Mechanical Equilibrium Dynamics Controlling Wetting State Transition at Low-Temperature Superhydrophobic Array-Microstructure Surfaces
Abstract
1. Introduction
2. Materials and Methods
3. Results
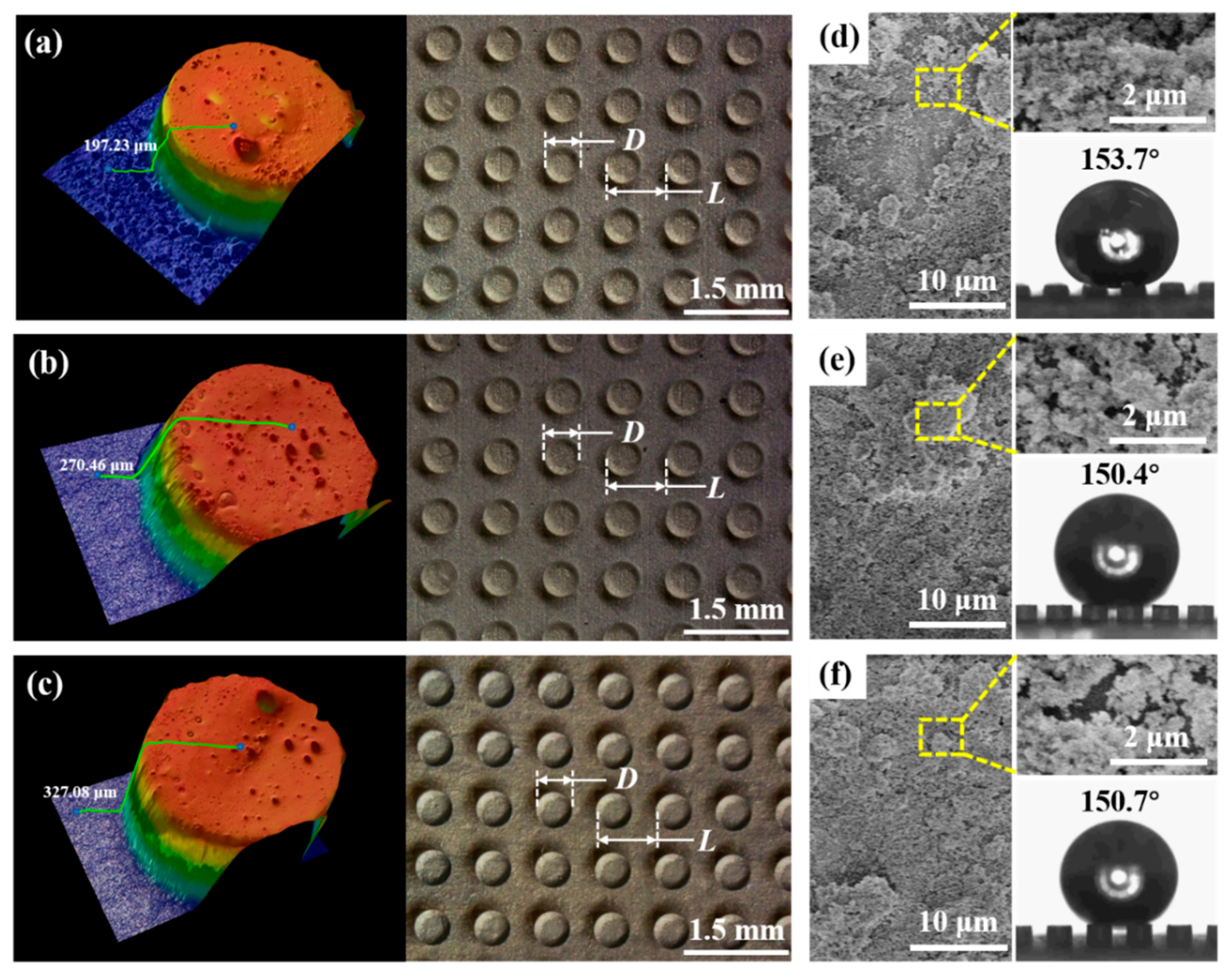
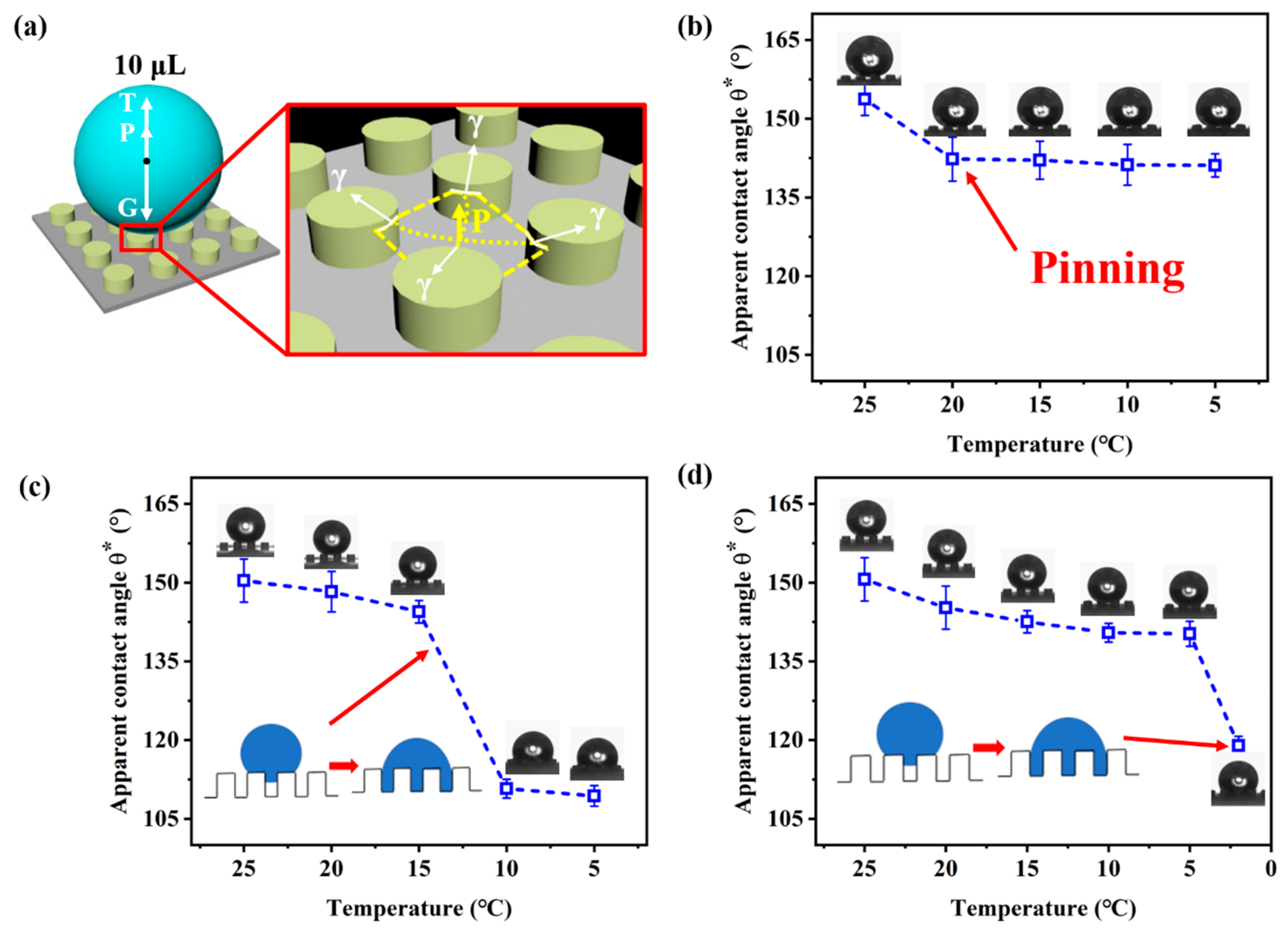
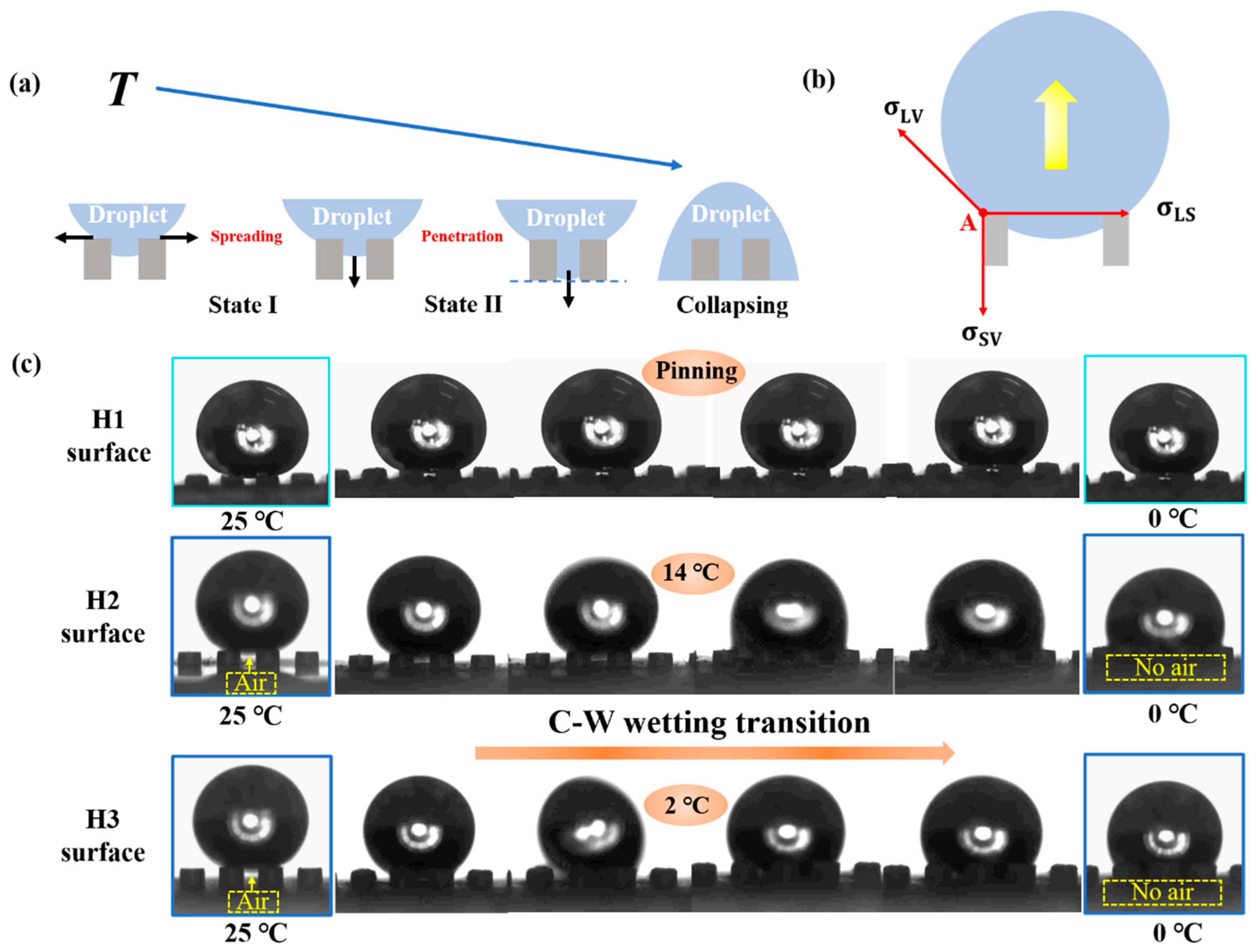
3.1. Non-Wetting Behavior at Low Temperature
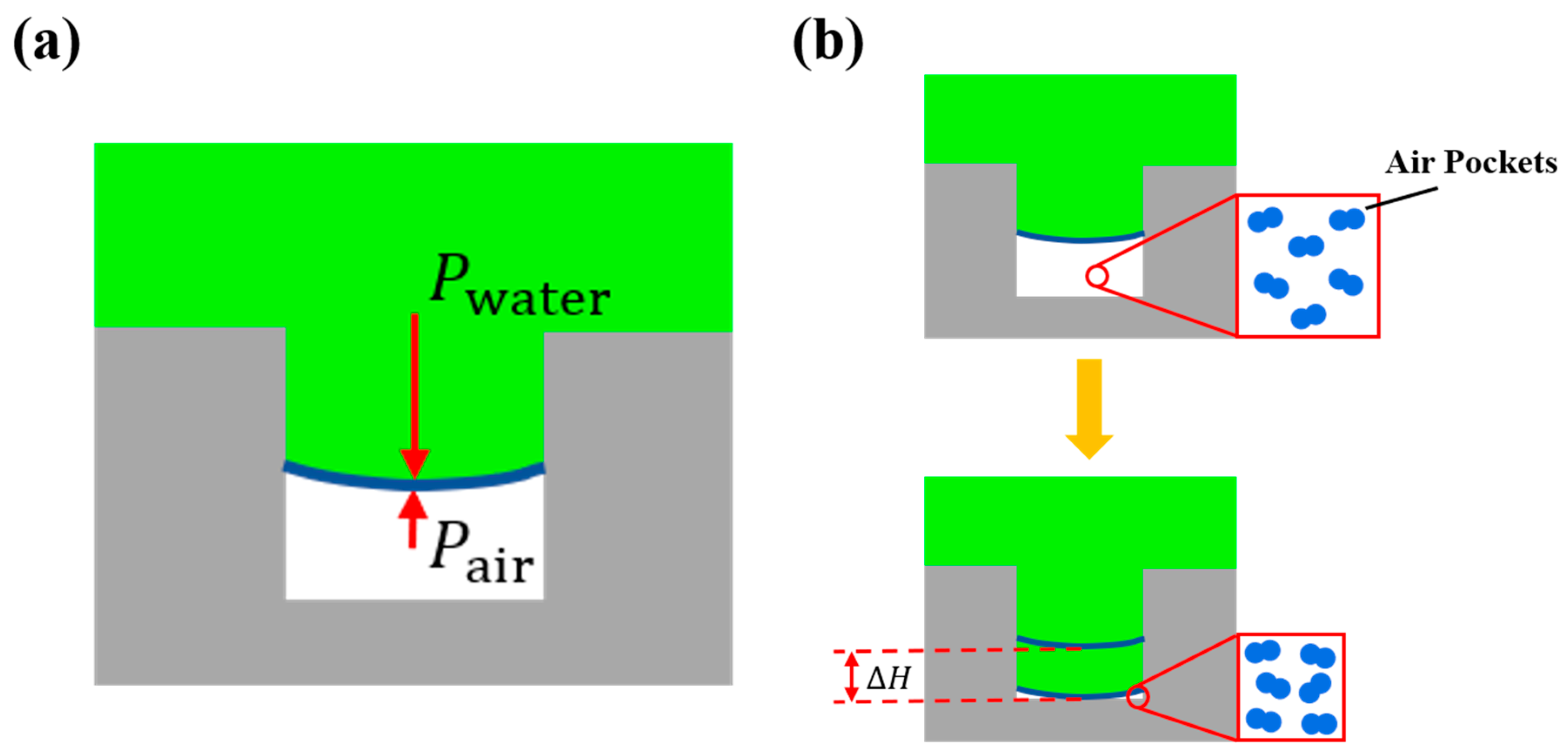
3.2. State of the Solid-Liquid Contact Interface at Low Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, J.C.; Coninck, J.D.; Wu, H.A.; Wang, F.C. Microscopic origin of capillary force balance at contact line. Phys. Rev. Lett. 2020, 124, 125502. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Fernandino, M.; Dorao, C.A. Conical micro-structures as a route for achieving super-repellency in surfaces with intrinsic hydrophobic properties. Appl. Phys. Lett. 2019, 115, 053703. [Google Scholar] [CrossRef]
- Li, S.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. A review on special wettability textiles: Theoretical models, fabrication technologies and multifunctional applications. J. Mater. Chem. A 2017, 5, 31–55. [Google Scholar] [CrossRef]
- Lai, Y.; Gao, X.; Zhuang, H.; Huang, J.; Lin, C.; Jiang, L. Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv. Mater. 2009, 21, 3799–3803. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Z.; Chen, T.; Xi, Y.; Zhang, J. Fabrication of superhydrophobic surface with desirable anti-icing performance based on micro/nano-structures and organosilane groups. Appl. Surf. Sci. 2020, 501, 144165. [Google Scholar] [CrossRef]
- Teisala, H.; Butt, H.J. Hierarchical structures for superhydrophobic and superoleophobic surfaces. Langmuir 2018, 35, 10689–10703. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.; Lammertink, R.G.; Wessling, M.; Lohse, D. Evaporation-triggered wetting transition for water droplets upon hydrophobic microstructures. Phys. Rev. Lett. 2010, 104, 116102. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Pogreb, R.; Whyman, G.; Bormashenko, Y.; Erlich, M. Vibration-induced Cassie-Wenzel wetting transition on rough surfaces. Appl. Phys. Lett. 2007, 90, 201917. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Yu, Y.; Zhao, Z.H. Effects of hydraulic pressure on the stability and transition of wetting modes of superhydrophobic surfaces. Langmuir 2005, 21, 12207–12212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, J.; Chen, L.; Zhai, J.; Song, Y.; Jiang, L. High-temperature wetting transition on micro- and nanostructured surfaces. Angew. Chem. 2011, 123, 5423–5426. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, X.; Kumar, D.; Ho, J.W.; Kanhere, P.D.; Srikanth, N.; Chen, Z. Development of sol-gel icephobic coatings: Effect of surface roughness and surface energy. ACS Appl. Mater. Interfaces 2014, 6, 20685–20692. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Li, X.; Sun, W. Mechanism study on transition of Cassie droplets to Wenzel state after meniscus touching substrate of pillars. J. Phys. Chem. C 2017, 121, 9802–9814. [Google Scholar] [CrossRef]
- Jiang, Y.; Lian, J.; Jiang, Z.; Li, Y.; Wen, C. Thermodynamic analysis on wetting states and wetting state transitions of rough surfaces. Adv. Colloid. Interface Sci. 2020, 278, 102136. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. From contact line structures to wetting dynamics. Langmuir 2019, 35, 10233–10245. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Cai, M.; Liu, W.; Luo, X.; Chen, C.; Zhang, H.; Zhong, M. Extremely high Cassie-Baxter state stability of superhydrophobic surfaces via precisely tunable dual-scale and triple-scale micro-nano structures. J. Mater. Chem. A 2019, 7, 18050–18062. [Google Scholar] [CrossRef]
- Gerber, J.; Lendenmann, T.; Eghlidi, H.; Schutzius, T.M.; Poulikakos, D. Wetting transitions in droplet drying on soft materials. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Papadopoulos, P.; Mammen, L.; Deng, X.; Vollmer, D.; Butt, H.J. How superhydrophobicity breaks down. Proc. Natl. Acad. Sci. USA 2013, 110, 3254–3258. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Guo, H.Y.; Li, B.; Li, Q.; Feng, X.Q. Revisiting the critical condition for the Cassie-Wenzel transition on micropillar-structured surfaces. Langmuir 2018, 34, 3838–3844. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Xie, X.; Tao, J.; Chen, H.; Cai, Z.; Liu, S.; Jiang, J. Mechanical Equilibrium Dynamics Controlling Wetting State Transition at Low-Temperature Superhydrophobic Array-Microstructure Surfaces. Coatings 2021, 11, 522. https://doi.org/10.3390/coatings11050522
Shen Y, Xie X, Tao J, Chen H, Cai Z, Liu S, Jiang J. Mechanical Equilibrium Dynamics Controlling Wetting State Transition at Low-Temperature Superhydrophobic Array-Microstructure Surfaces. Coatings. 2021; 11(5):522. https://doi.org/10.3390/coatings11050522
Chicago/Turabian StyleShen, Yizhou, Xinyu Xie, Jie Tao, Haifeng Chen, Zeyu Cai, Senyun Liu, and Jiawei Jiang. 2021. "Mechanical Equilibrium Dynamics Controlling Wetting State Transition at Low-Temperature Superhydrophobic Array-Microstructure Surfaces" Coatings 11, no. 5: 522. https://doi.org/10.3390/coatings11050522
APA StyleShen, Y., Xie, X., Tao, J., Chen, H., Cai, Z., Liu, S., & Jiang, J. (2021). Mechanical Equilibrium Dynamics Controlling Wetting State Transition at Low-Temperature Superhydrophobic Array-Microstructure Surfaces. Coatings, 11(5), 522. https://doi.org/10.3390/coatings11050522






