Validating Anti-Infective Activity of Pleurotus Opuntiae via Standardization of Its Bioactive Mycoconstituents through Multimodal Biochemical Approach
Abstract
1. Introduction
2. Materials and Methods
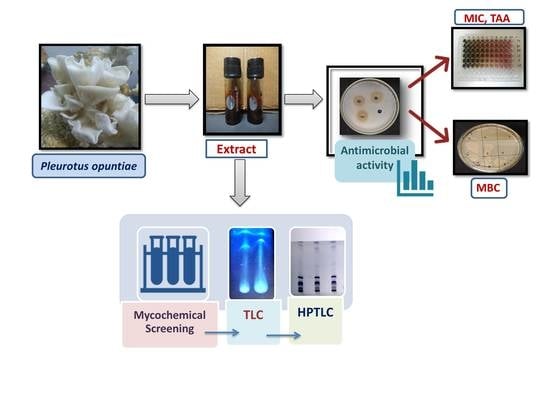
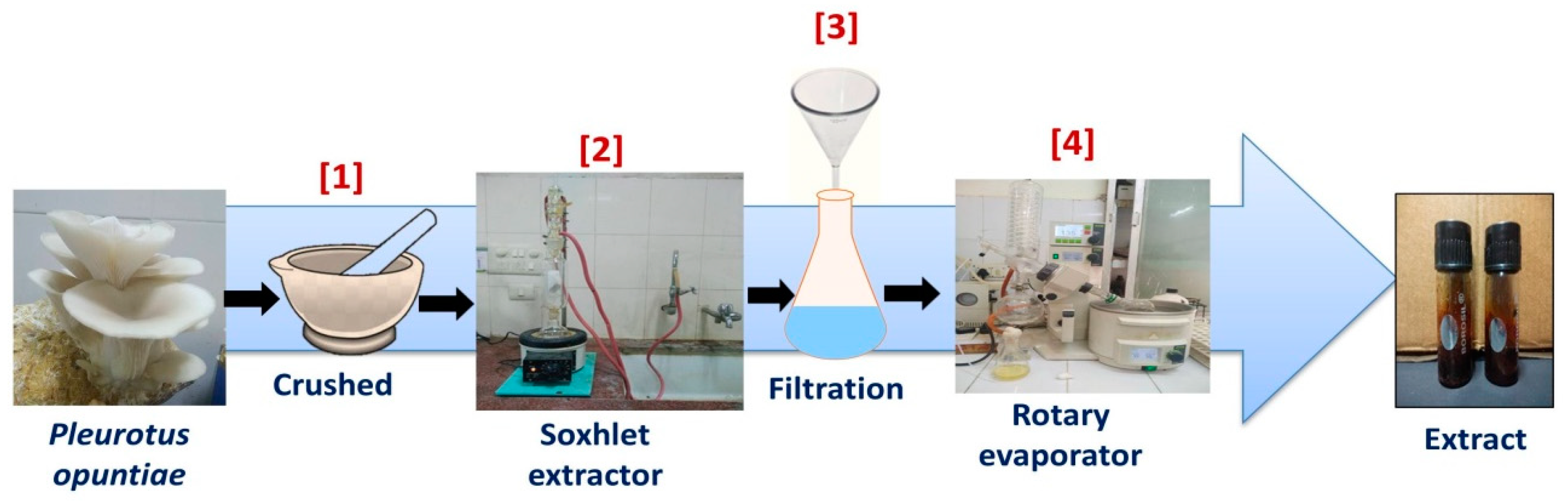
2.1. Collection of Mushroom
2.2. Preparation of Mushroom Extracts
2.3. Bacterial Tested Organisms
2.4. Determination of Antibacterial Screening Test
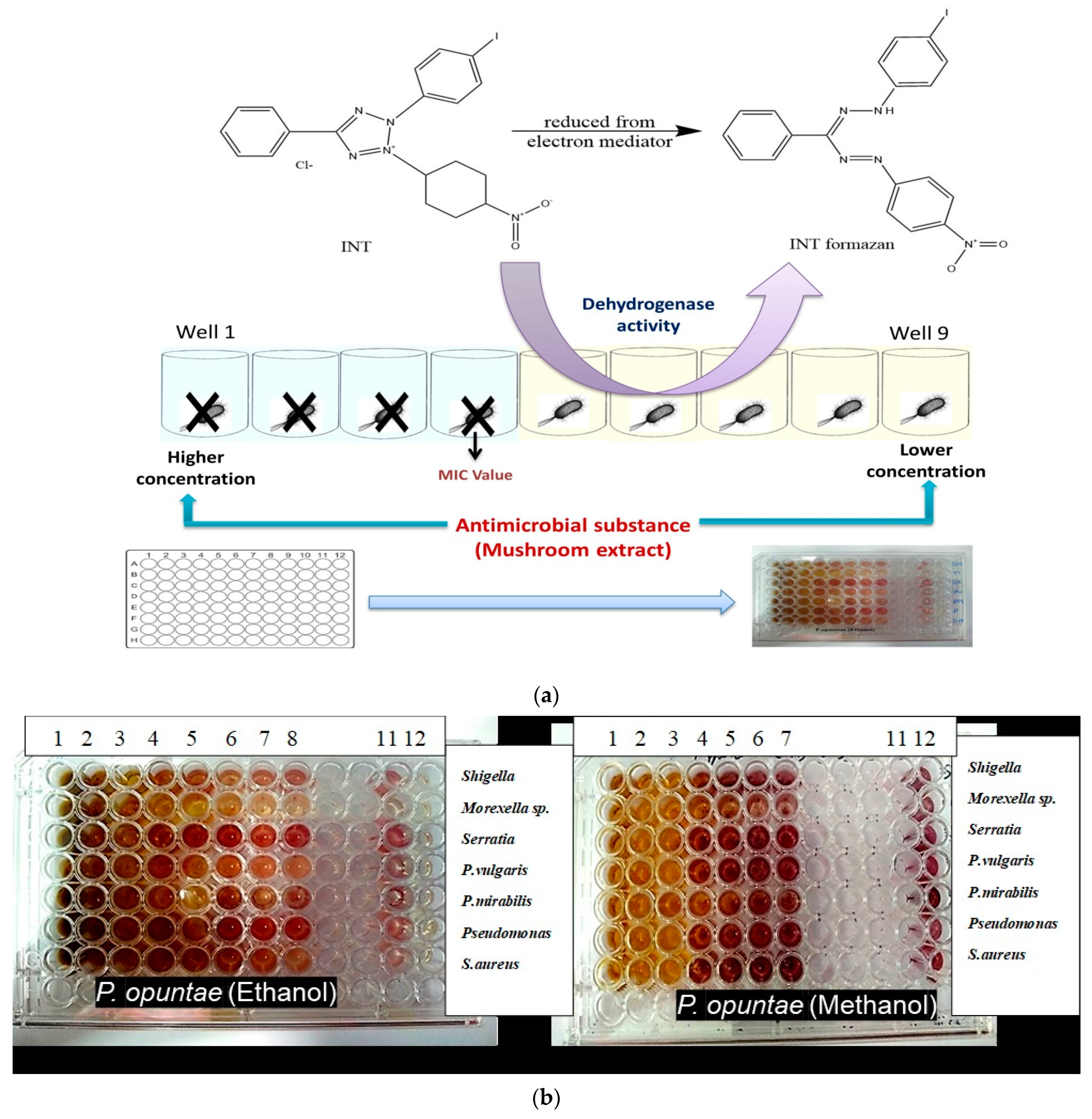
2.5. Determination of Minimum Inhibitory Concentration (MIC)
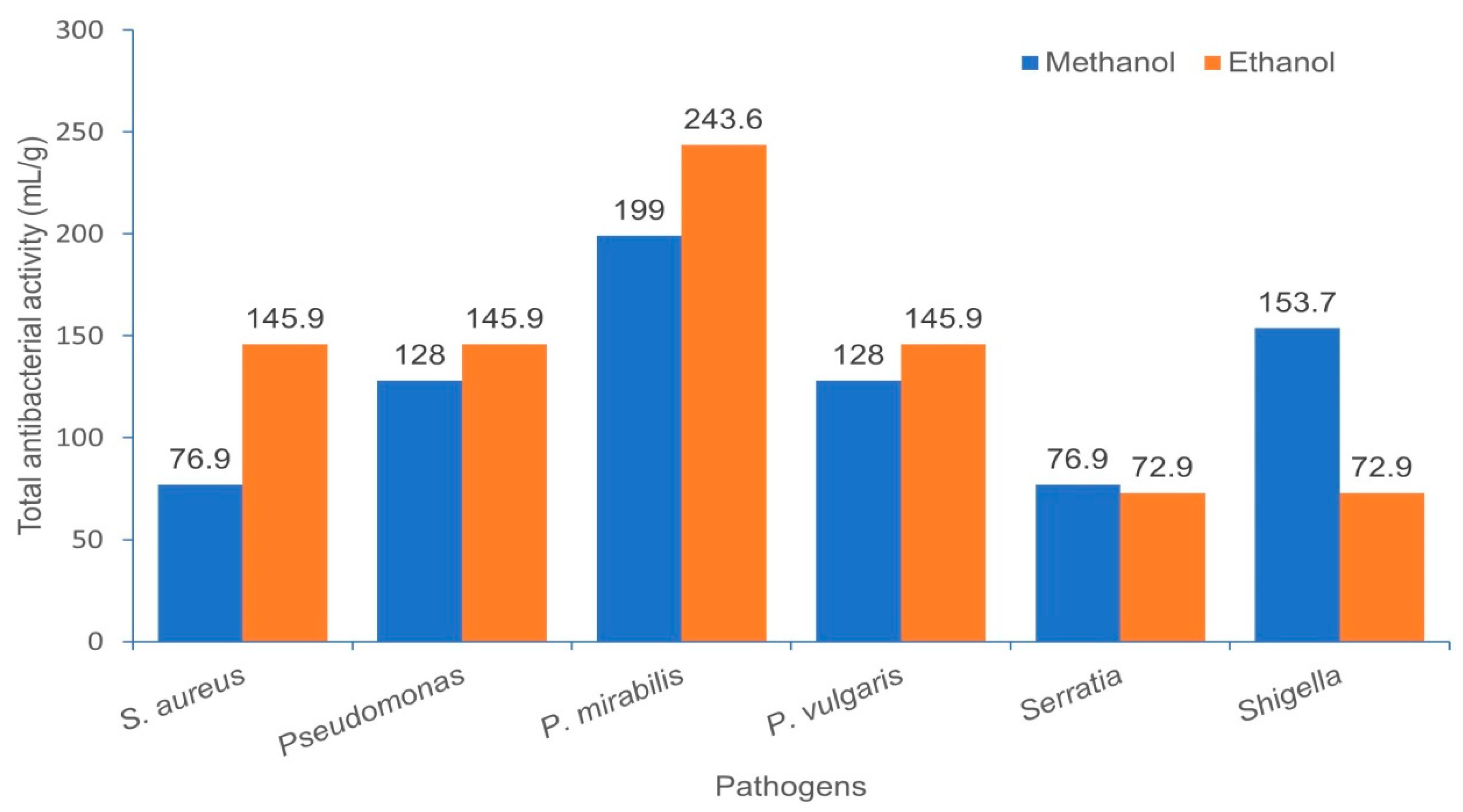
2.6. Total Antibacterial Activity (TAA)
2.7. Determination of Minimum Bactericidal Concentration (MBC)
2.8. Mycochemical Screening
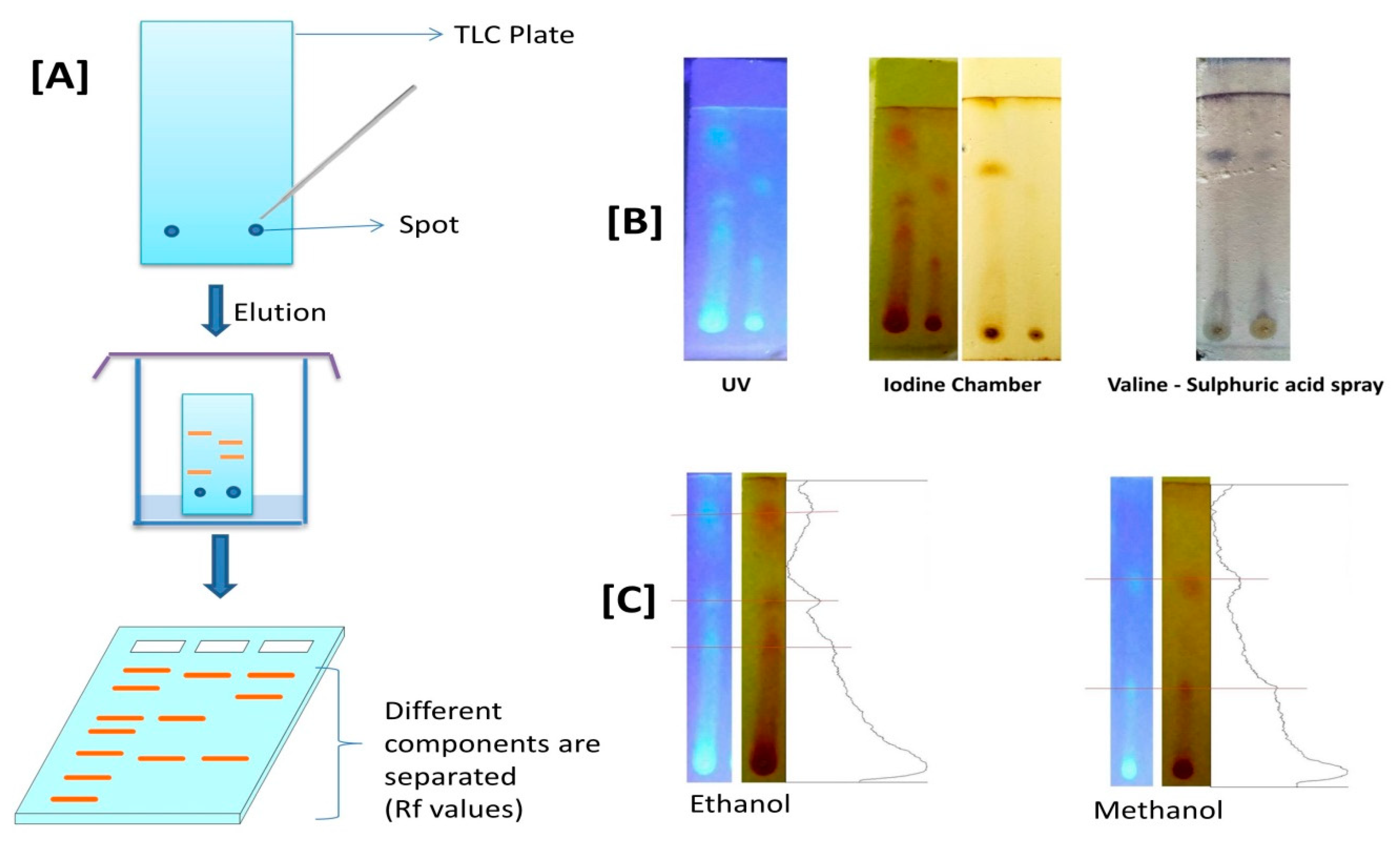
2.9. Thin-Layer Chromatography (TLC)
2.10. High-Performance Thin Layer Chromatography (HPTLC) Analysis
2.11. Statistical Analysis
3. Results
3.1. Antimicrobial Activity of P. opuntiae
3.2. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), MBC/MIC Ratio and Total Antimicrobial Activity (TAA)
3.3. Mycochemical Screening from Pleurotus opuntiae
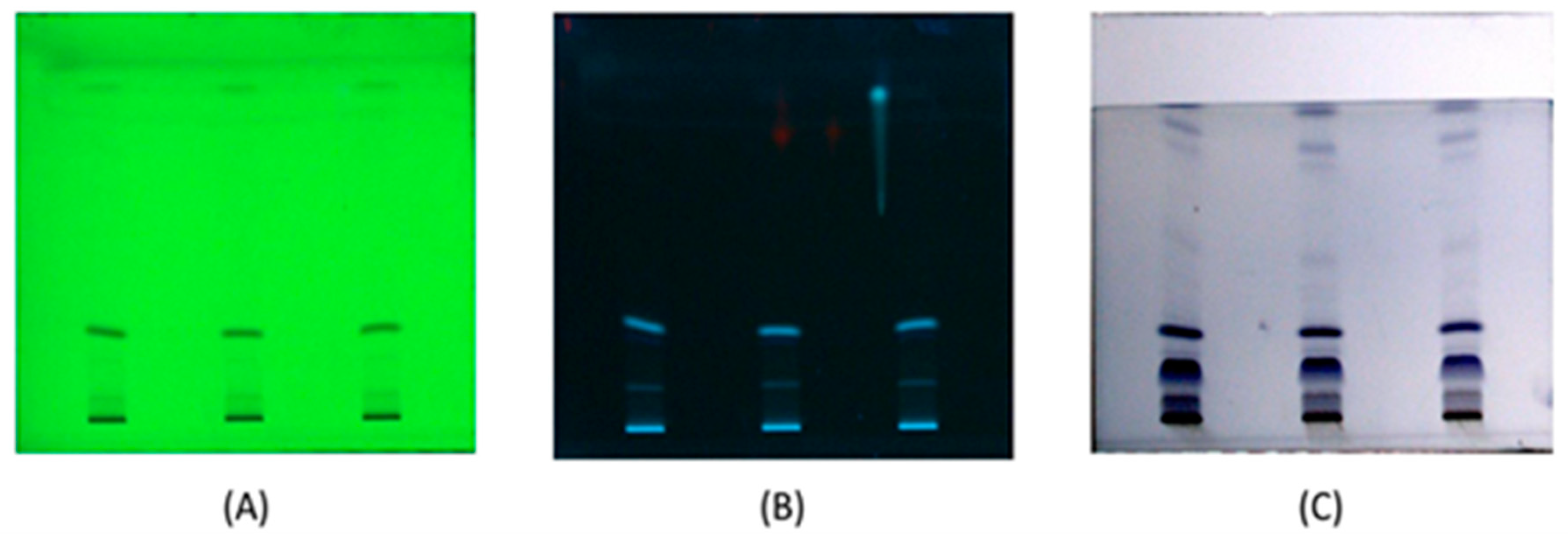
3.4. Thin Layer Chromatography (TLC) Profile
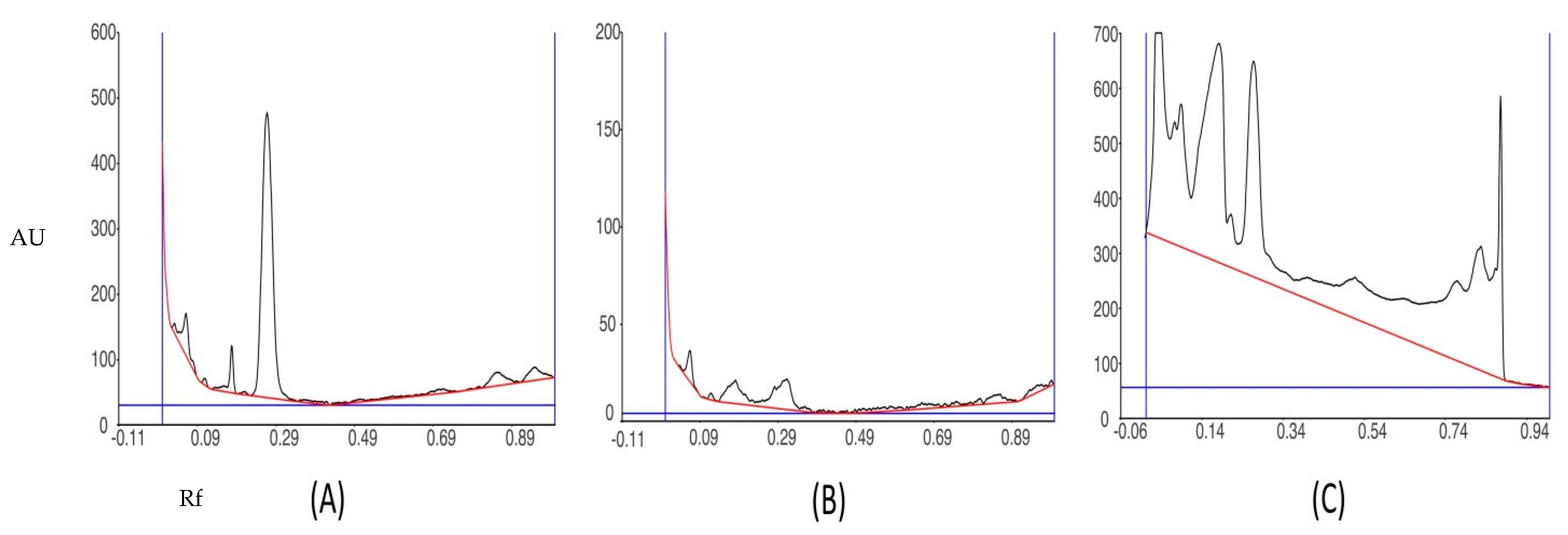
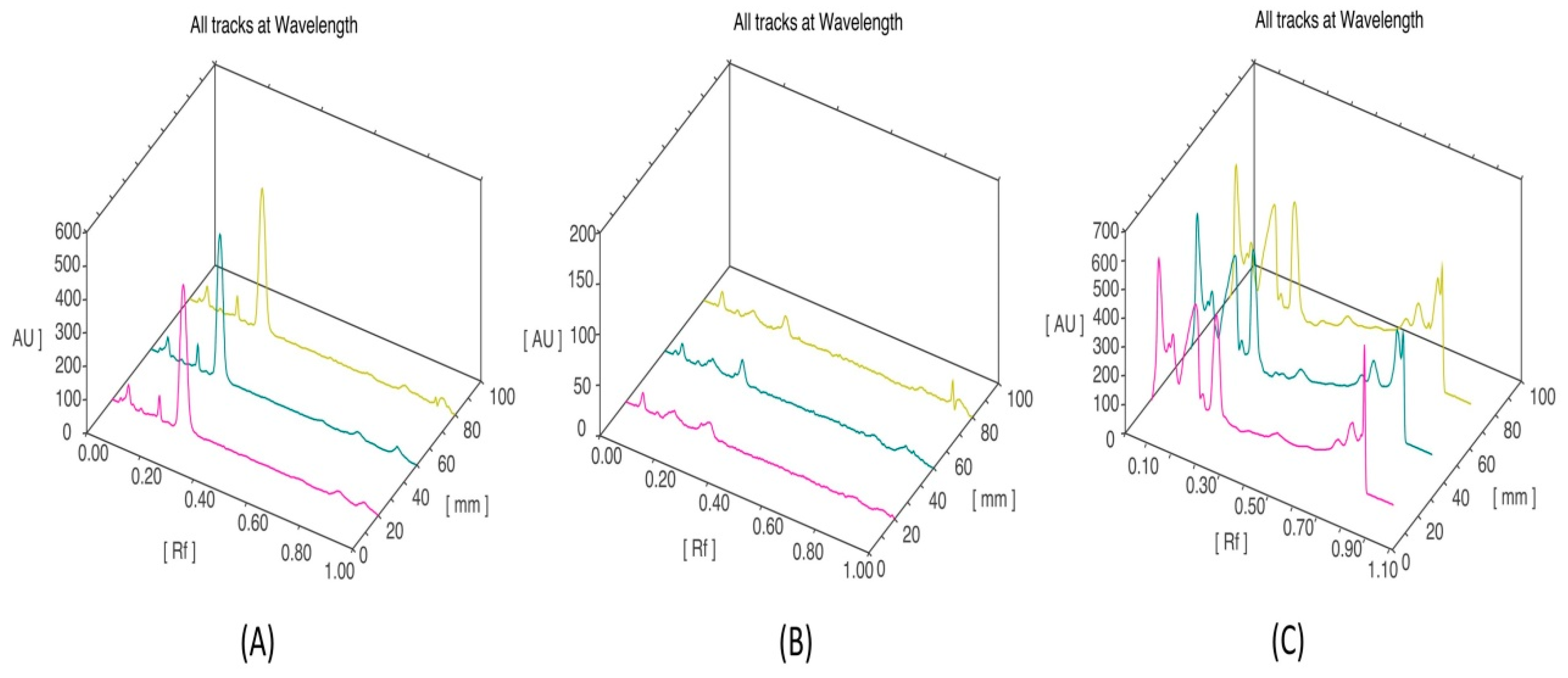
3.5. HPTLC Fingerprinting Analysis of Ethanol Extract of P. opuntiae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akova, M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 2016, 7, 252–266. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Pandey, A.T.; Pandey, I.; Hachenberger, Y.; Krause, B.C.; Haidar, R.; Laux, P.; Luch, A.; Singh, M.P.; Vikram Singh, V.A. Emerging paradigm against global antimicrobial resistance via bioprospecting of mushroom into novel nanotherapeutics development. Trends Food Sci. Technol. 2020, 106, 333–344. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Recommendations of CDC and health care infection control practices advisory committee. Atlanta 2003, 52, 1–42. [Google Scholar]
- Alves, M.J.; Ferreira, I.C.F.R.; Martins, A.; Pintado, M. Antimicrobial activity of wild mushroom extracts against clinical isolates resistant to different antibiotics. J. Appl. Microbiol. 2012, 113, 466–475. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Berhe, S.D. Determination of antimicrobial activity of extracts of indigenous wild mushrooms against pathogenic organisms. Evid.-Based Complementary Altern. Med. 2019, 2019, 6212673. [Google Scholar] [CrossRef]
- Wolff, E.R.; Wisbeck, E.; Silveira, M.L.; Gern, R.M.; Pinho, M.S.; Furlan, S.A. Antimicrobial and antineoplasic activity of Pleurotus ostreatus. Appl. Biochem. Biotechnol. 2008, 151, 402–412. [Google Scholar] [CrossRef]
- Vamanu, E.; Pelinescu, D.; Avram, I. Antioxidative effects of phenolic compounds of mushroom mycelia in simulated regions of the human colon, in vitro study. Pol. J. Food Nutr. Sci. 2018, 68, 83–90. [Google Scholar] [CrossRef]
- Panti, M. The antibacterial activity of coriolus versicolor methanol extract and its effect on ultrastructural changes of staphylococcus aureus and salmonella enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar]
- Elisashvili, V. Submerged cultivation of medicinal mushrooms: Bioprocesses and products (review). Int. J. Med. Mushrooms 2012, 14, 211–239. [Google Scholar] [CrossRef]
- Ren, L.; Hemar, Y.; Perera, C.O.; Lewis, G.; Krissansen, G.W.; Buchanan, P.K. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact. Carbohydr. Diet. Fibre. 2014, 3, 41–51. [Google Scholar] [CrossRef]
- Gashaw, G.; Fassil, A.; Redi, F. Evaluation of the antibacterial activity of Pleurotus spp. cultivated on different agricultural wastes in Chiro, Ethiopia. Int. J. Microbiol. 2020, 2020, 9312489. [Google Scholar] [CrossRef]
- Saritha, K.; Rajesh, A.; Manjulatha, K.; Setty, O.H.; Yenugu, S. Mechanism of antibacterial action of the alcoholic extracts of Hemidesmus indicus (L.) R. Br. ex Schult, Leucas aspera (Wild.), Plumbago zeylanica L., and Tridax procumbens (L.) R. Br. ex Schult. Front. Microbiol. 2015, 6, 577. [Google Scholar] [CrossRef]
- Camacho, M.; Guzmán, G.; Guzmán-Dávalos, L. Pleurotus opuntiae (Durieu et Lév.) Sacc. (higher Basidiomycetes) and other species related to Agave and Opuntia plants in Mexico-Taxonomy, distribution, and applications. Int. J. Med. Mushrooms 2012, 14, 65–78. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Venturella, G.; Fryssouli, V.; Inglese, P.; Polemis, E.; Gargano, M.L. Pleurotus opuntiae revisited—An insight to the phylogeny of dimitic Pleurotus species with emphasis on the P. djamor complex. Fungal Biol. 2019, 123, 188–199. [Google Scholar] [CrossRef]
- Oyetayo, V.O.; Dong, C.H.; Yao, Y.J. Antioxidant and antimicrobial properties of aqueous extract from Dictyophora indusiata. Open Mycol. J. 2009, 3, 20–26. [Google Scholar] [CrossRef]
- Boakye, Y.D. Anti-infective properties and time-kill kinetics of phyllanthus muellerianus and its major constituent, Geraniin. Med. Chem. 2016, 6, 95–104. [Google Scholar] [CrossRef]
- Eloff, J.N. Quantification the bioactivity of plant extracts during screening and bioassay guided fractionation. Phytomedicine 2004, 11, 370–371. [Google Scholar] [CrossRef]
- Elisha, I.L.; Botha, F.S.; McGaw, L.J.; Eloff, J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complementary Altern. Med. 2017, 17, 133. [Google Scholar] [CrossRef]
- Prakash, K.D.; Sayali, G. Current quality control methods for standardization of herbal drugs. Int. J. Pharm. 2017, 5, 82–95. [Google Scholar]
- Bains, A.; Tripathi, A. Antibacterial activity and phytochemical screening of wild edible mushroom pleurotus ostreatus collected from himachal pradesh. Int. J. Curr. Adv. Res. 2016, 4, 2320–5407. [Google Scholar]
- Appiah, T.; Boakye, Y.D.; Agyare, C. Antimicrobial activities and time-kill kinetics of extracts of selected ghanaian mushrooms. Evid.-Based Complementary Altern. Med. 2017, 2017, 4534350. [Google Scholar] [CrossRef] [PubMed]
- Bele, A.A.; Khale, A.; Archana, M.; Bele, A. An overview on thin layer chromatography. Int. J. Pharm. Sci. Res. 2011, 2, 256–267. [Google Scholar]
- Singh, A.V.; Ansari, M.H.D.; Rosenkranz, D.; Maharjan, R.S.; Kriegel, F.L.; Gandhi, K.; Kanase, A.; Singh, R.; Laux, P.; Luch, A. Artificial intelligence and machine learning in computational nanotoxicology: Unlocking and empowering nanomedicine. Adv. Healthc. Mater. 2020, 9, 1901862. [Google Scholar] [CrossRef]
- Suleiman, M.M.; McGaw, L.J.; Naidoo, V.; Eloff, J.N. Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected south african tree species. AJTCAM 2010, 1, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Monkai, J.; Bleha, R.; Macurkova, A.; Ruml, T.; Juhee, A.; Chukeatirote, E. Antimicrobial activity of crude extracts prepared from fungal mycelia. Asian Pac. J. Trop. Biomed. 2017, 7, 257–261. [Google Scholar] [CrossRef]
- Chowdhury, H.M.H.; Kubra, K.; Ahmed, R.R. Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 8. [Google Scholar] [CrossRef]
- Pandey, T.A.; Pandey, I.; Zamboni, P.; Gemmati, D.; Kanase, A.; Singh, A.V.; Singh, M.P. Traditional herbal remedies with a multifunctional therapeutic approach as an implication in COVID-19. Coatings 2020, 10, 761. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Keepers, T.R.; Gomez, M.; Celeri, C.; Nichols, W.W.; Krause, K.M. Bactericidal activity, absence of serum effect, and time-kill kinetics of ceftazidime-avibactam against β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 5297–5305. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Tellez, M.T.; Gupta, K.V.; Diaz-Godinez, G.; Soriano-Santos, J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Taee, H.A.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Bubueanu, C.; Grigore, A.; Serban, E.; Popa, G.; Cornea, P.C. HPTLC identification of bioactive compounds and antioxidant activity of Pleurotus ostreatus and Lentinus edodes extracts. Sci. Bull. Ser. F Biotechnol. 2017, XXI, 343–348. [Google Scholar]
- Gunalan, G.; Saraswathy, A.; Vijayalakshmi, K. HPTLC fingerprint profile of Bauhinia variegata Linn. leaves. Asian Pac. J. Trop. Dis. 2012, 2, S21–S25. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Yang, W.; Zhang, Z. Characterization of astragaloside I-IV based on the separation of HPTLC from Pleurotus ostreatus cultivated with Astragalus. J. Food Sci. 2020, 85, 3183–3190. [Google Scholar] [CrossRef]
- Lima, C.U.J.O.; Gris, E.F.; Karnikowski, M.G.O. Antimicrobial properties of the mushroom Agaricus blazei—Integrative review. Braz. J. Pharmacogn. 2016, 26, 780–786. [Google Scholar] [CrossRef][Green Version]
- Ong, G.; Kasi, R.; Subramaniam, R. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Ulbin-Figlewicz, N.; Zimoch, A.; Jarmoluk, A. Plant extracts as components of edible antimicrobial protective coatings. Czech J. Food Sci. 2013, 6, 596–600. [Google Scholar] [CrossRef]
- Irkin, R.; Esmer, O.K. Novel food packaging systems with natural antimicrobial agents. J. Food Sci. Technol. 2015, 10, 6095–6111. [Google Scholar] [CrossRef]
- Sudirman, I.L. Partial purification of antimicrobial compounds isolated from mycelia of tropical lentinus cladopus LC4. HAYATI 2010, 17, 63–67. [Google Scholar] [CrossRef]
- Murugesan, S.; Bhuvaneswari, S. HPTLC fingerprint profile of methanol extract of the marine red alga Portieria hornemannii (Lyngbye) (Silva). Int. J. Pharm. Anal. 2016, 3, 2320–4923. [Google Scholar]
- Dwivedi, C.; Pandey, I.; Pandey, H.; Patil, S.; Mishra, S.B.; Pandey, A.C.; Zamboni, P.; Ramteke, P.W.; Singh, A.V. In vivo diabetic wound healing with nanofibrous scaffolds modified with gentamicin and recombinant human epidermal growth factor. J. Biomed. Mater. Res. 2018, 106, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Ansari, M.H.D.; Laux, P.; Luch, A. Micro-nanorobots: Important considerations when developing novel drug delivery platforms. Expert Opin. Drug Del. 2019, 16, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Sitti, M. Targeted drug delivery and imaging using mobile milli/microrobots: A promising future towards theranostic pharmaceutical design. Curr. Pharm. Des. 2016, 22, 1418. [Google Scholar] [CrossRef]
- Singh, A.V.; Maharjan, R.; Kanase, A.; Siewert, K.; Rosenkranz, D.; Singh, R.; Laux, P.; Luch, A. Machine-learning-based approach to decode the influence of nanomaterial properties on their interaction with cells. ACS Appl. Mater. Interfaces 2021, 13, 1943−1955. [Google Scholar] [CrossRef]
- Singh, A.V.; Rosenkranz, D.; Hasan, M.; Ansari, D.; Singh, R.; Kanase, A.; Singh, S.P.; Johnston, B.; Tentschert, J.; Laux, P.; et al. Artificial intelligence and machine learning empower advanced biomedical material design to toxicity prediction. Adv. Intell. Syst. 2020, 2, 2000084. [Google Scholar] [CrossRef]
| Pathogens | Ethanol | Methanol | Antibiotics (Control) | ||||
|---|---|---|---|---|---|---|---|
| 100 mg/mL | 125 mg/mL | 150 mg/mL | 100 mg/mL | 125 mg/mL | 150 mg/mL | - | |
| Zone of Inhibition (mm) | |||||||
| S. aureus | 14.6 ± 0.3 | 17.3 ± 0.3 | 17.6 ± 0.3 | 10.0 ± 0.0 | 11.0 ± 0.0 | 11.6 ± 0.3 | 21.6 ± 0.3 |
| P. aeruginosa | 11.3 ± 0.3 | 12.6 ± 0.3 | 14.3 ± 0.3 | 18.6 ± 0.3 | 19.6 ± 0.3 | 21.6 ± 0.3 | 20.6 ± 0.3 |
| P. mirabilis | 14.6 ± 0.3 | 16.3 ± 0.3 | 19.3 ± 0.3 | 15.6 ± 0.3 | 17.6 ± 0.3 | 19.0 ± 0.0 | 24.3 ± 0.3 |
| P. vulgaris | 23.6 ± 0.3 | 24.6 ± 0.3 | 25.6 ± 0.3 | 16.3 ± 0.3 | 18.6 ± 0.3 | 19.6 ± 0.3 | 33.6 ± 0.3 |
| S. marcescens | 10.0 ± 0.0 | 14.0 ± 0.0 | 14.6 ± 0.3 | 10.0 ± 0.0 | 13.6 ± 0.3 | 15.3 ± 0.3 | 33.6 ± 0.3 |
| S. flexneri | 11.6 ± 0.3 | 13.6 ± 0.3 | 16.3 ± 0.3 | 17.6 ± 0.3 | 19.6 ± 0.3 | 19.6 ± 0.3 | 25.6 ± 0.3 |
| Morexella sp. | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 33.6 ± 0.3 |
| Pathogens | Ethanol | Methanol | ||||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | * MBC/MIC | MIC (mg/mL) | MBC (mg/mL) | * MBC/MIC | |
| S. aureus | 26.03 ± 7.3 | 52.08 ± 14.7 | 2.00 | 52.08 ± 14.7 | 62.5 ± 0.0 | 1.20 |
| P. aeruginosa | 26.03 ± 7.3 | 26.03 ± 7.3 | 1.00 | 31.25 ± 0.0 | 52.08 ± 14.7 | 1.66 |
| P. mirabilis | 15.60 ± 0.0 | 26.03 ± 7.3 | 1.66 | 20.81 ± 7.3 | 52.08 ± 14.7 | 2.50 |
| P. vulgaris | 26.03 ±7.3 | 62.5 ± 0.0 | 2.40 | 31.25 ± 0.0 | 52.08 ± 14.7 | 1.66 |
| S. marcescens | 52.08 ± 14.7 | 52.08 ± 14.7 | 1.00 | 52.08 ± 14.7 | 125.0 ± 0.0 | 2.40 |
| S. flexneri | 52.08 ± 14.7 | 62.5 ± 0.0 | 1.20 | 26.03 ± 7.3 | 52.08 ± 14.7 | 2.00 |
| Moraxella sp. | NA | NA | – | NA | NA | – |
| Mycoconstituents | Mycochemical Tests | Pleurotus opuntiae (Mushroom) | |
|---|---|---|---|
| Ethanol | Methanol | ||
| Alkaloids | Mayer’s test | ++ | + |
| Wagner’s test | ++ | + | |
| Dragendroff’s test | ++ | + | |
| Carbohydrate (Reducing sugar) | Benedict’s test | + | + |
| Barfold’s test | + | + | |
| Diterpenes | Copper acetate test | ++ | + |
| Phytosterol (triterpenes) | Salkowski’s test | − | + |
| Phenols | Ferric chloride test | + | + |
| Flavonoids | Alkaline reagent test | − | − |
| Proteins | Xanthoproteic test | + | + |
| Amino acids | Ninhydrin test | + | + |
| Tannins | Gelatin test | − | − |
| Braymer’s test | − | − | |
| Cardiac glycosides | Keller-Kilian’s test | − | − |
| Anthraquinones | Borntrager’s test | − | − |
| Phlobatamins | - | + | + |
| Terpens | Vanillin-sulfuric acid spray | + | + |
| Saponins | Froth test | + | + |
| Foam test | + | + | |
| Solvent System | Extracts | Rf Values | Active Band | Total Band |
|---|---|---|---|---|
| Visualised in Iodine Chamber | ||||
| 8 mL chloroform + 2 mL hexane | Ethanol | 0.6, 0.3, 0.7 | 3 | 5 |
| Methanol | 0.7, 0.5 | 2 | ||
| after Vanillin + Sulphuric Acid Spray | ||||
| Ethanol | 0.7, 0.4, 0.2 | 3 | 5 | |
| Methanol | 0.7, 0.2 | 2 | ||
| Peak | Start Rf | Start Height | Max Rf | Max Height | Max % | End Rf | End Height | Area | Area % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.02 | 0.5 | 0.03 | 15.7 | 2.46 | 0.04 | 9.6 | 105.1 | 0.83 |
| 2 | 0.04 | 10.2 | 0.06 | 65.0 | 10.18 | 0.09 | 0.2 | 867.6 | 6.87 |
| 3 | 0.10 | 0.2 | 0.11 | 10.5 | 1.64 | 0.12 | 0.1 | 77.3 | 0.61 |
| 4 | 0.14 | 3.6 | 0.18 | 71.9 | 11.24 | 0.19 | 0.2 | 628.2 | 4.98 |
| 5 | 0.23 | 0.3 | 0.27 | 435.2 | 68.09 | 0.33 | 2.4 | 9688.6 | 76.75 |
| 6 | 0.81 | 0.3 | 0.85 | 20.6 | 3.22 | 0.90 | 2.5 | 671.9 | 5.32 |
| 7 | 0.91 | 0.5 | 0.95 | 20.2 | 3.17 | 0.99 | 4.8 | 585.6 | 4.64 |
| Peak | Start Rf | Start Height | Max Rf | Max Height | Max % | End Rf | End Height | Area | Area % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.03 | 0.0 | 0.06 | 16.1 | 36.84 | 0.09 | 0.0 | 175.1 | 16.43 |
| 2 | 0.15 | 3.8 | 0.18 | 12.0 | 27.46 | 0.22 | 2.1 | 364.4 | 34.20 |
| 3 | 0.27 | 4.3 | 0.31 | 15.6 | 35.71 | 0.34 | 2.0 | 526.1 | 49.37 |
| Peak | Start Rf | Start Height | Max Rf | Max Height | Max % | End Rf | End Height | Area | Area % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.00 | 5.2 | 0.03 | 501.3 | 17.05 | 0.06 | 187.9 | 12,608.3 | 13.74 |
| 2 | 0.06 | 188.5 | 0.07 | 221.7 | 7.54 | 0.08 | 210.3 | 3122.3 | 3.40 |
| 3 | 0.08 | 212.3 | 0.09 | 259.3 | 8.82 | 0.11 | 96.8 | 5401.7 | 5.89 |
| 4 | 0.12 | 96.8 | 0.18 | 398.0 | 13.54 | 0.20 | 78.3 | 19,978.2 | 21.77 |
| 5 | 0.20 | 80.3 | 0.21 | 96.3 | 3.28 | 0.23 | 47.9 | 1657.3 | 1.81 |
| 6 | 0.23 | 48.1 | 0.27 | 391.2 | 13.31 | 0.34 | 31.0 | 12,969.8 | 14.13 |
| 7 | 0.37 | 24.2 | 0.41 | 39.9 | 1.36 | 0.42 | 38.1 | 1402.5 | 1.53 |
| 8 | 0.48 | 47.3 | 0.52 | 75.0 | 2.55 | 0.55 | 61.9 | 4264.3 | 4.65 |
| 9 | 0.61 | 60.5 | 0.65 | 74.1 | 2.52 | 0.66 | 72.5 | 3313.7 | 3.61 |
| 10 | 0.67 | 73.6 | 0.77 | 144.6 | 4.92 | 0.79 | 132.9 | 10,724.0 | 11.68 |
| 11 | 0.79 | 133.1 | 0.83 | 225.1 | 7.66 | 0.85 | 170.0 | 9183.0 | 10.01 |
| 12 | 0.85 | 170.1 | 0.88 | 513.4 | 17.46 | 0.89 | 0.0 | 7153.8 | 7.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari Pandey, A.; Pandey, I.; Kanase, A.; Verma, A.; Garcia-Canibano, B.; Dakua, S.P.; Balakrishnan, S.; Singh, M.P. Validating Anti-Infective Activity of Pleurotus Opuntiae via Standardization of Its Bioactive Mycoconstituents through Multimodal Biochemical Approach. Coatings 2021, 11, 484. https://doi.org/10.3390/coatings11040484
Tiwari Pandey A, Pandey I, Kanase A, Verma A, Garcia-Canibano B, Dakua SP, Balakrishnan S, Singh MP. Validating Anti-Infective Activity of Pleurotus Opuntiae via Standardization of Its Bioactive Mycoconstituents through Multimodal Biochemical Approach. Coatings. 2021; 11(4):484. https://doi.org/10.3390/coatings11040484
Chicago/Turabian StyleTiwari Pandey, Aprajita, Ishan Pandey, Anurag Kanase, Amita Verma, Beatriz Garcia-Canibano, Sarada Prasad Dakua, Shidin Balakrishnan, and Mohan Prasad Singh. 2021. "Validating Anti-Infective Activity of Pleurotus Opuntiae via Standardization of Its Bioactive Mycoconstituents through Multimodal Biochemical Approach" Coatings 11, no. 4: 484. https://doi.org/10.3390/coatings11040484
APA StyleTiwari Pandey, A., Pandey, I., Kanase, A., Verma, A., Garcia-Canibano, B., Dakua, S. P., Balakrishnan, S., & Singh, M. P. (2021). Validating Anti-Infective Activity of Pleurotus Opuntiae via Standardization of Its Bioactive Mycoconstituents through Multimodal Biochemical Approach. Coatings, 11(4), 484. https://doi.org/10.3390/coatings11040484