The Microstructure and Conductivity Evolution of Plasma-Sprayed (Mn, Co)3O4 Spinel Coatings during Conductivity Measurements at Elevated Temperature
Abstract
1. Introduction
2. Methodology
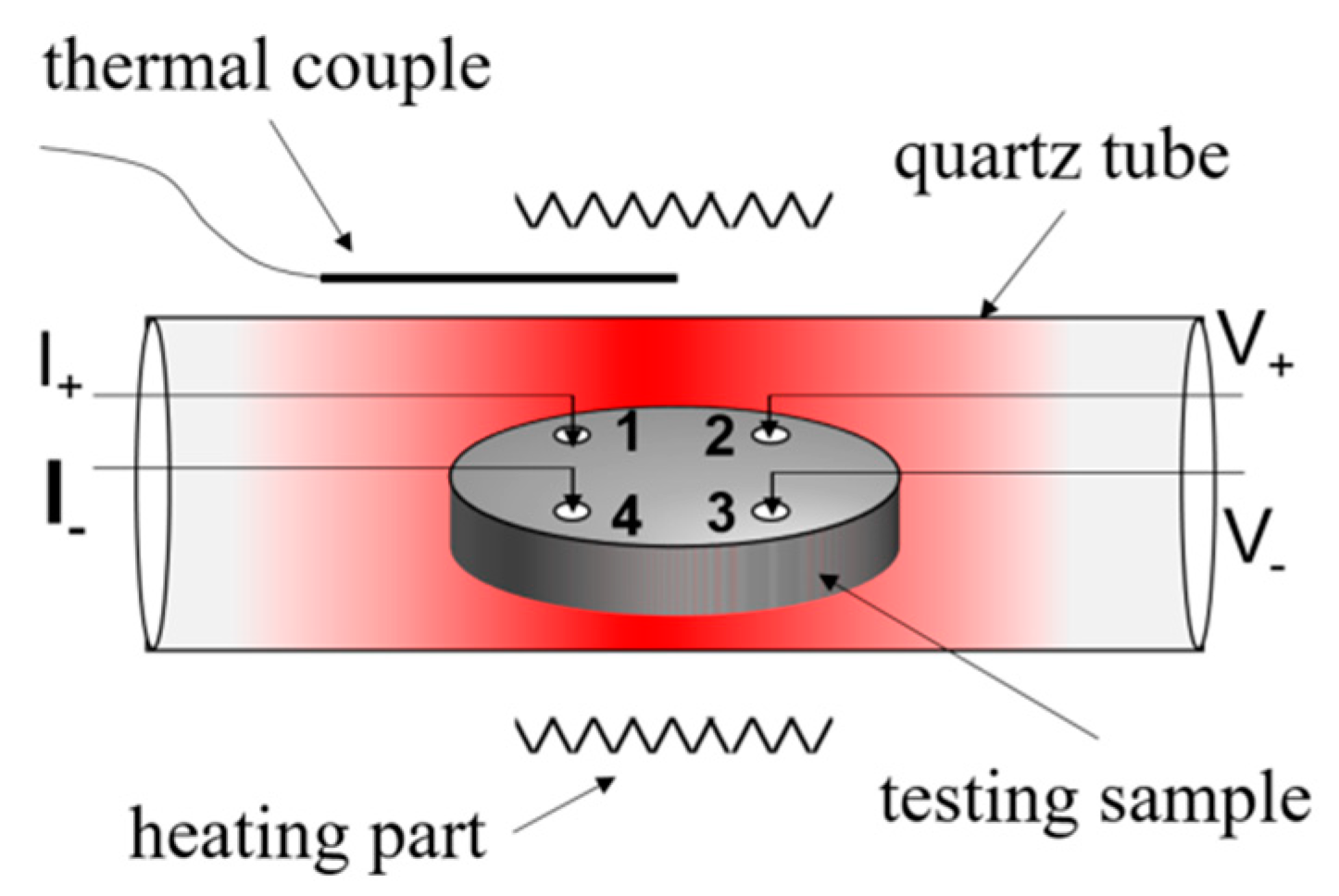
2.1. Experimental Setups and the Preparation of Coatings
2.2. Characterization of MnCoO Spinel Coatings
3. Results and Discussion
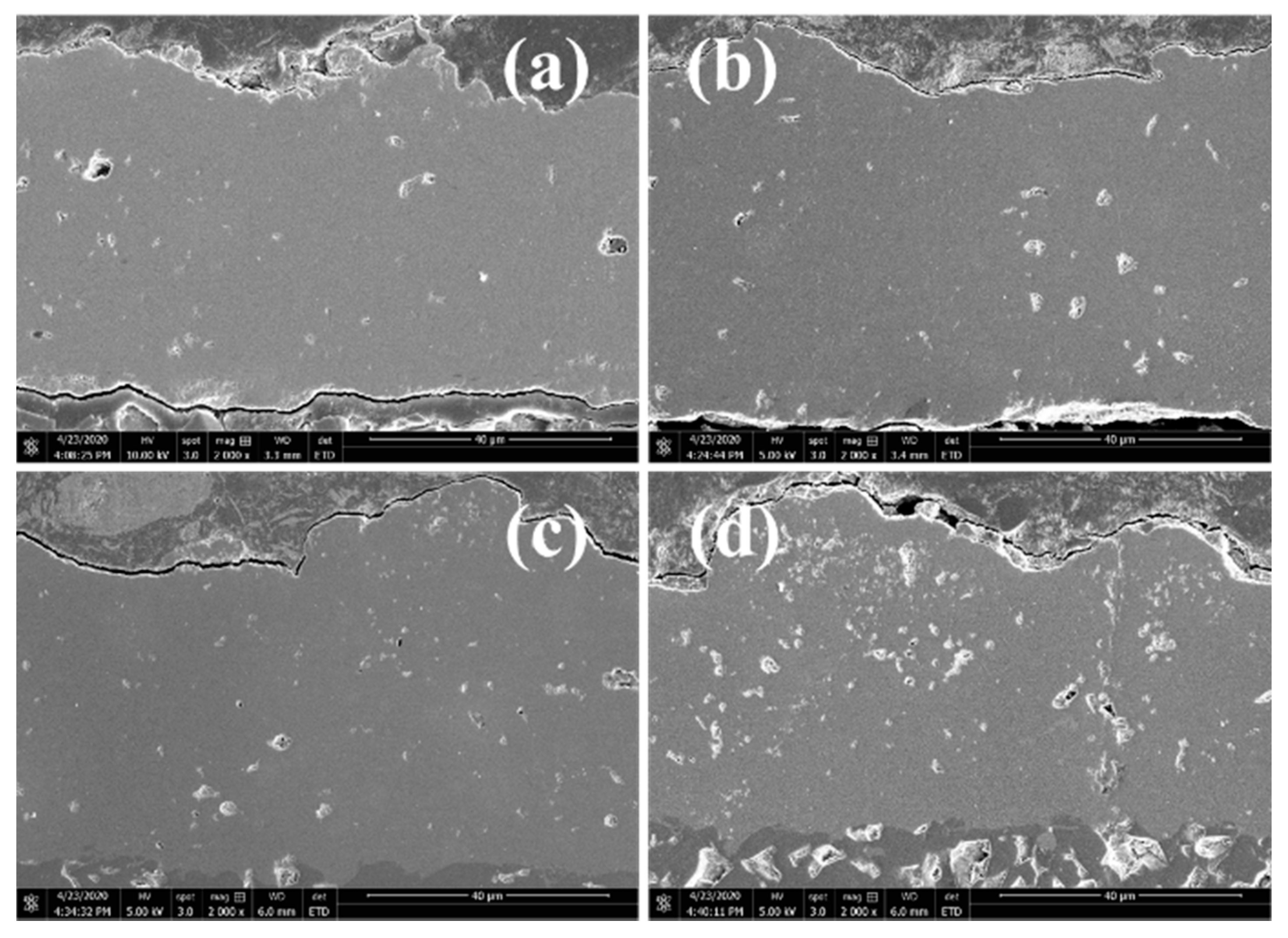
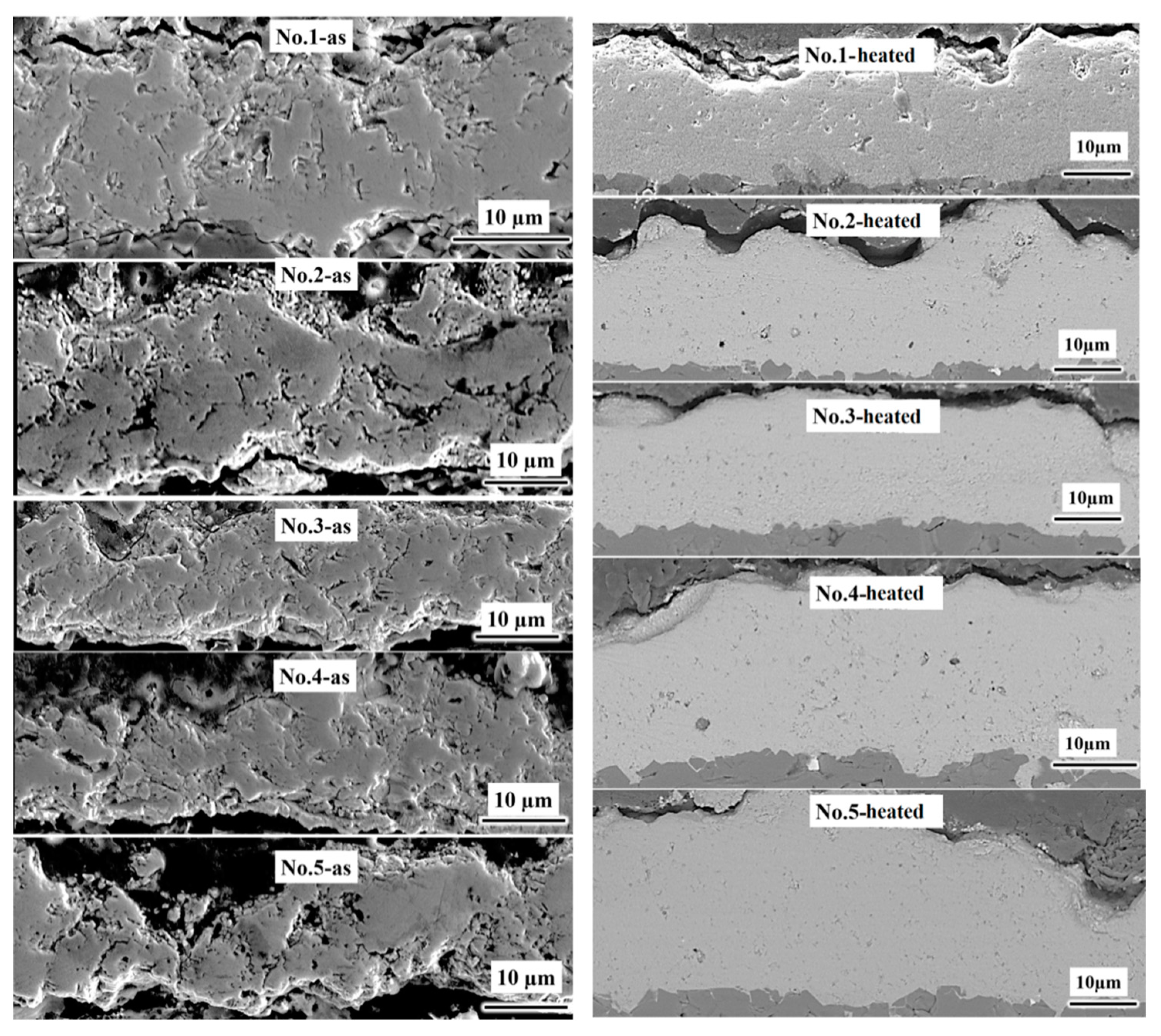
3.1. Morphology of MnCoO Spinel Coatings
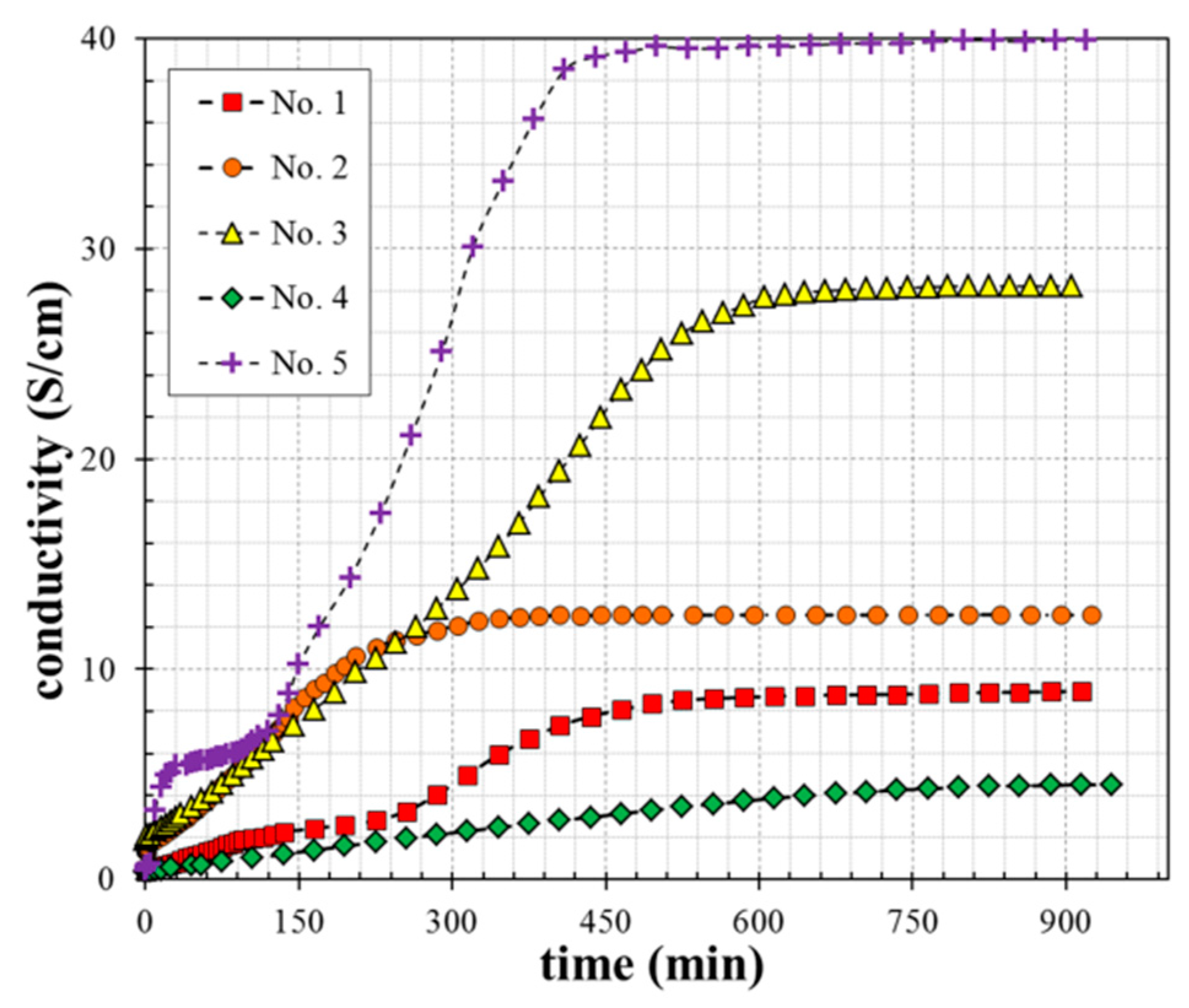
3.2. Electrical Conductivity
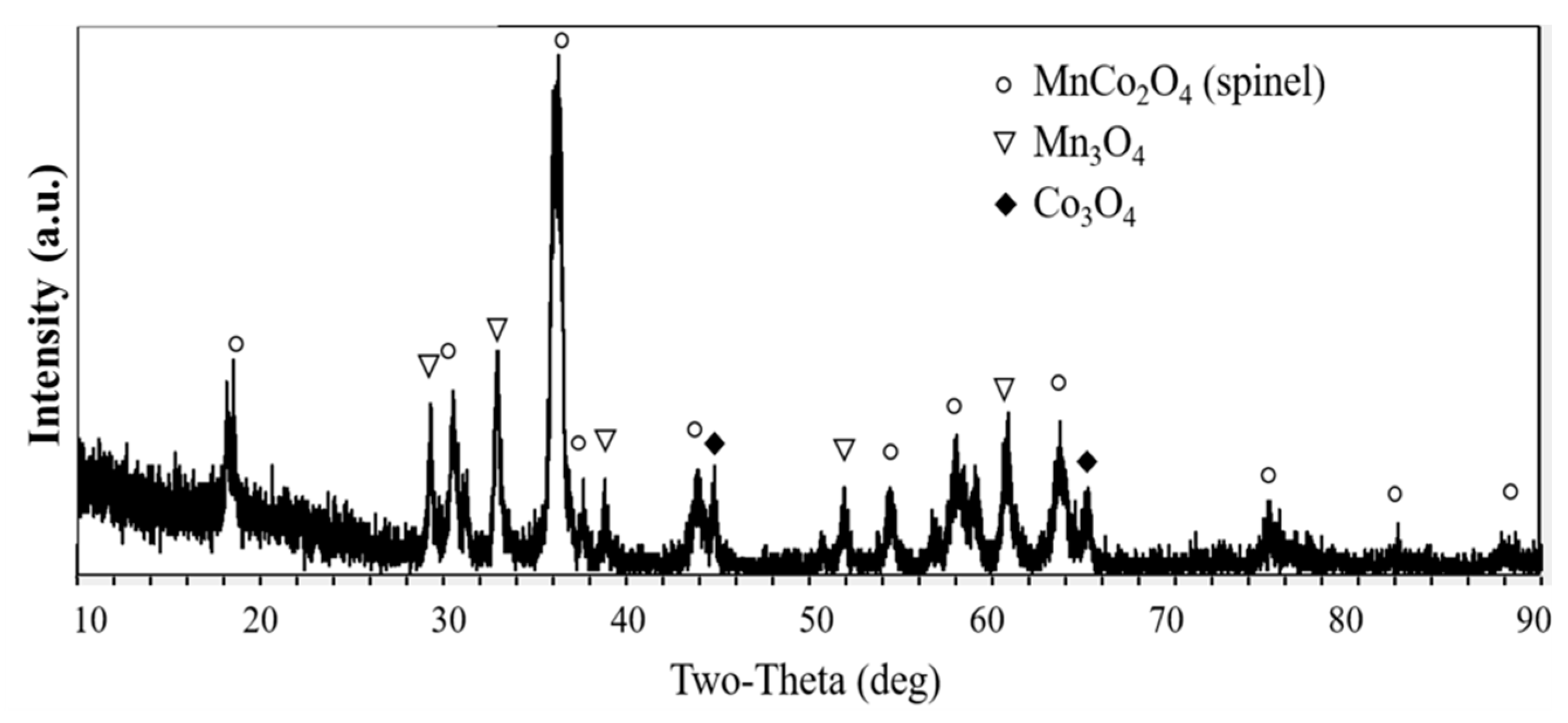
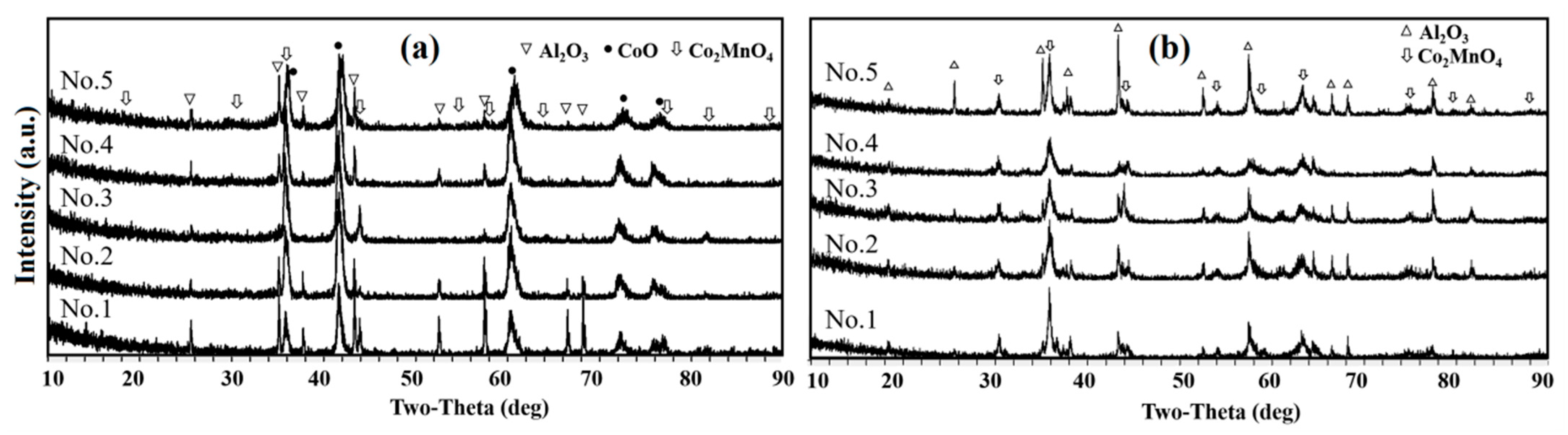
3.3. Phase Composition
3.4. Elemental Composition
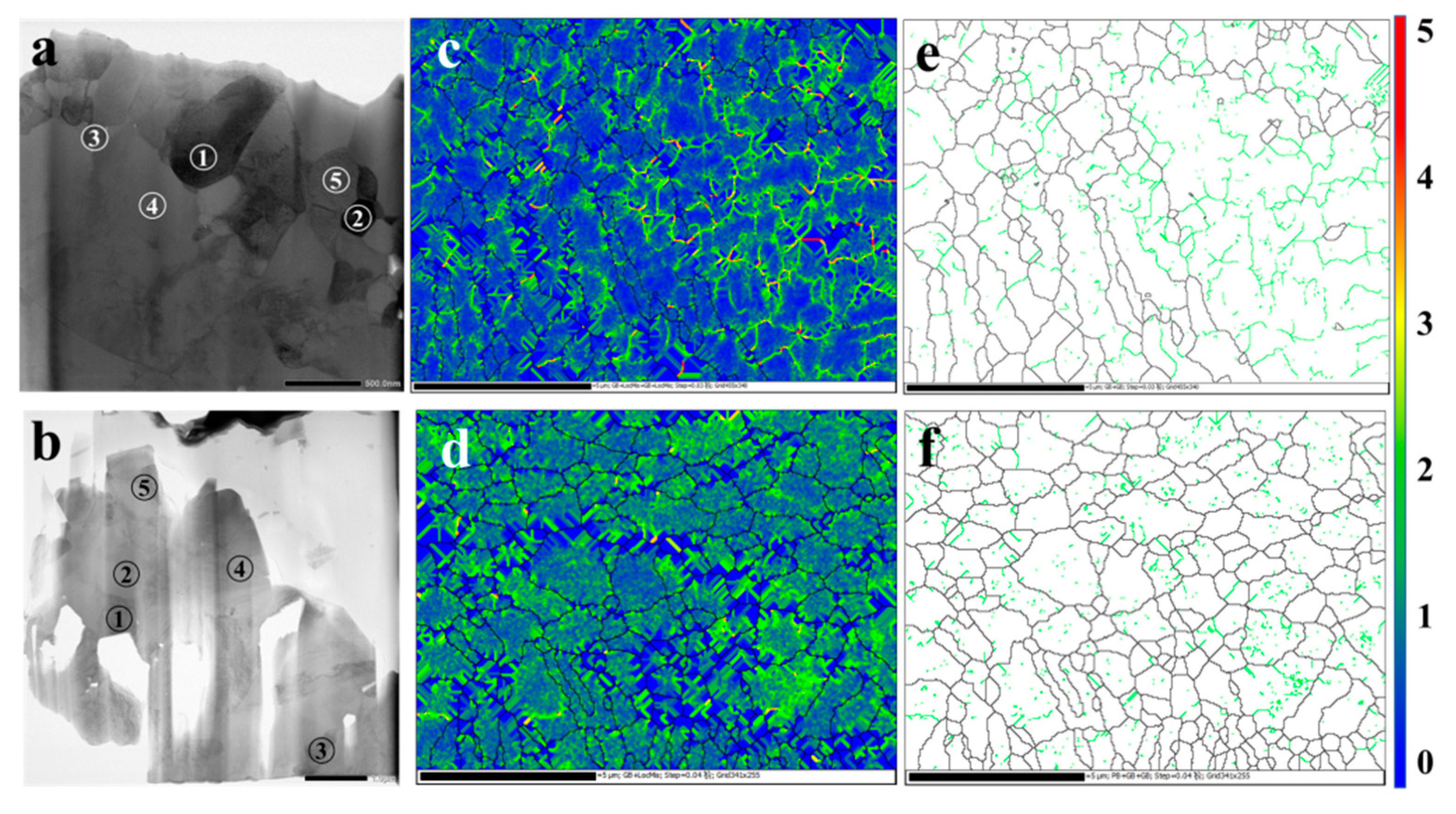
3.5. The Microstructure of Sliced Samples
3.6. Effects of Spray Conditions on Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
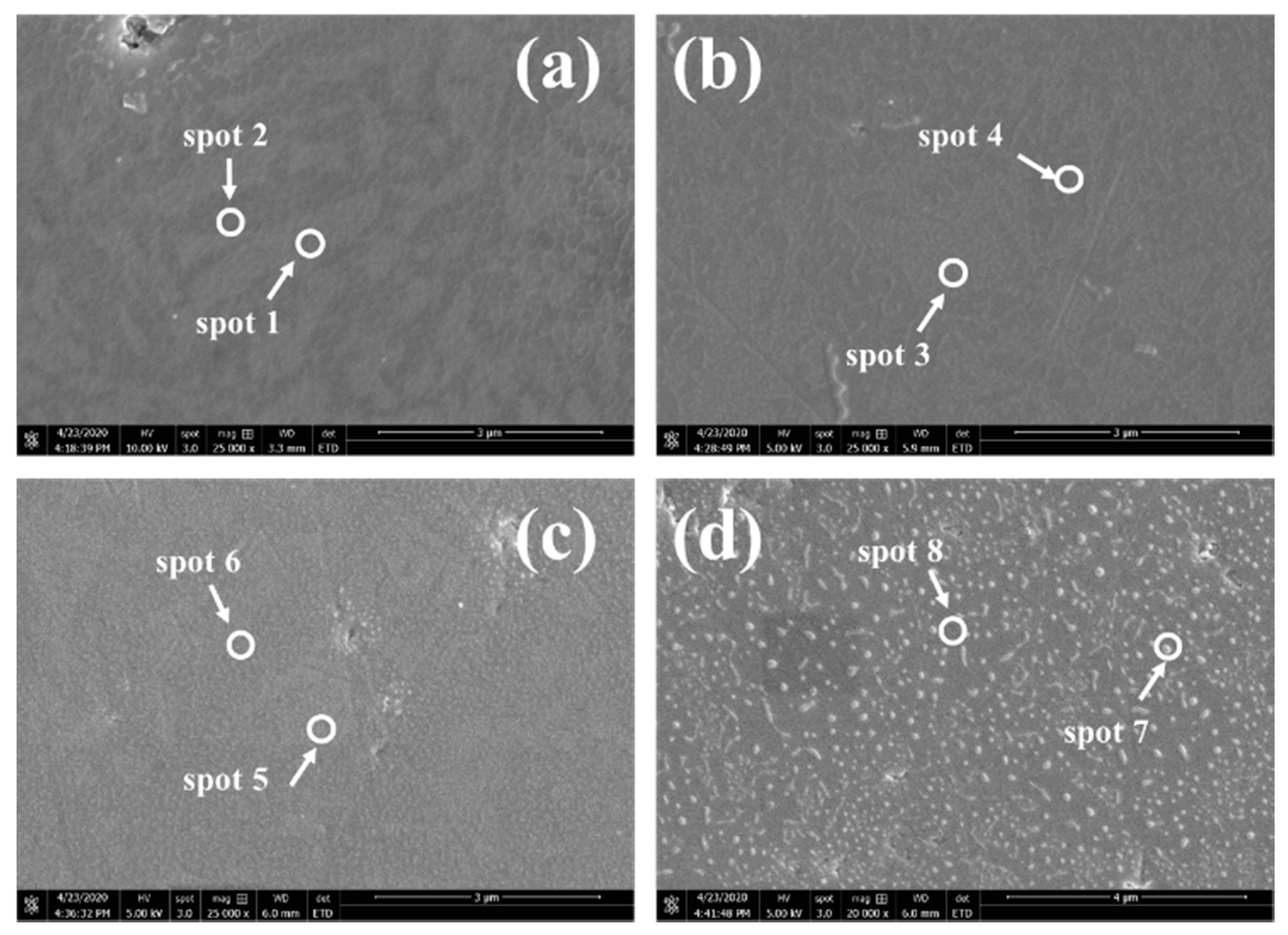
| at.% | 2 h | 5 h | 11 h | 20 h | ||||
|---|---|---|---|---|---|---|---|---|
| Spot 1 | Spot 2 | Spot 3 | Spot 4 | Spot 5 | Spot 6 | Spot 7 | Spot 8 | |
| Mn | 29.12 | 51.67 | 3.05 | 16.48 | 0.03 | 14.60 | 4.81 | 13.70 |
| Co | 24.04 | 3.97 | 28.78 | 3.52 | 39.27 | 5.00 | 23.02 | 6.82 |
| O | 46.84 | 44.35 | 68.17 | 80.00 | 61.33 | 80.40 | 72.17 | 79.48 |


References
- Yang, Z. Recent Advances in Metallic Interconnects for Solid Oxide Fuel Cells. Int. Mater. Rev. 2008, 53, 39–54. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Deevi, S.C. Development of Interconnect Materials for Solid Oxide Fuel Cells. Mater. Sci. Eng. A 2003, 348, 227–243. [Google Scholar] [CrossRef]
- Fergus, J.W. Metallic Interconnects for Solid Oxide Fuel Cells. Mater. Sci. Eng. A 2005, 397, 271–283. [Google Scholar] [CrossRef]
- Jo, K.H.; Kim, J.H.; Kim, K.M.; Lee, I.-S.; Kim, S.-J. Development of a New Cost Effective Fe–Cr Ferritic Stainless Steel for SOFC Interconnect. Int. J. Hydrog. Energy 2015, 40, 9523–9529. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, Y.; Yang, J.; Chen, J.; Chi, B.; Pu, J.; Li, J. A Novel Low Cr-Containing Fe–Cr–Co Alloy for Metallic Interconnects in Planar Intermediate Temperature Solid Oxide Fuel Cells. J. Power Sources 2014, 271, 25–31. [Google Scholar] [CrossRef]
- Geng, S.; Zhu, J. Promising Alloys for Intermediate-Temperature Solid Oxide Fuel Cell Interconnect Application. J. Power Sources 2006, 160, 1009–1016. [Google Scholar] [CrossRef]
- Geng, S.J.; Zhu, J.H.; Lu, Z.G. Evaluation of Several Alloys for Solid Oxide Fuel Cell Interconnect Application. Scr. Mater. 2006, 55, 239–242. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X. Recent Development of SOFC Metallic Interconnect. J. Mater. Sci. Technol. 2010, 26, 293–305. [Google Scholar] [CrossRef]
- Yang, Z.; Weil, K.S.; Paxton, D.M.; Stevenson, J.W. Selection and Evaluation of Heat-Resistant Alloys for SOFC Interconnect Applications. J. Electrochem. Soc. 2003, 150, A1188. [Google Scholar] [CrossRef]
- Hilpert, K.; Das, D.; Miller, M.; Peck, D.H.; Weiß, R. Chromium Vapor Species over Solid Oxide Fuel Cell Interconnect Materials and Their Potential for Degradation Processes. J. Electrochem. Soc. 2019, 143, 3642–3647. [Google Scholar] [CrossRef]
- Jiang, S.P.; Zhen, Y. Mechanism of Cr Deposition and Its Application in the Development of Cr-Tolerant Cathodes of Solid Oxide Fuel Cells. Solid State Ion. 2008, 179, 1459–1464. [Google Scholar] [CrossRef]
- Yokokawa, H.; Horita, T.; Sakai, N.; Yamaji, K.; Brito, M.E.; Xiong, Y.-P.; Kishimoto, H. Thermodynamic Considerations on Cr Poisoning in SOFC Cathodes. Solid State Ion. 2006, 177, 3193–3198. [Google Scholar] [CrossRef]
- Zeng, Z.; Natesan, K. Corrosion of Metallic Interconnects for SOFC in Fuel Gases. Solid State Ion. 2004, 167, 9–16. [Google Scholar] [CrossRef]
- Mosbaek, R.R.; Hjelm, J.; Barfod, R.; Høgh, J.; Hendriksen, P.V. Electrochemical Characterization and Degradation Analysis of Large SOFC Stacks by Impedance Spectroscopy. Fuel Cells 2013, 13, 605–611. [Google Scholar] [CrossRef]
- Qu, W.; Jian, L.; Hill, J.M.; Ivey, D.G. Electrical and Microstructural Characterization of Spinel Phases as Potential Coatings for SOFC Metallic Interconnects. J. Power Sources 2006, 153, 114–124. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A Review of Recent Progress in Coatings, Surface Modifications and Alloy Developments for Solid Oxide Fuel Cell Ferritic Stainless Steel Interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Bateni, M.R.; Wei, P.; Deng, X.; Petric, A. Spinel Coatings for UNS 430 Stainless Steel Interconnects. Surf. Coat. Technol. 2007, 201, 4677–4684. [Google Scholar] [CrossRef]
- Zhu, J.H.; Zhang, Y.; Basu, A.; Lu, Z.G.; Paranthaman, M.; Lee, D.F.; Payzant, E.A. LaCrO3-Based Coatings on Ferritic Stainless Steel for Solid Oxide Fuel Cell Interconnect Applications. Surf. Coat. Technol. 2004, 177–178, 65–72. [Google Scholar] [CrossRef]
- Johnson, C.; Gemmen, R.; Orlovskaya, N. Nano-Structured Self-Assembled LaCrO3 Thin Film Deposited by RF-Magnetron Sputtering on a Stainless Steel Interconnect Material. Compos. Part B Eng. 2004, 35, 167–172. [Google Scholar] [CrossRef]
- Qiu, P.; Li, J.; Jia, L.; Chi, B.; Pu, J.; Li, J.; Chen, F. LaCoO3-δ Coated Ba0.5Sr0.5Co0.8Fe0.2O3-δ Cathode for Intermediate Temperature Solid Oxide Fuel Cells. Electrochim. Acta 2019, 319, 981–989. [Google Scholar] [CrossRef]
- Molin, S.; Kusz, B.; Gazda, M.; Jasinski, P. Protective Coatings for Stainless Steel for SOFC Applications. J. Solid State Electrochem. 2009, 13, 1695–1700. [Google Scholar] [CrossRef]
- Fujita, K.; Ogasawara, K.; Matsuzaki, Y.; Sakurai, T. Prevention of SOFC Cathode Degradation in Contact with Cr-Containing Alloy. J. Power Sources 2004, 131, 261–269. [Google Scholar] [CrossRef]
- Kamecki, B.; Karczewski, J.; Miruszewski, T.; Jasiński, G.; Szymczewska, D.; Jasiński, P.; Molin, S. Low Temperature Deposition of Dense MnCo2O4 Protective Coatings for Steel Interconnects of Solid Oxide Cells. J. Eur. Ceram. Soc. 2018, 38, 4576–4579. [Google Scholar] [CrossRef]
- Hu, Y.-Z.; Li, C.-X.; Yang, G.-J.; Li, C.-J. Evolution of Microstructure during Annealing of Mn1.5Co1.5O4 Spinel Coatings Deposited by Atmospheric Plasma Spray. Int. J. Hydrog. Energy 2014, 39, 13844–13851. [Google Scholar] [CrossRef]
- Dayaghi, A.M.; Askari, M.; Rashtchi, H.; Gannon, P. Fabrication and High-Temperature Corrosion of Sol–Gel Mn/Co Oxide Spinel Coating on AISI 430. Surf. Coat. Technol. 2013, 223, 110–114. [Google Scholar] [CrossRef]
- Yang, Z.; Simner, S.P.; Singh, P.; Xia, G.; Stevenson, J.W. Development of (Mn, Co)3O4 Protection Layers for Ferritic Stainless Steel Interconnects; PNNL-15303; 1042570–97; Pacific Northwest National Laboratory: Washington, WA, USA, 2005. [Google Scholar]
- Zhang, Y.; Javed, A.; Zhou, M.; Liang, S.; Xiao, P. Fabrication of Mn-Co Spinel Coatings on Crofer 22 APU Stainless Steel by Electrophoretic Deposition for Interconnect Applications in Solid Oxide Fuel Cells. Int. J. Appl. Ceram. Technol. 2014, 11, 332–341. [Google Scholar] [CrossRef]
- Kruk, A.; Stygar, M.; Brylewski, T. Mn–Co Spinel Protective–Conductive Coating on AL453 Ferritic Stainless Steel for IT-SOFC Interconnect Applications. J. Solid State Electrochem. 2013, 17, 993–1003. [Google Scholar] [CrossRef]
- Miccoli, I.; Edler, F.; Pfnür, H.; Tegenkamp, C. The 100th Anniversary of the Four-Point Probe Technique: The Role of Probe Geometries in Isotropic and Anisotropic Systems. J. Phys. Condens. Matter 2015, 27, 223201. [Google Scholar] [CrossRef]
- Aukrust, E.; Muan, A. Phase Relations in the System Cobalt Oxide-Manganese Oxide in Air. J. Am. Ceram. Soc. 1963, 46, 511. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Morphology-Controllable Synthesis of Cobalt Oxalates and Their Conversion to Mesoporous Co3O4 Nanostructures for Application in Supercapacitors. Inorg. Chem. 2011, 50, 6482–6492. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Li, X.; Qian, Y. A Facile Route to Synthesize Multiporous MnCo2O4 and CoMn2O4 Spinel Quasi-Hollow Spheres with Improved Lithium Storage Properties. Nanoscale 2013, 5, 2045. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiong, D.; Wang, L.; Hirbod, M.K.S.; Li, X. High-Performance Self-Assembly MnCo2O4 Nanosheets for Asymmetric Supercapacitors. J. Energy Chem. 2019, 37, 66–72. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, S.; Yao, M.; Yang, L.; Zhu, Y.; Liu, P.; Xing, O.; Zhou, J.; Wang, M.; Luo, H.; et al. A Low-Cost, Self-Standing NiCo2O4@CNT/CNT Multilayer Electrode for Flexible Asymmetric Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702160. [Google Scholar] [CrossRef]


| Sets | Current (A) | Voltage (V) | Input Power (kW) | Primary Gas (Ar, L/min) | Secondary Gas (H2, L/min) |
|---|---|---|---|---|---|
| 1 | 450 | 79 | 35 | 50 | 11 |
| 2 | 500 | 79 | 40 | 50 | 11 |
| 3 | 650 | 79 | 50 | 50 | 11 |
| 4 | 650 | 69 | 45 | 40 | 7 |
| 5 | 650 | 84 | 55 | 70 | 7 |
| Sample | As-Sprayed/(%) | Heated/(%) |
|---|---|---|
| No. 1 | 8.37 ± 0.72 | 3.04 ± 0.66 |
| No. 2 | 7.70 ± 1.10 | 1.82 ± 0.65 |
| No. 3 | 7.80 ± 1.43 | 3.05 ± 0.39 |
| No. 4 | 9.90 ± 0.01 | 2.16 ± 0.19 |
| No. 5 | 7.83 ± 0.84 | 2.02 ± 0.66 |
| Sample | Conductivity in Stage I | Conductivity in Stage II | Conductivity in Stage III |
|---|---|---|---|
| No. 1 | 3 S/cm | 9 S/cm | 9 S/cm |
| No. 2 | 6 S/cm | 12 S/cm | 12.5 S/cm |
| No. 3 | 14 S/cm | 28 S/cm | 28 S/cm |
| No. 4 | 2 S/cm | 4 S/cm | 4.5 S/cm |
| No. 5 | 10 S/cm | 39 S/cm | 40 S/cm |
| Elements | Proportions of As-Sprayed Samples (%) | Proportions of HEATED SAMPles (%) | ||
|---|---|---|---|---|
| No. 4 | No. 5 | No. 4 | No. 5 | |
| Co3+ | 72.65 | 69.47 | 47.43 | 82.59 |
| Co2+ | 27.35 | 30.52 | 52.57 | 17.41 |
| Mn2+ | 14.27 | 13.56 | 1.90 | 13.00 |
| Mn3+ | 38.04 | 45.17 | 57.34 | 29.04 |
| Mn4+ | 47.69 | 42.27 | 40.76 | 57.96 |
| Percentage (at.%) | Spot 1 | Spot 2 | Spot 3 | Spot 4 | Spot 5 | Average | Mn:Co:O | |
|---|---|---|---|---|---|---|---|---|
| Sample No. 4 | Mn | 26.68 | 25.77 | 23.75 | 25.17 | 24.93 | 25.26 | 1 |
| Co | 30.39 | 30.31 | 30.11 | 27.96 | 29.76 | 29.71 | 1.18 | |
| O | 42.93 | 43.92 | 46.14 | 46.87 | 45.31 | 45.03 | 1.78 | |
| Sample No. 5 | Mn | 18.23 | 17.58 | 17.39 | 25.27 | 25.65 | 20.82 | 1 |
| Co | 19.18 | 16.99 | 17.81 | 26.82 | 28.21 | 21.80 | 1.05 | |
| O | 62.59 | 65.43 | 64.8 | 47.91 | 46.14 | 57.37 | 2.76 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Song, C.; Wen, K.; Liu, T.; Deng, C.; Liu, M.; Yang, C. The Microstructure and Conductivity Evolution of Plasma-Sprayed (Mn, Co)3O4 Spinel Coatings during Conductivity Measurements at Elevated Temperature. Coatings 2021, 11, 533. https://doi.org/10.3390/coatings11050533
Zou J, Song C, Wen K, Liu T, Deng C, Liu M, Yang C. The Microstructure and Conductivity Evolution of Plasma-Sprayed (Mn, Co)3O4 Spinel Coatings during Conductivity Measurements at Elevated Temperature. Coatings. 2021; 11(5):533. https://doi.org/10.3390/coatings11050533
Chicago/Turabian StyleZou, Jianbo, Chen Song, Kui Wen, Taikai Liu, Chunming Deng, Min Liu, and Chenghao Yang. 2021. "The Microstructure and Conductivity Evolution of Plasma-Sprayed (Mn, Co)3O4 Spinel Coatings during Conductivity Measurements at Elevated Temperature" Coatings 11, no. 5: 533. https://doi.org/10.3390/coatings11050533
APA StyleZou, J., Song, C., Wen, K., Liu, T., Deng, C., Liu, M., & Yang, C. (2021). The Microstructure and Conductivity Evolution of Plasma-Sprayed (Mn, Co)3O4 Spinel Coatings during Conductivity Measurements at Elevated Temperature. Coatings, 11(5), 533. https://doi.org/10.3390/coatings11050533






