Abstract
Melamine sponge (MS) has the characteristics of multilayer network structure, high porosity, adjustable pore structure, and low price, which is considered to be an ideal material for oil leakage treatment. Here, a facile, economical, environmental friendly, and one step reaction was developed to fabricate Ag nanoparticles (NPs) decorated MS composite with tannic acid (TA) as a reducing agent. By reduction reaction, the super-hydrophobic and super-oleophylic surface of the MS–TA–Ag composite was formed, demonstrating a single selectivity for oil and water. Thus, the adsorption capacity of MS–TA–Ag composites for various oils/organic solvents can reach 48~129 times its own weight and and display superior efficiency to separate oil/organic solvent from the water. In addition, the MS–TA–Ag composite has stable super-hydrophobicity and reusability. It is exciting that modified MS exhibits excellent chemical stability properties after a long processing time with strong alkali, strong acids, and salt solutions. The simple strategy provides a method for the preparation of large-scale oil spill cleaning and recovery materials.
1. Introduction
With the development of economy and society, chemical reagent leakage and oil spill accidents happen frequently, which seriously harm the ecological environment and the healthy development of human beings [1,2]. Therefore, it is urgent to find an efficient method for oil–water separation. Because oil and water are incompatible, designing functional materials with special wettability has attracted considerable attention in recent years [3,4]. Super-hydrophobic materials are widely used in anti-fog, self-cleaning, liquid transportation, oil–water separation, water collection, anti-corrosion, and so on. In particular, super-hydrophobic super-oleophilic materials have been widely used in the field of oil–water separation and have become a hot topic in current research [5,6,7]. Super-hydrophobic materials can be constructed by creating suitable surface roughness with hierarchical microstructures/nanostructures and chemically modifying them with low surface energy materials. 3D porous materials have been studied as the substrate for super-hydrophobic materials owing to their specific surface area and strong adsorption [8,9,10]. Compared with the existing two-dimensional membrane and powder materials, 3D porous materials can be used to deal with oil spills and organic pollutants in a simple, rapid, and large-scale manner. Among the 3D porous materials, melamine sponge (MS) presents a promising prospect for practical applications because of its high porosity, large production scale, and low price [11,12,13,14]. However, pristine MS absorbs both water and oil at the same time. The functional modification needs be used to endow the MS with stable super-hydrophobicity and super-oleophilicity. Thus, the modified sponge can easily absorb oil from water thanks to its special selective wettability. The following two key techniques should be considered in preparing super-hydrophobic coatings on MS: introduce low surface energy materials and construct micro-nano rough surfaces. The general modified methods include dip coating, chemical vapor deposition, in situ chemical reaction, carbonization, atom transfer radical polymerization, radical copolymerization, and rapid microwave method [15,16,17,18]. These modified materials can skillfully switch the surface of hydrophilic sponge to hydrophobic, and the modified sponge material is expected to be the preferred material for oil leakage cleaning. To this end, various materials were selected to decorate commercial sponges, including silane [19,20], hydrophobic polymers [21,22], hydrophobic nanoparticles [23,24,25], carbon materials [26], and so on. However, in the process of constructing super-hydrophobic coatings on sponges, most of these approaches suffer from the disadvantages of complex equipment and tedious fabrication procedures [27,28,29]. Hence, it is of great significance to develop a simple and large-scale strategy for preparing super-hydrophobic MS for oil spill cleanup. Moreover, functional super-hydrophobic MS with high stability and reusability as well as excellent selectivity are worthy of further study [30]. Recently, it has been reported that superhydrophobic pure metal surfaces can be prepared by hydrophobic methods without the functionalization of hydrophobic molecules [31,32,33]. Under heating conditions, the surface energy of metals can be effectively reduced by spontaneous adsorption of carbon pollutants; it may provide a new, low-cost green pathway to fabricate superhydrophobic MS, without using any additional low surface energy materials.
In this study, the micro-nano hierarchical tannic acid (TA) metal complexes were deposited on MS through one step room temperature reaction, as shown in Figure 1. TA is a kind of plant extract, which has the advantage of wide source and low price [34,35]. The TA–Ag modified coating was developed through a TA reduction process. The modified coatings endow the MS substrate with stable super-hydrophobicity, super-oleophylicity, and reusability. It is exciting that the modified MS exhibits excellent hydrophobic properties after a long processing time with strong alkali, strong acids, and salt solutions. The simple strategy provides new methods for the preparation of large-scale oil spill cleanup and recovery materials.
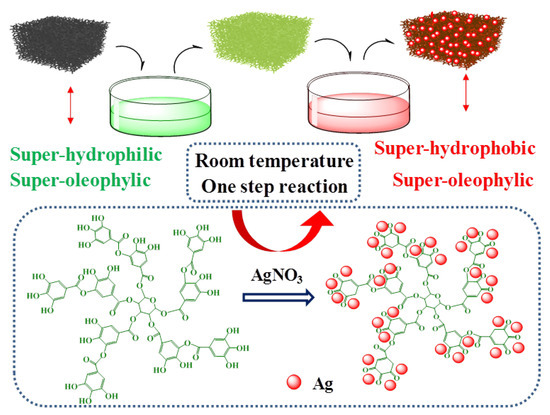
Figure 1.
Schematic of the preparation process of superhydrophobic sponge.
2. Experimental
2.1. Materials
MS was provided from an online store of Alibaba Co., Ltd. (Hangzhou, China). TA, silver nitrate (AgNO3), methyl blue, oil red, sodium chloride (NaCl), sodium hydroxide (NaOH), ethanol (99.5%), toluene (>99.8%), petroleum ether (>99%), dichloroethane (>99%), and hydrochloric acid (HCl, 32%) were purchased from Sigma-Aldrich (Shanghai, China). Gasoline, diesel oil, heat-conductive silicone oil, and corn oil were purchased from a local store. All chemicals were used as received without further purification.
2.2. Preparation of Super-Hydrophobic and Super-Oleophylic 3D Porous Nanocomposites
MS was washed sequentially with ethanol and deionized water in ultrasonic oscillator (Yumeng, YM-040S, Shenzhen, China) for three minutes to remove oil and dust from the surface of the substrate and dried in an oven (Heng Ruitong, DHG-9003, Haimen, China) at 65 °C. The dried sponge was first immersed in TA solution for a period of time and then used with deionized water to remove the excess TA. Finally, it was immersed in AgNO3 for 15 min, then cleaned with deionized water and dried at 65 °C. So far, the super-hydrophobic and super-oleophylic sponge was obtained.
2.3. Characterizations and Measurements
2.3.1. Structure and Morphology Characterizations
Infrared spectra of the samples were performed by Fourier transform infrared spectroscopy (FT-IR, Thermo Nicolet Corporationcompany, Nicolet-Nexus 670, Waltham, MA, USA) under the attenuated total reflection (ATR) mode. The samples were recorded with wavenumbers ranging from 400 to 4000 cm−1. X-ray photoelectron spectroscopy (XPS, Physical Electronics Inc., PHI-5700, Chanhassen, MN, USA) analyses were measured using a Kratos AXIS Ultra DLD spectrometer with a monochromatized Al Kα X-ray source at the photoelectron take-off angle of 90°. Surface morphologies, internal skeleton structure, and element distribution of the samples were characterized by scanning electron microscopy (SEM, ZEISS, Gemini 300, Jena, Germany) and energy-dispersive spectroscopy (EDS). The voltage was 5 kV and the surface of samples should be sputtered with platinum for 6 min. The static and dynamic contact angles tests of the samples were measured by a contact angle measuring system (Sindin, SDC-100, Shenzhen, China). Each sample was measured three times at different locations and the results were averaged. Test temperature at room temperature. Contact angle test was used to further characterize the stability of the coating. The specific method was to dip the modified sponges into 0.1 mol/L NaCl, 35 g/L NaCl, 0.1 mol/L HCl, and 0.1 mol/L NaOH solutions for different times (1 h, 3 h, and 5 h, respectively) and observe the change of water contact angle.
2.3.2. Oil Absorption Test
The prepared sponge samples were taken out afterwards by completely immersing them in different oil or organic solvents, and then taking them out. The adsorption capacity was evaluated according to the mass change before and after the adsorption. The oil adsorption capacity and recycling ability of the material were tested by repeated adsorption and extrusion. The material was first sufficiently adsorbed to the oil phase or the organic solvent, and then extruded by an external force to release the absorbed oil phase or organic solvent. The absorption capacity was calculated using Equation (1) [9,36]:
where and are the quality of the sponge before and after oil absorption, respectively.
3. Results and Discussion
3.1. Structural Analysis of Hydrophobic Coating
Figure 2a–c show the influence of the MS–TA–Ag substrate immersion time in TA solution, TA concentration, and silver nitrate concentration on the hydrophobic property of modified MS. The results show that, when the MS is soaked in TA solution for 5 h, the AgNO3 solution concentration is 1 mol/L and the TA solution concentration is 10 g/L, and the modified sponge has the best hydrophobicity. The test samples were prepared based on the above optimal reaction conditions; the structure of unmodified and modified samples was compared and analyzed. Figures S1 and S2 are the FT-IR and XPS spectra of the unmodified MS and modified MS. Figure 2d–f are the the corresponding local graphs of FT-IR and XPS spectra. In the FT-IR spectrum of MS–TA, the absorption peaks at 1310 cm−1 are associated with the C–O bending vibration. Additionally, the peaks at 869 and 759 cm−1 are associated with the out plane bending vibration of –OH and C–H, respectively. Those changes prove that the TA immobilized on the surface of MS. Meanwhile, the intensity of the above absorption peak become weaker than that of MS-TA after being deposited with Ag in the FT-IR spectrum of MS–TA–Ag. Ag is produced because of the transfer of π electrons from the TA carbonyl group to free orbital formation of Ag+ (see Figure 2g). XPS was carried out to determine the electronic properties of MS–TA–Ag. Figure 2e is the full spectrum of MS–TA–Ag and Figure 2f is the core-level spectra of Ag3d regions. The XPS results indicate that the Ag elements are in Ag (0) state by identifying an intense doublet at the 374 (Ag 3d3/2) and 368 (Ag 3d5/2) eV binding energies. The XPS results directly demonstrate that Ag+ is reduced to silver Ag (0) [37]. The SEM test was used to determine the surface morphology of MS–TA–Ag. The SEM image is illustrated in Figure 3. As shown in Figure 3a–c, the MS–TA–Ag surface displays uniform roughness compared with the surface of MS and MS–TA. This phenomenon is more pronounced in larger images (Figure 3d–f). Figure 3i (Amplification in the Fig.3f) shows the evenly distributed Ag nano particles on the MS–TA–Ag surface. For further confirmation, the distribution of the detected elements in the MS–TA–Ag EDS detector was coupled to the SEM system. The compositional maps of the N, O, and Ag elements are depicted in Figure 4b–d. Based on the EDS figure, the Ag nano particles are dispersed homogeneously in the surface of MS–TA–Ag. The FT-IR, XPS, and EDS spectra visually verified the successful deposition of Ag nano particles on the surface of MS.
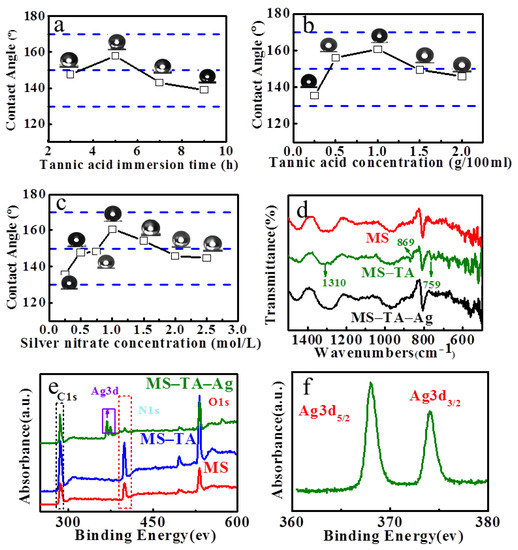
Figure 2.
The effect of the melamine sponge (MS)─tannic acid (TA)─Ag substrate soaking time in TA solution (a), the concentration of TA solution (b), and the concentration of AgNO3 solution (c) on the hydrophilic properties of the modified MS surface. The Fourier transform infrared spectroscopy (FT-IR) spectra of MS, MS–TA, and MS–TA–Ag (d). The full spectrum of MS–TA–Ag (e). The core-level spectrum of Ag3d regions (f). The production process of Ag (0) (g).
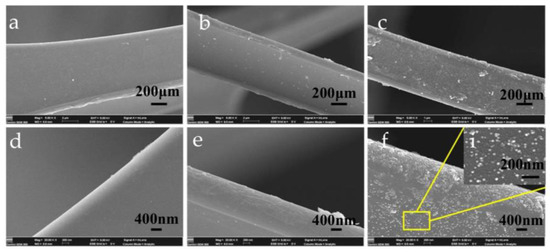
Figure 3.
The scanning electron microscopy (SEM) image of the MS (a,d), the MS–TA (b,e), and the MS–TA–Ag (c,f). Built-in image for Fig.3f local magnification image.

Figure 4.
The energy-dispersive spectroscopy (EDS) map of the MS–TA–Ag (a–d).
3.2. Hydrophobic Properties Analysis of the MS–TA–Ag
In Figure 5a, the deionized water stained with methyl blue and the dichloroethane stained with oil red O are dropped onto the MS, and the water and dichoroethane instantly infiltrate into the sponge. The water contact angle was 0°, which shows the characteristics of superhydrophilicity and superoleophilicity. However, after the introduction of Ag, MS–TA–Ag exhibits superhydrophobicity with a water contact angle of 156°. Figure 5b shows the water droplets quickly roll down from the sloping surface of the MS–TA–Ag and no water marks remain (see Movie S1 in the ESI†). Figure 5c demonstrates that the water droplet can be compressed and separated completely from the surface of the MS–TA–Ag without any residual attachment. This indicates ultralow adhesion between the MS–TA–Ag and water (see Movie S2 in the ESI†). The superhydrophobicity of the MS–TA–Ag can further proved by Figure 5d,e. The superhydrophobic property of the modified sponges is shown in Figure 5d. The modified sponge can float on the water while the original sponge sinks to the bottom of the water spontaneously. As shown in Figure 5e, with the experience of absorbing the droplets of dichloromethane at the bottom of the water, the hydrophobicity and lipophilicity of the modified sponge can be intuitively perceived (see Movie S3 in the ESI†). In Figure 5f, the water and dichloromethane mixture could be perfectly separated by the modified melamine sponge under a single gravity condition (see Movie S4 in the ESI†), which indicates that the modified MS has superhydrophobic and superoleophilic characteristics [38].
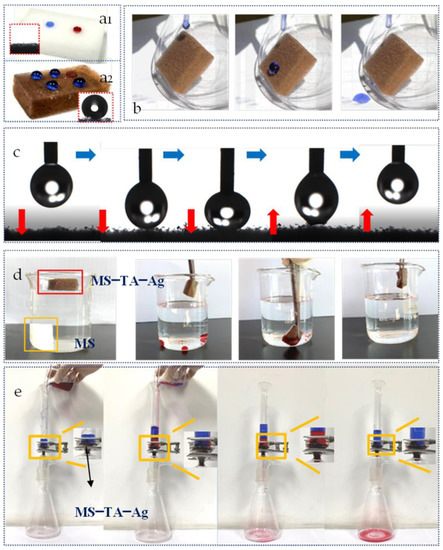
Figure 5.
Photographs of dripping water and dichloromethane on the MS (a1) and MS–TA–Ag (a2). The rolling photos of water droplets on modified MS (b). Digital photograph of dynamic contact modified sponge (c). The state of sponges in water (d). The oil absorption experiment under water for modified MS (e). The separation test of dichloromethane and water for the modified MS (f).
3.3. Oil Adsorption Performance Analysis of Superhydrophobic MS
Figure 6 is a comprehensive evaluation of the absorption capacity for modified sponge on different solvents and oils. As shown in Figure 6a, the modified sponge has a good absorption capacity for a wide range of organic solvents and oils, even in high density solvents (e.g., dichloromethane), and viscous oils (e.g., corn oil and silicone oil) can be easily recovered by gently squeezing. Figure 6b–e are the cyclic serviceability of the MS–TA–Ag for various types of oils/organic solvents. The recovery rate of various oils was still almost more than 50% after repeated use of modified sponge for 10 times. The decrease of cyclic absorptivity of oil and organic solvent is mainly caused by the collapse of sponge skeleton and the decrease of sponge porosity [36]. Figure 6f is the water contact angle of the MS–TA–Ag after absorbing and extruding different oil/organic solvent for 10 and 50 cycles. As seen in Figure 6f, the water contact angle of the MS–TA–Ag gets through a small change after absorbing and extruding different oils/organic solvent for 10 cycles and 50 cycles repeatedly, which indicates that they still possess the superhydrophobic characteristics. These results show that the modified sponge has wide adaptability to organic solvents and oils, showing excellent recycling performance and mechanical stability.
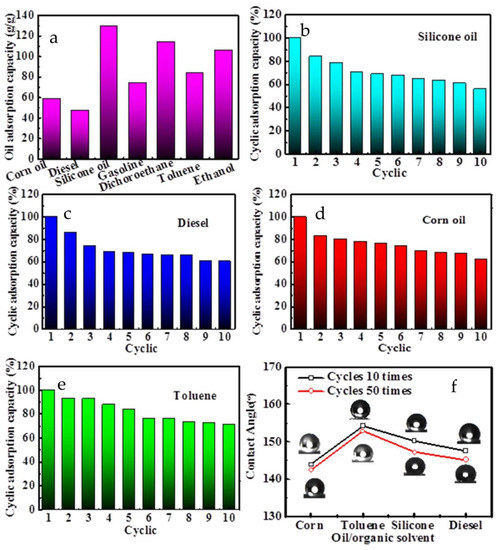
Figure 6.
The oil adsorption capacity of the MS–TA–Ag (a) and the cyclic serviceability (b–e) of the MS–TA–Ag for various types of oils/organic solvent. (f) The water contact angle of the MS–TA-Ag after absorbing and extruding different oil/organic solvent for 10 and 50 cycles.
In order to further appraise the performance of modified sponge in harsh environment, the contact angle of the modified sponge after immersion was tested to characterize the stability of the coating on the modified sponges. As shown in Figure 7, the modified sponges still show strong hydrophobic characteristic with water contact angles of 148°, 147°, and 135° after being immersed into 0.1 mol/L NaCl, 0.1 mol/L HCl, and 0.1 mol/L NaOH solution for 5 h, respectively. The practical properties of modified sponge were further evaluated by simulated seawater environment in Figure 7 (NaCl concentration is 35 g/L). After soaking in simulated seawater for 5 h, the water contact angle of the modified sponge was 143°. The results show that the coating has good stability without falling off and dissolving. The stability of modified sponges in harsh environment makes it possible to deal with oil leakage accidents and practical applications.

Figure 7.
Contact angle of modified sponge treated with corresponding solution for different times.
4. Conclusions
The superoleophilic and superhydrophobic sponge was prepared by a simple environment-friendly and energy-saving method. Specifically, the hydrophobicity and rough structure of the surface were obtained by one step reduction reaction of silver ions. Notably, the modified sponge has a wide range adaptability for various organic solvents and oils. The adsorption capacity of modified sponge for various oils and organic solvents is as much as 48–129 times its own weight. Significantly, the modified sponge shows strong stability in strong alkali, strong acid, and high salt solution. The modified sponge has the advantages of simple preparation method, excellent oil adsorption performance, and strong stability, which provide the possibility for large-scale oil spill recovery at sea.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/coatings11050521/s1, Figure S1: The FT-IR spectrum of the unmodified MS and modified MS, Figure S2: The XPS spectrum of the unmodified MS and modified MS, Moive S1: The dichloroethane droplets absorption of the modified melamine sponge, Moive S2: Dynamic contact between water droplets and modified sponges, Moive S3: Oil adsorption experiment of modified sponge, Moive S4: The separation of an oil/water mixture via modified melamine sponges.
Author Contributions
Data curation, J.S.; Investigation, J.S. and Z.C.; Methodology, Z.C. and Y.L.; Project administration, P.Z. and Y.L.; Writing—original draft, L.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by large instruments open foundation of nantong university (KFJN2028).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, X.; Hong, J. Recent advances in robust superwettable membranes for oil–Water separation. Adv. Mater. Interfaces 2019, 6, 1900126. [Google Scholar] [CrossRef]
- Yan, C.; Ji, Z.; Ma, S.; Wang, X.; Zhou, F. 3D printing as feasible platform for on-site building oil-skimmer for oil collection from spills. Adv. Mater. Interfaces 2016, 3, 1600015. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, J.; Du, R.; Xie, Z.; Deng, S.; Liu, R.; Liu, Z.; Zhang, J. Robust superhydrophobic foam: A graphdiyne-based hierarchical architecture for oil/water separation. Adv. Mater. 2016, 28, 168–173. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation mechanism and construction of surfaces with special wettability for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Shinde, T.B.; Pawar, S.B.; Khot, T.M.; Bhosale, A.K.; Sadasivuni, K.K.; Xing, R.; Mao, L.; Liu, S. Superhydrophobic leaf mesh decorated with SiO2 nanoparticle–polystyrene nanocomposite for oil–water separation. ACS Appl. Nano Mater. 2019, 2, 799–805. [Google Scholar] [CrossRef]
- Latthe, S.S.; Kodag, V.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Kulal, S.R.; Liu, S.; Xing, R. Sawdust-based superhydrophobic pellets for efficient oil-water separation. Mater. Chem. Phys. 2020, 243, 122634. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Aly, M.A.S.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S. Recent advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Khalilifard, M.; Javadian, S. Magnetic superhydrophobic polyurethane sponge loaded with Fe3O4@oleic acid@graphene oxide as high performance adsorbent oil from water. Chem. Eng. J. 2021, 408, 127369. [Google Scholar] [CrossRef]
- Lin, B.; Chen, J.; Li, Z.-T.; He, F.-A.; Li, D.-H. Superhydrophobic modification of polyurethane sponge for the oil-water sep-aration. Surf. Coat. Technol. 2019, 359, 216–226. [Google Scholar] [CrossRef]
- Guselnikova, O.; Barras, A.; Addad, A.; Sviridova, E.; Szunerits, S.; Postnikov, P.; Boukherroub, R. Magnetic polyurethane sponge for efficient oil adsorption and separation of oil from oil-in-water emulsions. Sep. Purif. Technol. 2020, 240, 116627. [Google Scholar] [CrossRef]
- Xue, J.; Zhu, L.; Zhu, X.; Li, H.; Ma, C.; Yu, S.; Sun, D.; Xia, F.; Xue, Q. Tetradecylamine-mxene functionalized melamine sponge for effective oil/water separation and selective oil adsorption. Sep. Purif. Technol. 2021, 259, 118106. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, J.; Yan, H.; Xiao, J.; Wang, J. Reversible wettability switching of melamine sponges for oil/water separation. Mater. Chem. Phys. 2021, 257, 123772. [Google Scholar] [CrossRef]
- Lei, Z.; Zheng, P.; Niu, L.; Yang, Y.; Shen, J.; Zhang, W.; Wang, C. Ultralight, robustly compressible and super-hydrophobic biomass-decorated carbonaceous melamine sponge for oil/water separation with high oil retention. Appl. Surf. Sci. 2019, 489, 922–929. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Yang, Y.; Chen, Z.; Jia, G.; Zhang, L. Silk fibroin-graphene oxide functionalized melamine sponge for efficient oil absorption and oil/water separation. Appl. Surf. Sci. 2019, 497, 143762. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.; Kumar, A.M.; Sadasivuni, K.K.; Xing, R.; Liu, S. Self-cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Jing, Y.; Jiang, X.; Yu, L.; Yan, X. Surface design of durable and recyclable superhydrophobic materials for oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 128–138. [Google Scholar] [CrossRef]
- Jamsaz, A.; Goharshadi, E.K. An environmentally friendly superhydrophobic modified polyurethane sponge by seashell for the efficient oil/water separation. Process. Saf. Environ. Prot. 2020, 139, 297–304. [Google Scholar] [CrossRef]
- Pethsangave, D.A.; Wadekar, P.H.; Khose, R.V.; Some, S. Super-hydrophobic carrageenan cross-linked graphene sponge for recovery of oil and organic solvent from their water mixtures. Polym. Test. 2020, 90, 106743. [Google Scholar] [CrossRef]
- Li, L.; Hu, T.; Yang, Y.; Zhang, J. Strong, compressible, bendable and stretchable silicone sponges by solvent-controlled hy-drolysis and polycondensation of silanes. J. Colloid Interface Sci. 2019, 540, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Liu, Q.; Zhou, J.; Ju, P.; Waterhouse, G.I.; Zhou, S.; Ai, S. Superhydrophobic sponge containing silicone oil-modified layered double hydroxide sheets for rapid oil-water separations. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 339–346. [Google Scholar] [CrossRef]
- Lu, S.; Liu, Q.; Han, R.; Shi, J.; Guo, M.; Song, C.; Ji, N.; Lu, X.; Ma, D. Core-shell structured Y zeolite/hydrophobic organic polymer with improved toluene adsorption capacity under dry and wet conditions. Chem. Eng. J. 2021, 409, 128194. [Google Scholar] [CrossRef]
- Yu, J.; Gou, S.; Li, Q.; Peng, C.; Zhou, L.; Liu, L.; Tang, L.; He, Y.; Duan, M. A graft-modification of chitosan with twin-tail hydrophobic association polymer for enhance oil recovery. Chem. Phys. Lett. 2021, 763, 138164. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Y.; Hu, X.; Duan, M.; Wang, X. Recycle of oil waste via hydrophobic sponge prepared from toner waste. J. Hazard. Mater. 2018, 360, 615–622. [Google Scholar] [CrossRef]
- Gao, H.; Sun, P.; Zhang, Y.; Zeng, X.; Wang, D.; Zhang, Y.; Wang, W.; Wu, J. A two-step hydrophobic fabrication of melamine sponge for oil absorption and oil/water separation. Surf. Coat. Technol. 2018, 339, 147–154. [Google Scholar] [CrossRef]
- Yue, J.; Yuan, M.; Zhang, X.; Wen, G.; Ren, G.; Ge, B.; Zhao, L.; Shao, X. Fabrication of novel superhydrophobic ZIF-8 modified directly Z-scheme bismuth oxyiodide/cadmium sulfide melamine sponge for efficient oil/water separation and visible-light photo-degradation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124992. [Google Scholar] [CrossRef]
- Mao, M.; Xu, H.; Guo, K.-Y.; Zhang, J.-W.; Xia, Q.-Q.; Zhang, G.-D.; Zhao, L.; Gao, J.-F.; Tang, L.-C. Mechanically flexible, super-hydrophobic and flame-retardant hybrid nano-silica/graphene oxide wide ribbon decorated sponges for efficient oil/water separation and fire warning response. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106191. [Google Scholar] [CrossRef]
- Peng, Z.; Song, J.; Gao, Y.; Liu, J.; Lee, C.; Chen, G.; Wang, Z.; Chen, J.; Leung, M.K. A fluorinated polymer sponge with superhydrophobicity for high-performance biomechanical energy harvesting. Nano Energy 2021, 85, 106021. [Google Scholar] [CrossRef]
- Zhan, Y.; He, S.; Hu, J.; Zhao, S.; Zeng, G.; Zhou, M.; Zhang, G.; Sengupta, A. Robust super-hydrophobic/super-oleophilic sandwich-like UIO-66-F4@rGO composites for efficient and multitasking oil/water separation applications. J. Hazard. Mater. 2020, 388, 121752. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, G.; Deng, Y.; Wang, C. Surface modification of melamine sponges for pH-responsive oil absorption and de-sorption. Appl. Surf. Sci. 2017, 416, 798–804. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Hosseini, E.; Motamedi, E.; Moosavi-Movahedi, A.A.; Salekdeh, G.H. Application of carboxymethyl cellu-lose-g-poly(acrylic acid-co-acrylamide) hydrogel sponges for improvement of efficiency, reusability and thermal stability of a re-combinant xylanase. Chem. Eng. J. 2019, 375, 122022. [Google Scholar] [CrossRef]
- Xu, P.; Wang, F.; Yang, C.; Ou, J.; Li, W.; Amirfazli, A. Reversible transition between superhydrophobicity and superhy-drophilicity of a silver surface. Surf. Coat. Technol. 2016, 294, 47–53. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Pu, F.; Yin, X.; Xia, Y.; Huang, W.; Li, Z. Facile fabrication of superhydrophobic Bi/Bi2O3 surfaces with hi-erarchical micro-nanostructures by electroless deposition or electrodeposition. Appl. Surf. Sci. 2014, 288, 558–563. [Google Scholar] [CrossRef]
- Liu, P.; Cao, L.; Zhao, W.; Xia, Y.; Huang, W.; Li, Z. Insights into the superhydrophobicity of metallic surfaces prepared by electrodeposition involving spontaneous adsorption of airborne hydrocarbons. Appl. Surf. Sci. 2015, 324, 576–583. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.; Torres-Acosta, J.; Sandoval-Castro, C.A.; Capetillo-Leal, C.; Alonso-Dıaz, M.; Torres-Acosta, J.F.D.J. Amino acid profile of the protein from whole saliva of goats and sheep and its interaction with tannic acid and tannins extracted from the fodder of tropical plants. Small Rumin. Res. 2012, 103, 69–74. [Google Scholar] [CrossRef]
- Li, R.; Dai, T.; Zhou, W.; Fu, G.; Wan, Y.; McClements, D.J.; Li, J. Impact of pH, ferrous ions, and tannic acid on lipid oxidation in plant-based emulsions containing saponin-coated flaxseed oil droplets. Food Res. Int. 2020, 136, 109618. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, M.; Lu, H.; He, H.; Liu, X.; Wang, Y. 3D multiscale sponges with plant-inspired controllable superhydrophobic coating for oil spill cleanup. Prog. Org. Coat. 2021, 151, 106075. [Google Scholar] [CrossRef]
- Veisi, H.; Moradi, S.B.; Saljooqi, A.; Safarimehr, P. Silver nanoparticle-decorated on tannic acid-modified magnetite nano-particles (Fe3O4@TA/Ag) for highly active catalytic reduction of 4-nitrophenol, Rhodamine B and Methylene blue. Mater. Sci. Eng. C 2019, 100, 445–452. [Google Scholar] [CrossRef]
- Parsaie, A.; Tamsilian, Y.; Pordanjani, M.R.; Abadshapoori, A.K.; McKay, G. Novel approach for rapid oil/water separation through superhydrophobic/ superoleophilic zinc stearate coated polyurethane sponges. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126395. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).