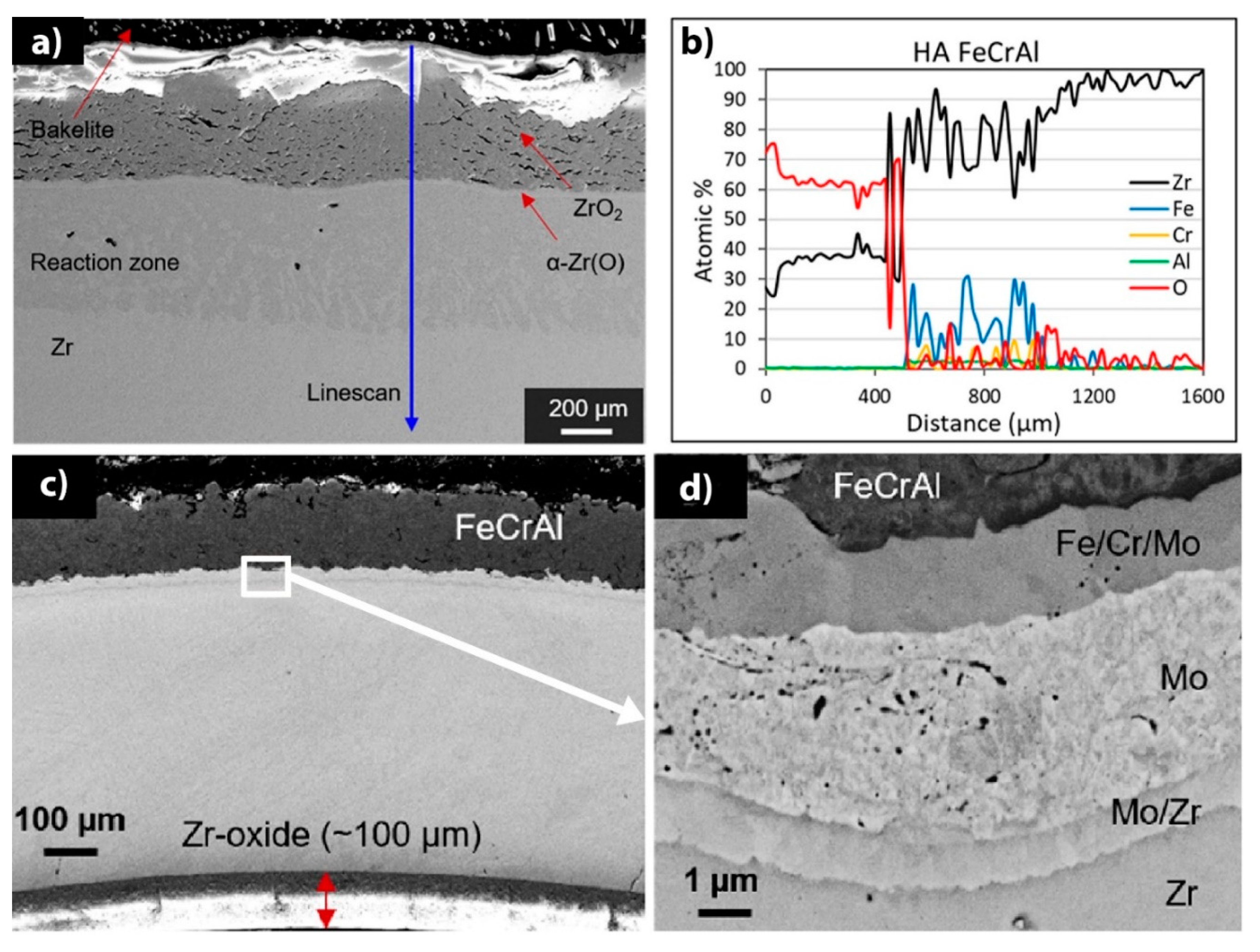
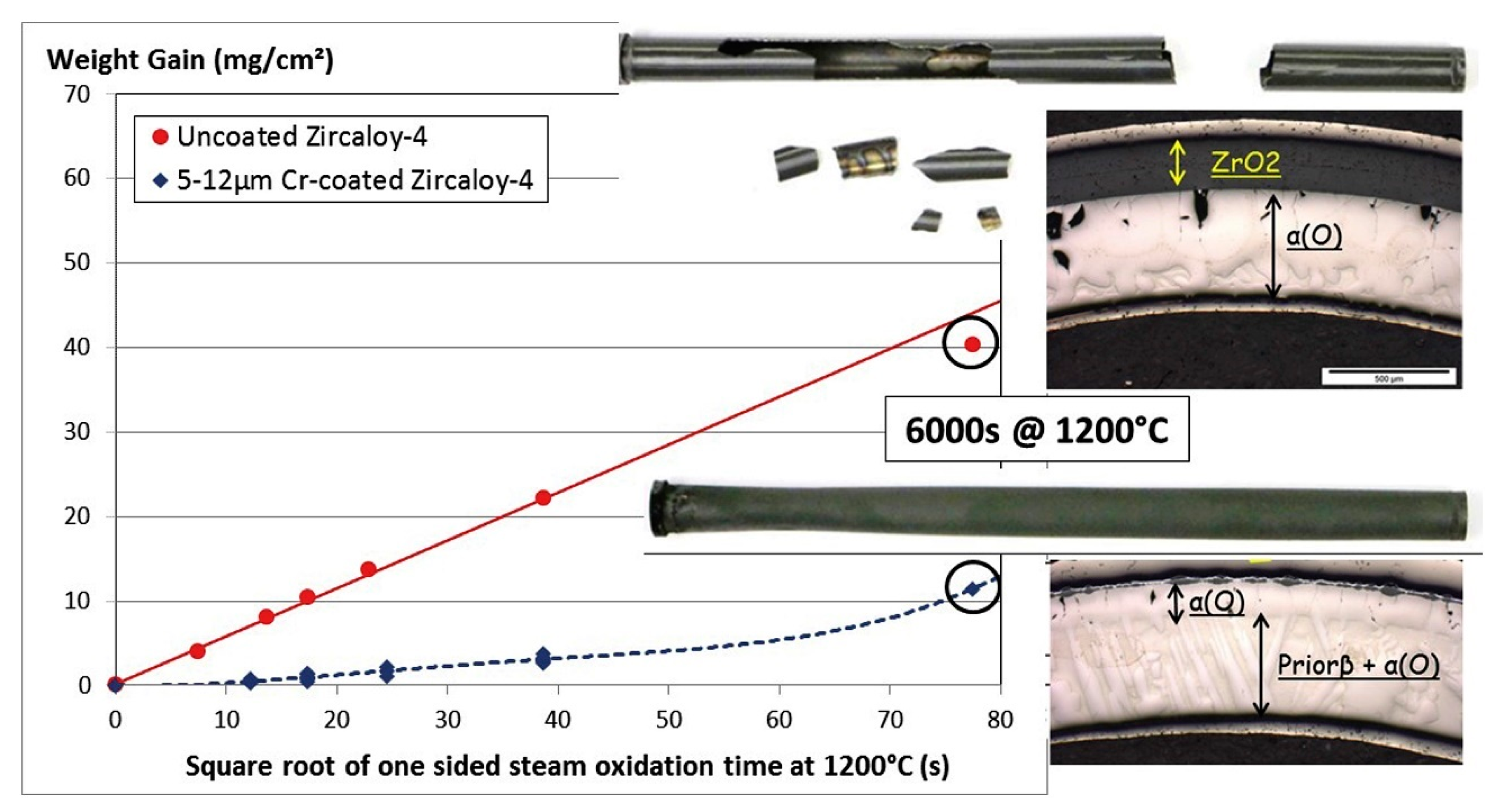
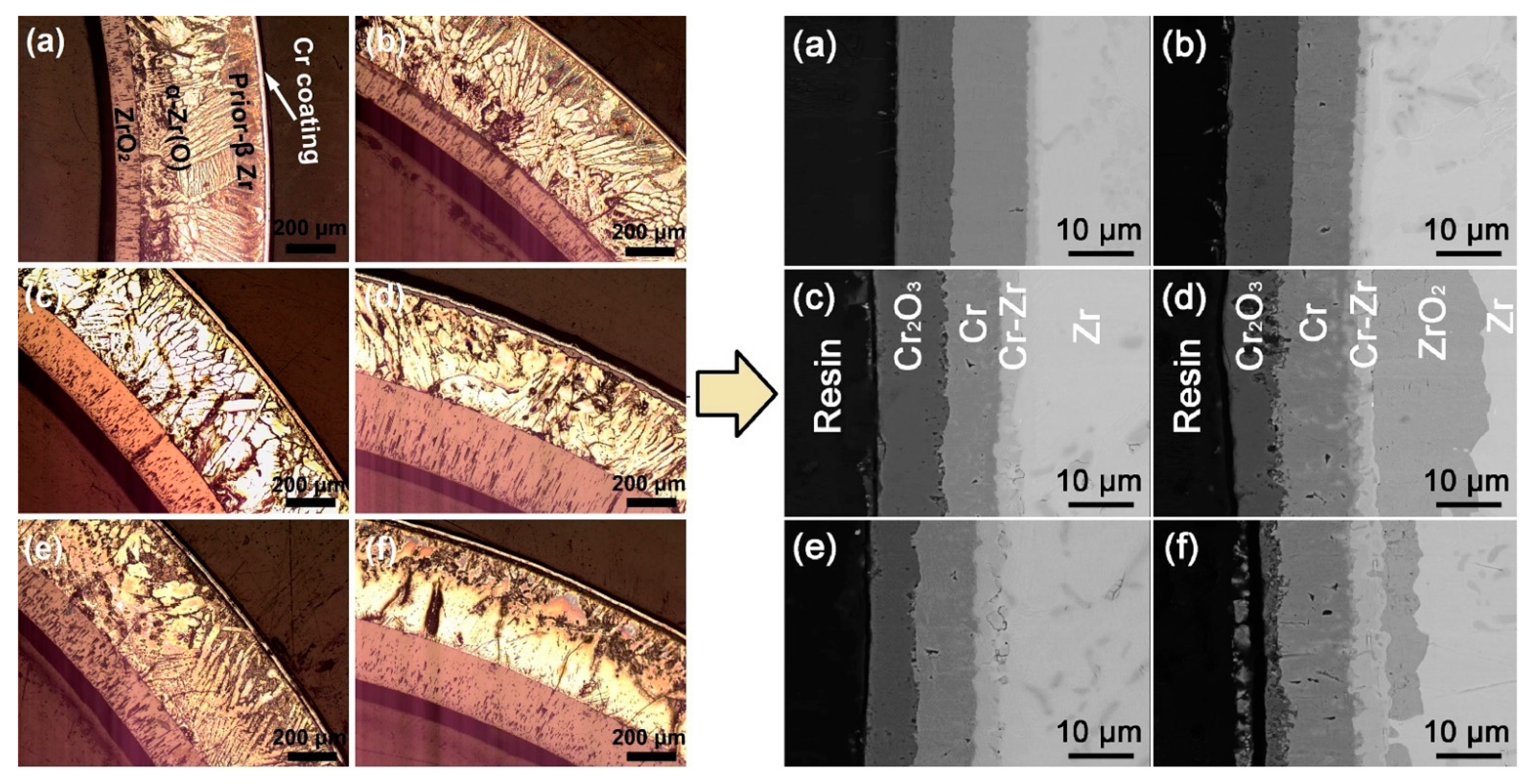
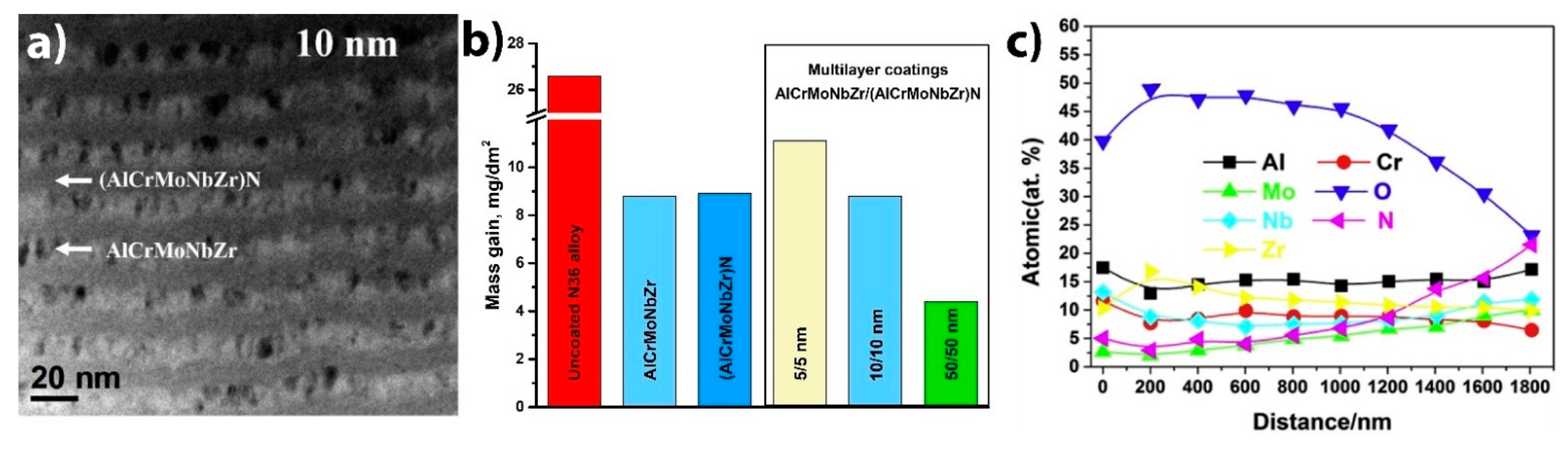
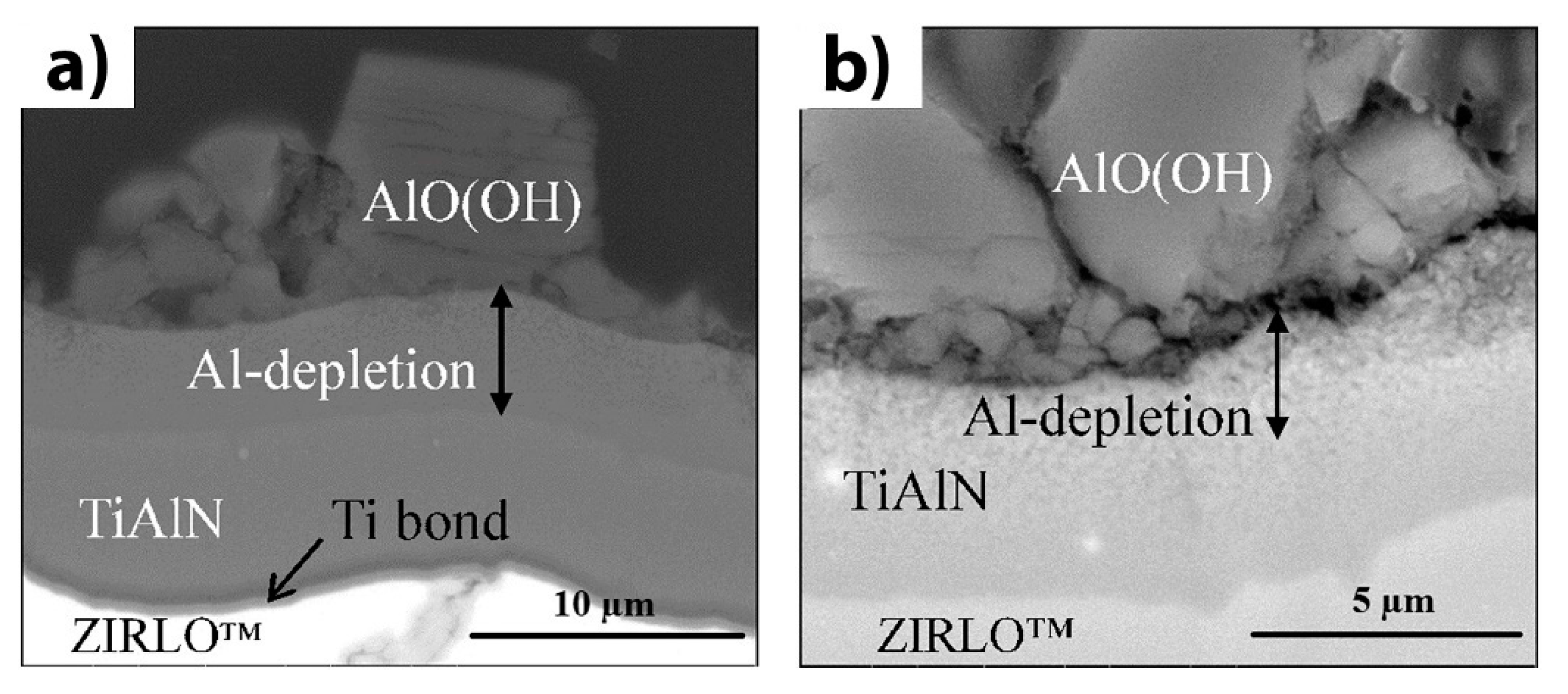
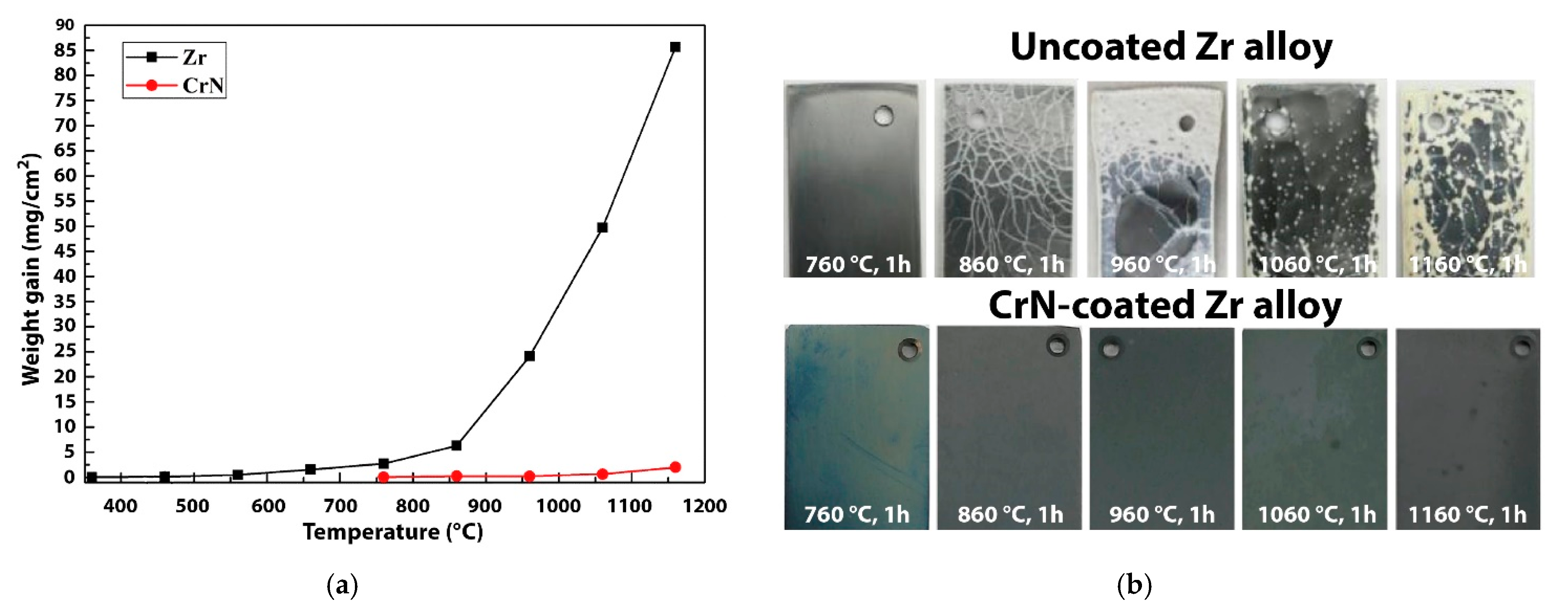
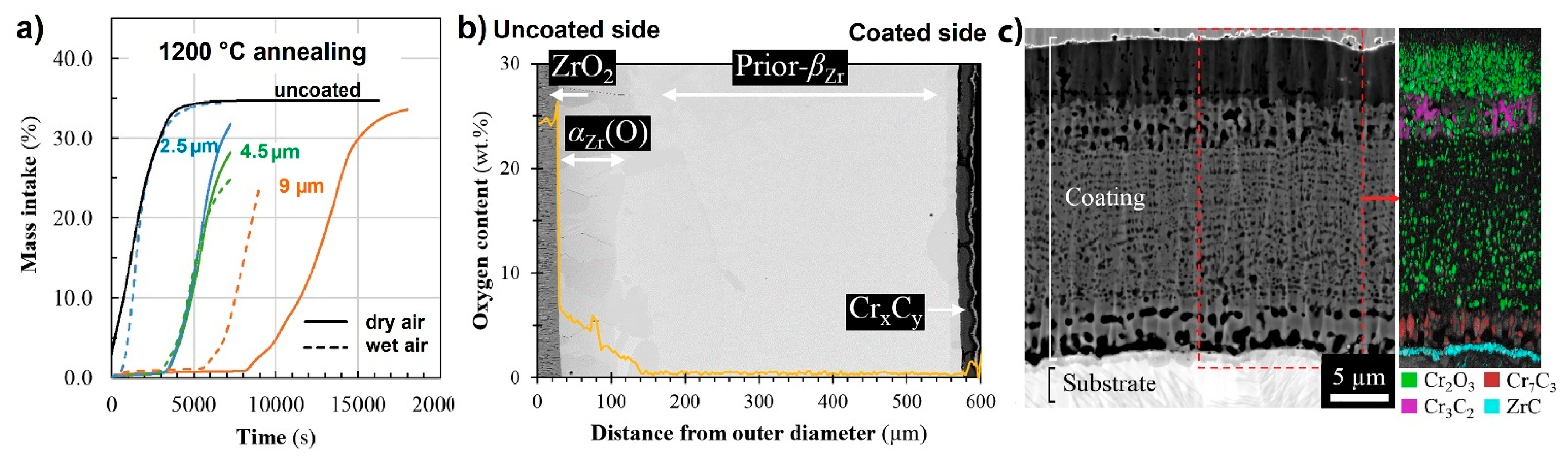
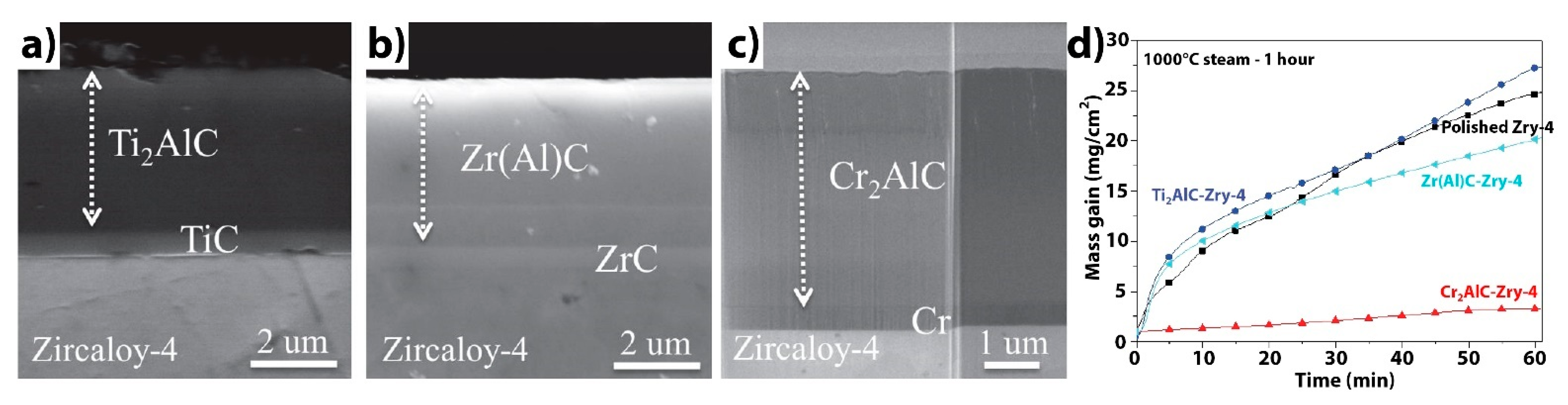
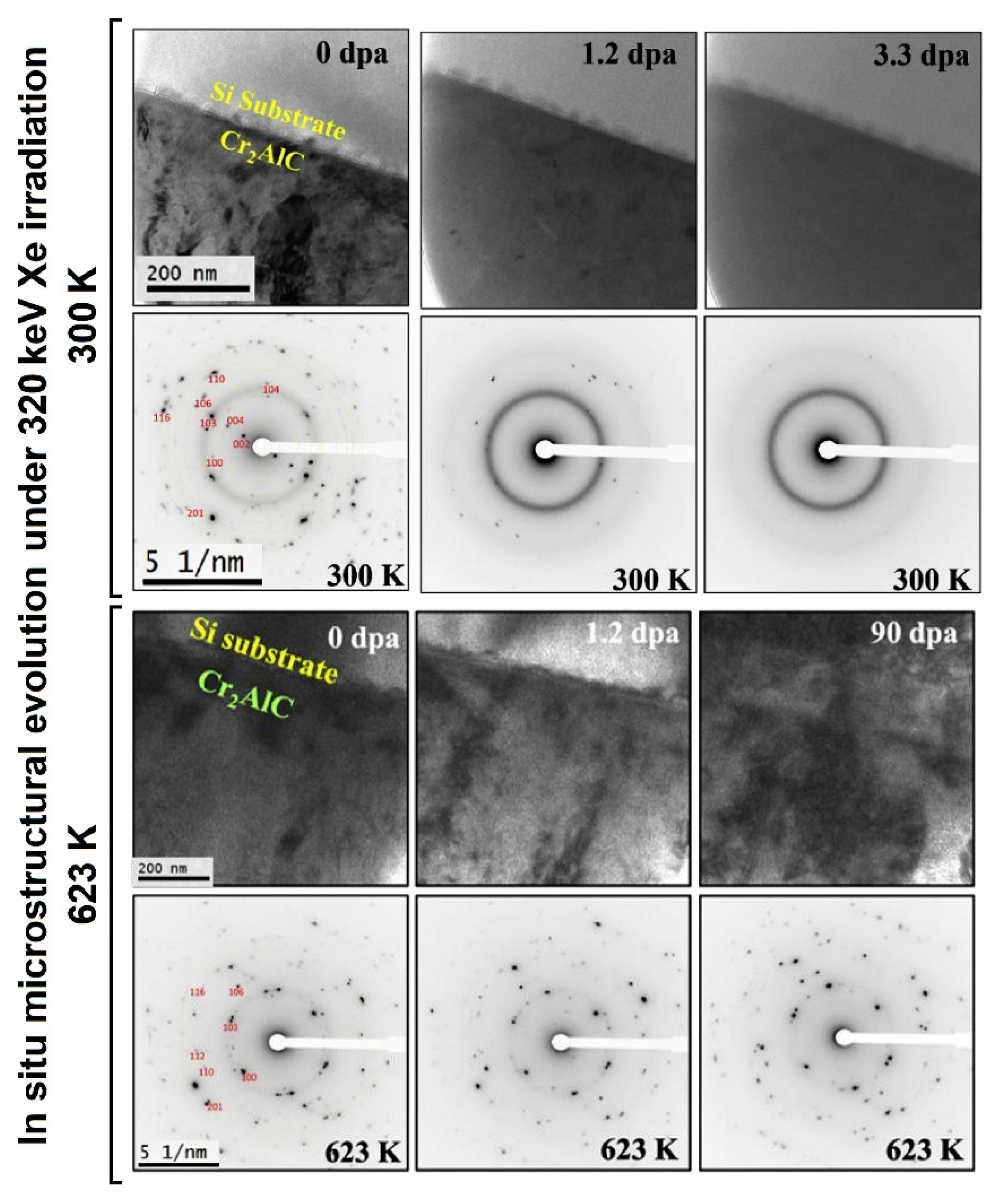
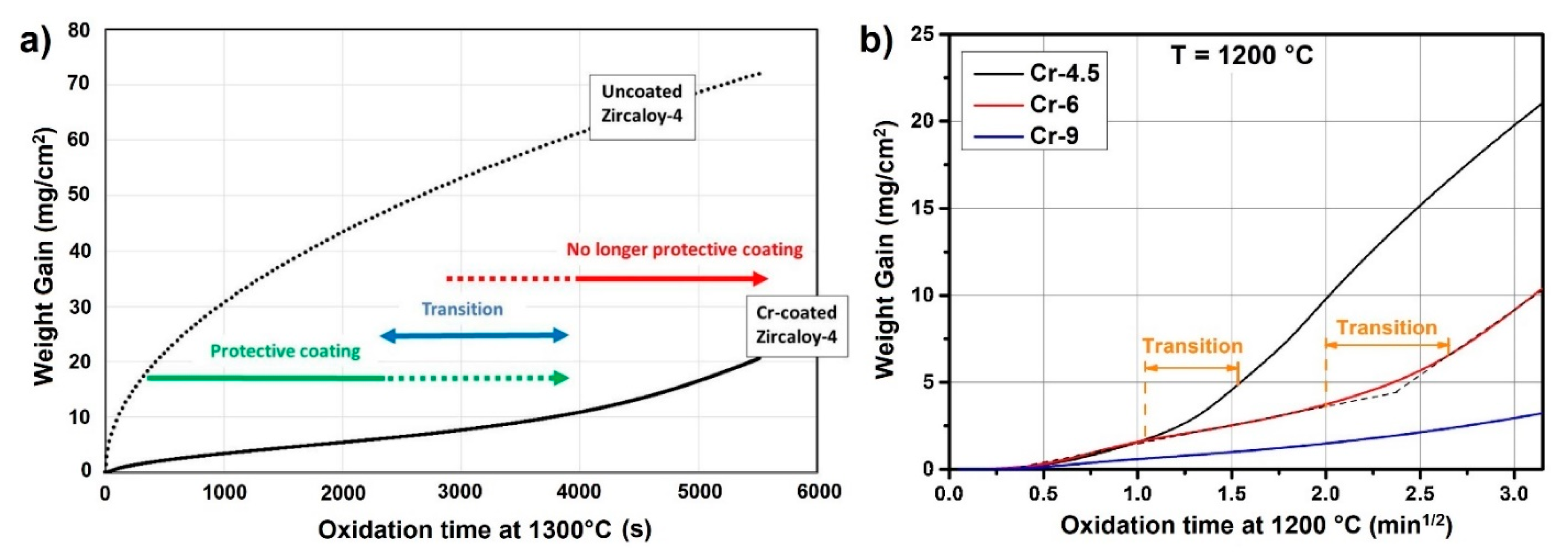
| Alloys | Sn | Fe | Cr | Ni | Nb |
|---|---|---|---|---|---|
| Zircaloy-2 | 1.50 | 0.12 | 0.10 | 0.05 | – |
| Zircaloy-4 | 1.50 | 0.20 | 0.10 | – | – |
| ZIRLO | 1.02 | 0.10 | – | – | 1.01 |
| OPT-ZIRLO | 0.66 | 0.11 | – | – | 1.04 |
| M5 | – | 0.05 | 0.015 | – | 1.0 |
| E110 | – | – | – | – | 0.95–1.05 |
| E110 opt. | – | 0.025–0.07 | – | – | 0.90–1.10 |
| E110M | – | 0.07–0.15 | – | – | 0.90–1.10 |
| E125 | – | – | – | – | 2.20–2.60 |
| E125 opt. | – | 0.025–0.05 | – | – | 2.40–2.70 |
| E635 | 1.1–1.3 | 0.30–0.40 | – | – | 2.40–1.05 |
| E635M | 0.70–0.90 | 0.30–0.40 | – | – | 0.70–0.90 |
| Interlayer | Possible Eutectics | Tm, °C | Diffusion Coefficient in β-Zr | Solubility in β-Zr at 1300 °C | CTE at RT, 10−6 K−1 | λ, W/m/K | Phase Transitions | Mechanical Behavior |
|---|---|---|---|---|---|---|---|---|
| Zr | – | 1855 | – | – | 7.2 (hcp) 9.6 (bcc) | 22 | αhcp → βbcc at 862 °C | Ductile |
| Cr | 1330 °C (Cr-Zr) | 1907 | High | <8 at.% | 6.5 | 93.7 | no | Ductile/Brittle |
| Mo | 1550 °C (Mo-Zr) | 2623 | Low | <30 at.% | 5.5 | 138 | no | Ductile |
| Ta | 1820 °C (Ta-Zr) 1760 °C (Ta-Cr) | 3017 | Low | ~15 at.% | 6.5 | 57.5 | Only for metastable β → α at 750–775 °C | Ductile |
| Nb | 1668 °C (Nb-Cr) | 2477 | High | 100 at.% | 7.1 | 53.7 | no | Ductile |
| Re | 1600 °C (Re-Zr) | 3186 | Unknown | ~10 at.% | 6.1-6.6 | 39.6 | no | Ductile |
| CrN (Cr2N) | – | 1770 | N diffusion due to CrN → Cr2N | – | 9.0-9.4 | 2-5 (CrN); 12 (Cr2N) | CrN → Cr2N at HT | Brittle |
| Cr2O3 | – | 2330 | – | – | 7.1 | 12 | no | Brittle |
| ZrO2 | – | 2715 | – | – | 7 (m-ZrO2) 12 (t-ZrO2) | 1.7 (m-ZrO2) | t → m-ZrO2 (high volume expansion) | Brittle |
| ZrC | – | 3532 | – | – | 6.7 | 20.5 | no | Brittle |
| 3C-SiC | 1370 °C (Zr-Si) | 2830 | – | – | 4.3 | 270 | no | Brittle |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashkarov, E.; Afornu, B.; Sidelev, D.; Krinitcyn, M.; Gouws, V.; Lider, A. Recent Advances in Protective Coatings for Accident Tolerant Zr-Based Fuel Claddings. Coatings 2021, 11, 557. https://doi.org/10.3390/coatings11050557
Kashkarov E, Afornu B, Sidelev D, Krinitcyn M, Gouws V, Lider A. Recent Advances in Protective Coatings for Accident Tolerant Zr-Based Fuel Claddings. Coatings. 2021; 11(5):557. https://doi.org/10.3390/coatings11050557
Chicago/Turabian StyleKashkarov, Egor, Bright Afornu, Dmitrii Sidelev, Maksim Krinitcyn, Veronica Gouws, and Andrey Lider. 2021. "Recent Advances in Protective Coatings for Accident Tolerant Zr-Based Fuel Claddings" Coatings 11, no. 5: 557. https://doi.org/10.3390/coatings11050557
APA StyleKashkarov, E., Afornu, B., Sidelev, D., Krinitcyn, M., Gouws, V., & Lider, A. (2021). Recent Advances in Protective Coatings for Accident Tolerant Zr-Based Fuel Claddings. Coatings, 11(5), 557. https://doi.org/10.3390/coatings11050557









