Synthesis of Zinc Oxide Nanomaterials via Sol-Gel Process with Anti-Corrosive Effect for Cu, Al and Zn Metallic Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Modified ZnO-Nanoparticles
2.2. Metallic Coatings
2.3. Characterization Methods
2.3.1. Dynamic Light Scattering Measurements
2.3.2. SEM Microscopy
2.3.3. FTIR Analysis
2.3.4. Contact Angle Measurement
2.3.5. Electrochemical Characterization
2.3.6. Spectroscopic Ellipsometry (SE)
3. Results and Discussion
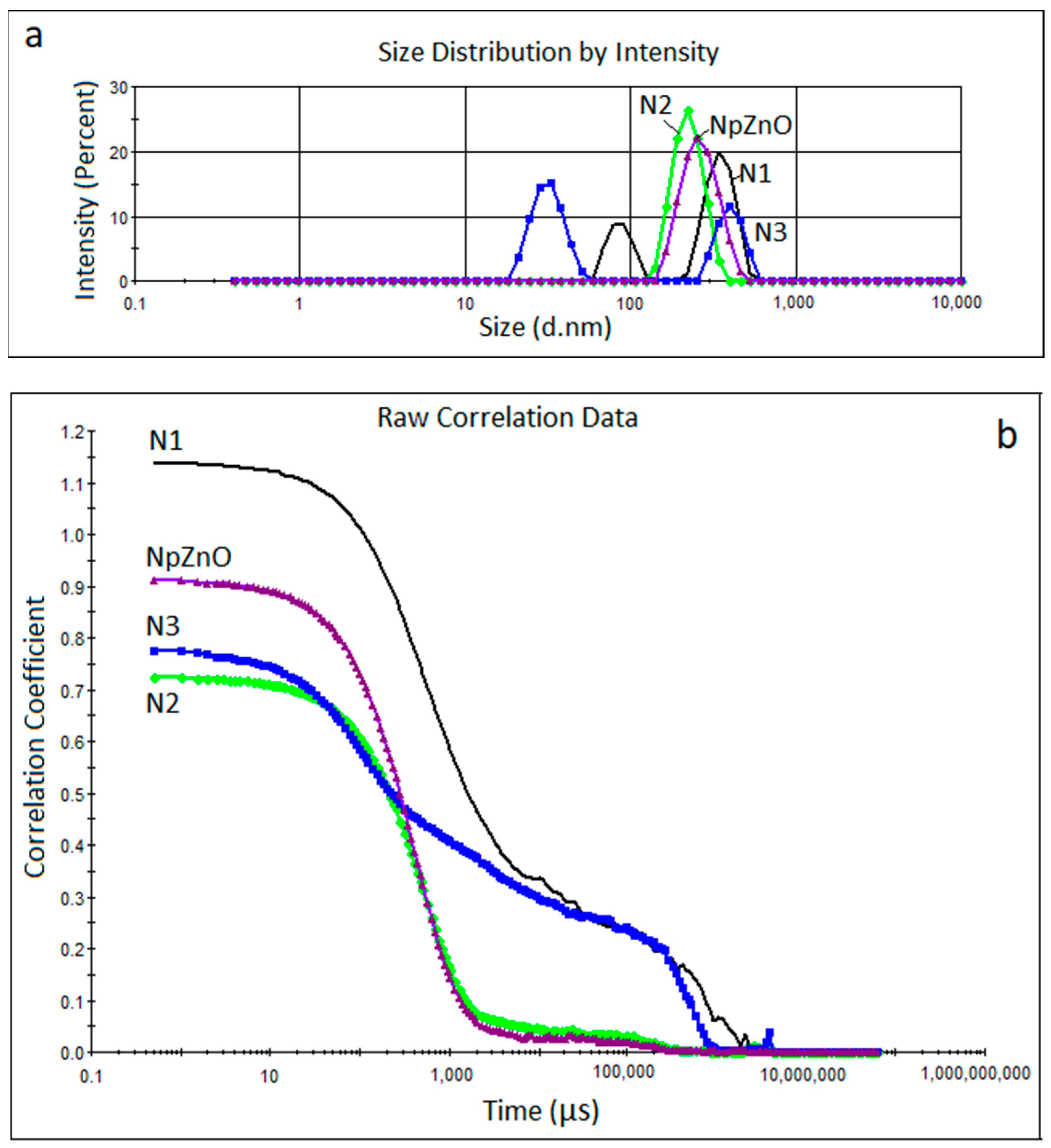
3.1. DLS Measurements
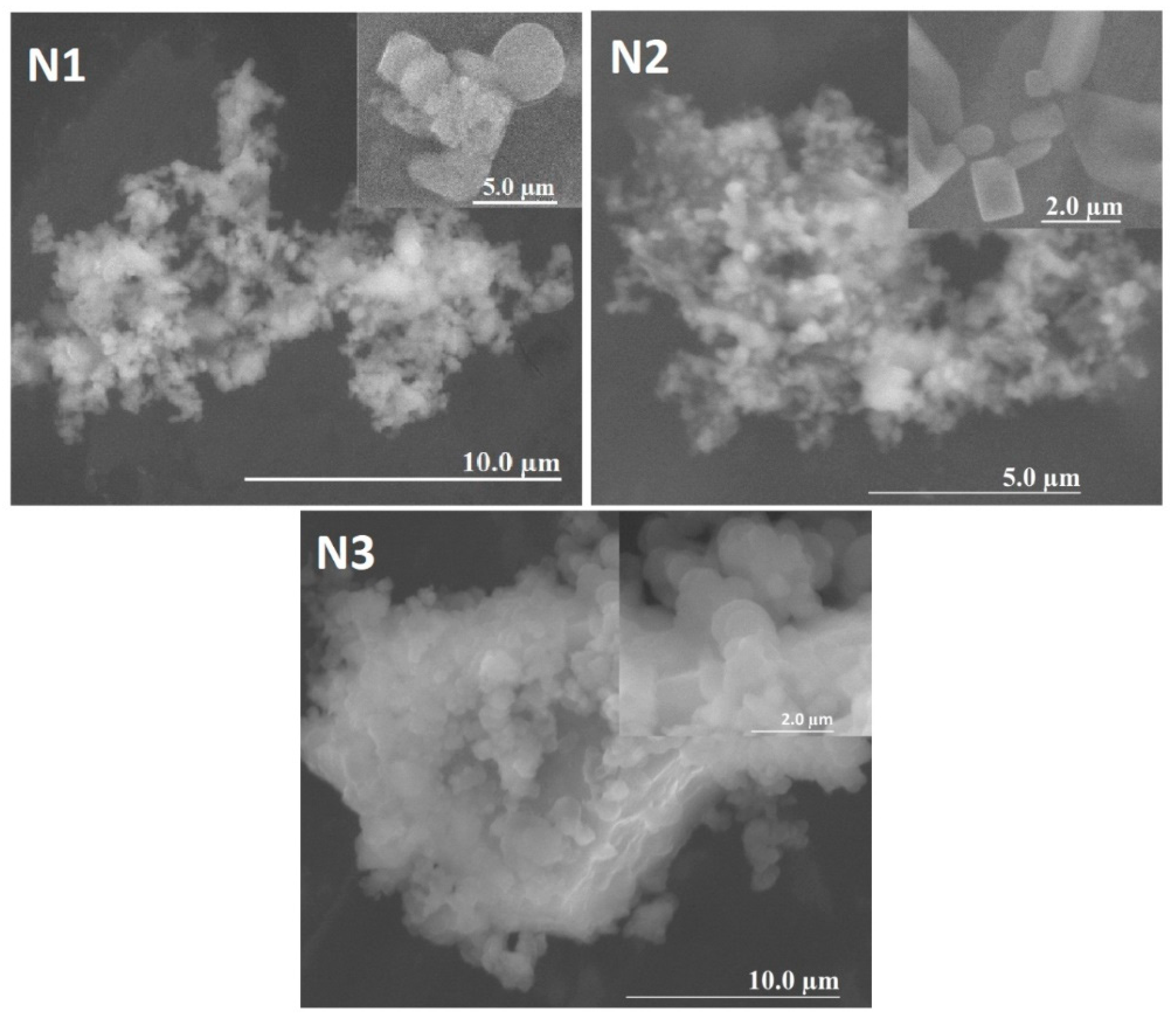
3.2. Morphological Structure
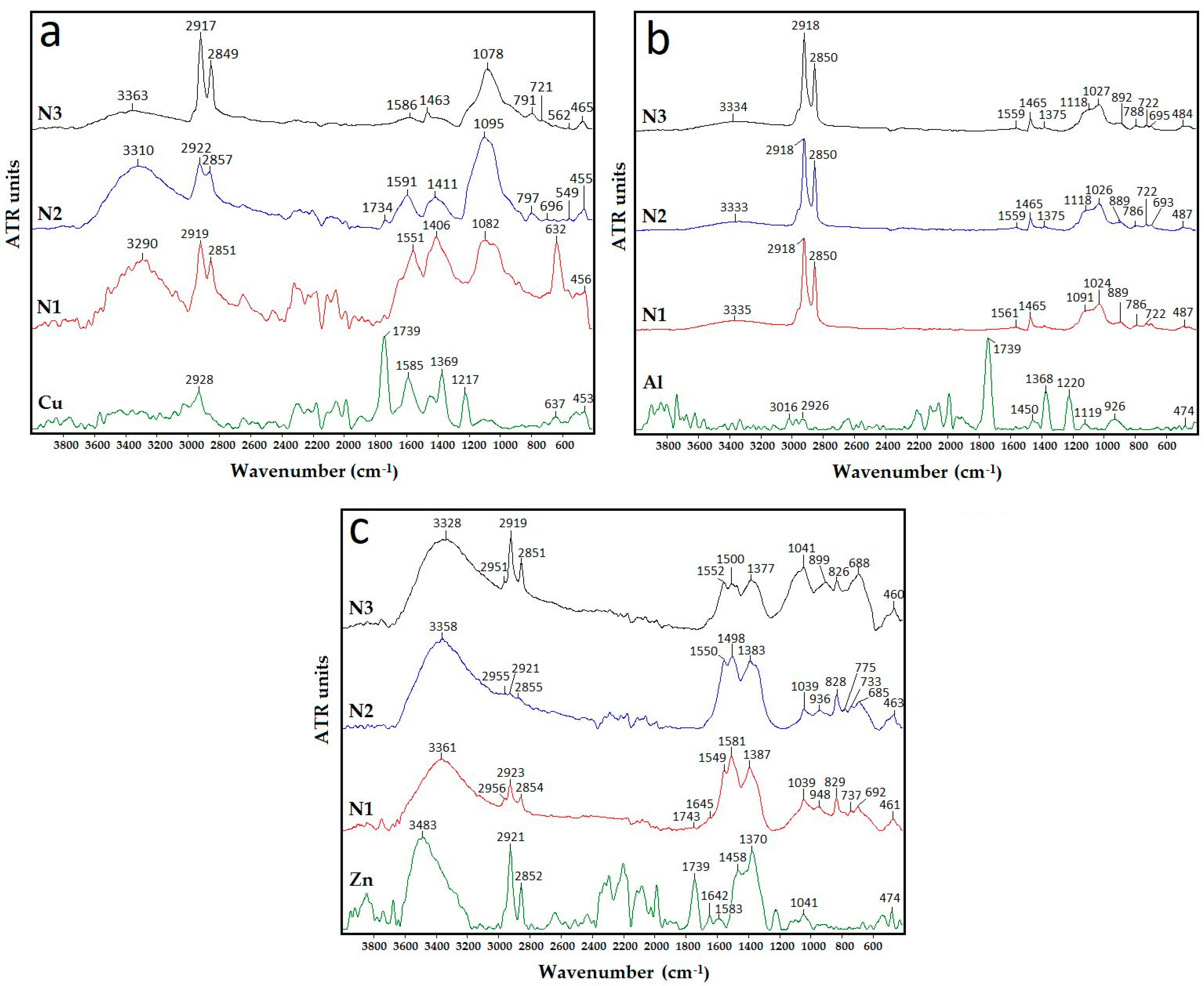
3.3. FTIR Analysis
3.4. Contact Angle Measurements
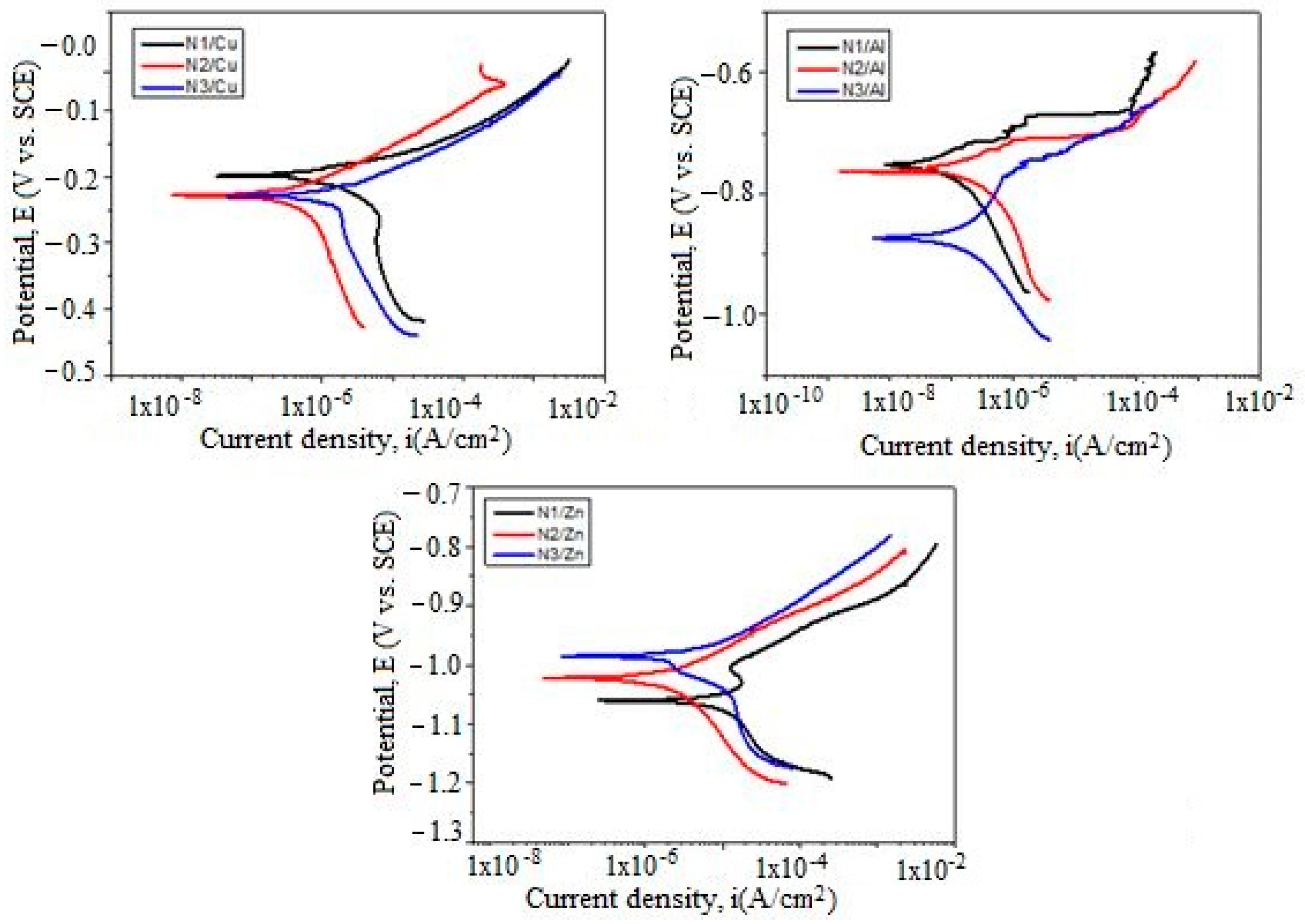
3.5. Potentiodynamic Polarization Measurements
Polarization Resistance (Rp)
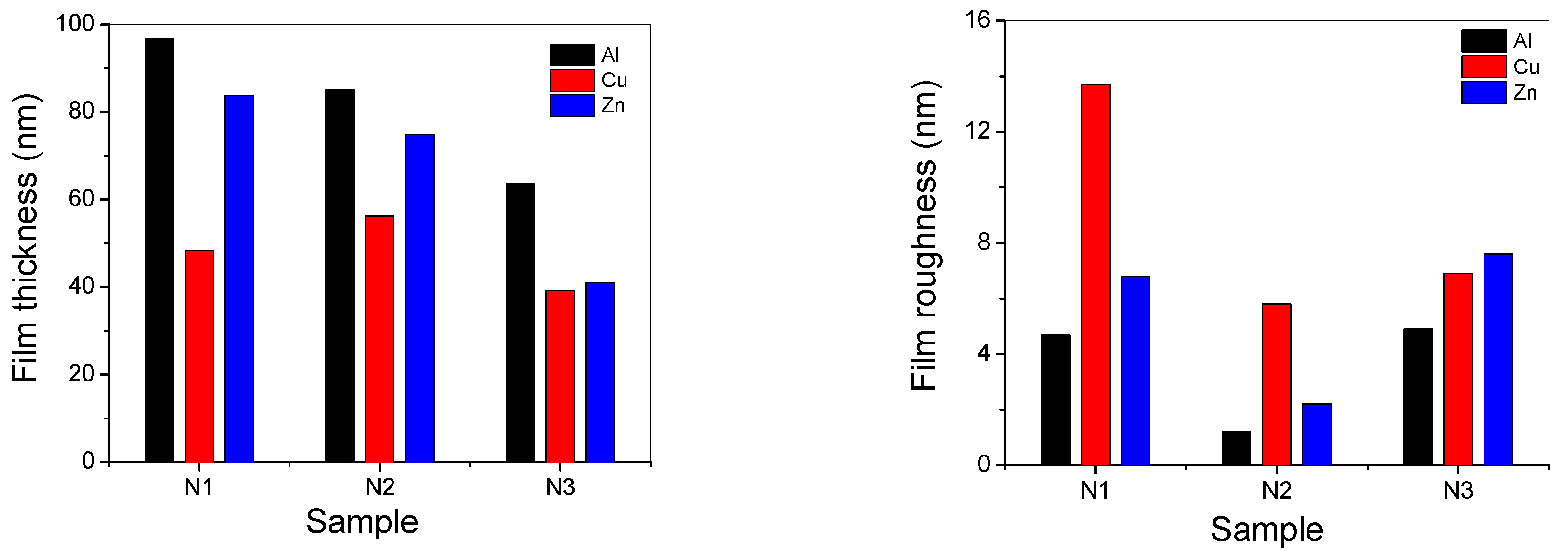
3.6. Films Thickness and Roughness Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41; Springer: Berlin/Heidelberg, Germany, 2020; pp. 83–99. [Google Scholar]
- Czyżowska, A.; Barbasz, A. A review: Zinc oxide nanoparticles—Friends or enemies? Int. J. Environ. Health Res. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aboorvakani, R.; Kennady Vethanathan, S.J.; Madhu, K.U. Influence of zn concentration on zinc oxide nanoparticles and their anti-corrosion property. J. Alloys Compd. 2020, 834, 155078. [Google Scholar] [CrossRef]
- Rivero, P.J.; Maeztu, J.D.; Berlanga, C.; Miguel, A.; Palacio, J.F.; Rodriguez, R. Hydrophobic and corrosion behavior of sol-gel hybrid coatings based on the combination of TIO2 nps and fluorinated chains for aluminum alloys protection. Metals 2018, 8, 1076. [Google Scholar] [CrossRef]
- Rashvand, M.; Ranjbar, Z. Effect of nano-zno particles on the corrosion resistance of polyurethane-based waterborne coatings immersed in sodium chloride solution via eis technique. Prog. Org. Coat. 2013, 76, 1413–1417. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M. Studying the effects of micro and nano sized zno particles on the corrosion resistance and deterioration behavior of an epoxy-polyamide coating on hot-dip galvanized steel. Prog. Org. Coat. 2011, 71, 314–328. [Google Scholar] [CrossRef]
- Fayomi, O.S. Effect of composite particulate reinforcement on the morphology, anti-corrosion and hardness properties of fabricated zn-zno coatings. J. Mater. Environ. Sci. 2015, 6, 963–968. [Google Scholar]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid sol-gel coatings: Smart and green materials for corrosion mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Ou, J.F.; Liu, M.Z.; Li, W.; Wang, F.J.; Xue, M.S.; Li, C.Q. Corrosion behavior of superhydrophobic surfaces of ti alloys in nacl solutions. Appl. Surf. Sci. 2012, 258, 4724–4728. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, T.; Chen, S.; Liu, T.; Cheng, S. Structure stability and corrosion inhibition of super-hydrophobic film on aluminum in seawater. Appl. Surf. Sci. 2008, 255, 2978–2984. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Longhi, M.; Kunsta, S.R.; Beltrami, L.V.R.; Kerstner, E.K.; Silva Filho, C.I.; Sarmento, V.H.V.; Malfatti, C. Effect of tetraethoxy-silane (teos) amounts on the corrosion prevention properties of siloxane-pmma hybrid coatings on galvanized steel substrates. Mater. Res. 2015, 18, 1140–1155. [Google Scholar] [CrossRef]
- Foo, K.L.; Hashim, U.; Muhammad, K.; Voon, C.H. Sol–gel synthesized zinc oxide nanorods and their structural and optical investigation for optoelectronic application. Nanoscale Res. Lett. 2014, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Jurablu, S.; Farahmandjou, M.; Firoozabadi, T.P. Sol-gel synthesis of zinc oxide (zno) nanoparticles: Study of structural and optical properties. J. Sci. Islamic Repub. Iran 2015, 26, 281–285. [Google Scholar]
- Heakal, F.E.-T.; Elkholy, A.E. Gemini surfactants as corrosion inhibitors for carbon steel. J. Mol. Liquids 2017, 230, 395–407. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Jungwirth, S.; Seely, N.; Fang, Y.; Shi, X. The application of anti-corrosion coating for preserving the value of equipment asset in chloride-laden environments: A. Int. J. Electrochem. Sci 2015, 10, 10756–10780. [Google Scholar]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liquids 2017, 233, 403–414. [Google Scholar] [CrossRef]
- El Hamdani, N.; Fdil, R.; Tourabi, M.; Jama, C.; Bentiss, F. Alkaloids extract of retama monosperma (l.) boiss. Seeds used as novel eco-friendly inhibitor for carbon steel corrosion in 1m hcl solution: Electrochemical and surface studies. Appl. Surf. Sci. 2015, 357, 1294–1305. [Google Scholar] [CrossRef]
- Al-Dahiri, R.H.; Turkustani, A.M.; Salam, M.A. The application of zinc oxide nanoparticles as an eco-friendly inhibitor for steel in acidic solution. Int. J. Electrochem. Sci 2020, 15, 442–457. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.K.; Kuhn, S.; Hammad, T.; Hempelmann, R.; Al Bhaisi, S. Synthesis & characterization of silica coated and functionalized silica coated zinc oxide nanomaterials. Powder Technol. 2016, 287, 439–446. [Google Scholar]
- Laurenti, M.; Stassi, S.; Canavese, G.; Cauda, V. Surface engineering of nanostructured zno surfaces. Adv. Mater. Interfaces 2017, 4, 1600758. [Google Scholar] [CrossRef]
- Topala, P.; Ojegov, A.; Besliu, V. Formation of anticorrosive structures and thin films on metal surfaces by applying edm. In Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Zanna, S.; Salmi, E.; Ritala, M.; Marcus, P. Corrosion protection of aluminium by ultra-thin atomic layer deposited alumina coatings. Corros. Sci. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Krishnan, M.A.; Aneja, K.S.; Shaikh, A.; Bohm, S.; Sarkar, K.; Bohm, H.L.M.; Raja, V.S. Graphene-based anticorrosive coatings for copper. RSC Adv. 2018, 8, 499–507. [Google Scholar] [CrossRef]
- Lapeire, L.; Lombardia, E.M.; De Graeve, I.; Terryn, H.; Verbeken, K. Influence of grain size on the electrochemical behavior of pure copper. J. Mater. Sci. 2017, 52, 1501–1510. [Google Scholar] [CrossRef]
- Prosek, T.; Hagström, J.; Persson, D.; Fuertes, N.; Lindberg, F.; Chocholatý, O.; Taxén, C.; Šerák, J.; Thierry, D. Effect of the microstructure of zn-al and zn-al-mg model alloys on corrosion stability. Corros. Sci. 2016, 110, 71–81. [Google Scholar] [CrossRef]
- Goo, B.J.; Lee, B.R.; Moon, M.B.; Oh, S.J. Corrosion properties of Zn alloy coated steel sheet for automotive panels. In Proceedings of the 19th International Corrosion Congress, Jeju, Korea, 2–6 November 2014. [Google Scholar]
- Prosek, T.; Nazarov, A.; Le Gac, A.; Thierry, D. Coil-coated zn–mg and zn–al–mg: Effect of climatic parameters on the corrosion at cut edges. Prog. Org. Coat. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Alwan, R.M.; Kadhim, Q.A.; Sahan, K.M.; Ali, R.A.; Mahdi, R.J.; Kassim, N.A.; Jassim, A.N. Synthesis of zinc oxide nanoparticles via sol–gel route and their characterization. Nanosci. Nanotechnol. 2015, 5, 1–6. [Google Scholar]
- Vasconcelos, D.C.L.; Nunes, E.H.M.; Vasconcelos, W.L. Aes and ftir characterization of sol–gel alumina films. J. Non-Crystalline Solids 2012, 358, 1374–1379. [Google Scholar] [CrossRef]
- Betancourt-Galindo, R.; Reyes-Rodriguez, P.Y.; Puente-Urbina, B.A.; Avila-Orta, C.A.; Rodríguez-Fernández, O.S.; Cadenas-Pliego, G.; Lira-Saldivar, R.H.; García-Cerda, L.A. Synthesis of copper nanoparticles by thermal decomposition and their antimicrobial properties. J. Nanomater. 2014, 2014, 980545. [Google Scholar] [CrossRef]
- Włodarski, M.; Putkonen, M.; Norek, M. Infrared absorption study of zn–s hybrid and zns ultrathin films deposited on porous aao ceramic support. Coatings 2020, 10, 459. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The effect of different coupling agents on nano-zno materials obtained via the sol-gel process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef]
- Álvarez, D.; Collazo, A.; Nóvoa, X.R.; Pérez, C. Assessment of zno nanoparticles as anticorrosive pigment in hybrid sol–gel films. Prog. Org. Coat. 2016, 96, 3–12. [Google Scholar] [CrossRef]
- Ghosh, S.P. Synthesis and Characterization of Zinc Oxide Nanoparticles by Sol-Gel Process. Master’s Thesis, National Institute of Technology, Orissa, India, 2012. [Google Scholar]
- El Saeed, A.M.; Abd El-Fattah, M.; Azzam, A.M. Synthesis of zno nanoparticles and studying its influence on the antimicrobial, anticorrosion and mechanical behavior of polyurethane composite for surface coating. Dyes Pigment. 2015, 121, 282–289. [Google Scholar] [CrossRef]
- Rissi, N.C.; Hammer, P.; Chiavacci, L.A. Surface modification of zno quantum dots by organosilanes and oleic acid with enhanced luminescence for potential biological application. Mater. Res. Express 2017, 4, 015027. [Google Scholar] [CrossRef]
- Gopal, N.O.; Narasimhulu, K.V.; Rao, J.L. Epr, optical, infrared and raman spectral studies of actinolite mineral. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.-Y.; Wu, L.-K.; Hu, J.-M.; Zhang, J.-Q. Silane-incorporated epoxy coatings on aluminum alloy (aa2024). Part 1: Improved corrosion performance. Corros. Sci. 2015, 92, 118–126. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. Use of silane coupling agent for surface modification of zinc oxide as inorganic filler and preparation of poly(amide-imide)/zinc oxide nanocomposite containing phenylalanine moieties. Bull. Mater. Sci. 2012, 35, 333–339. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast fourier transform ir characterization of epoxy gy systems crosslinked with aliphatic and cycloaliphatic eh polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef]
- Tinio, J.V.G.; Simfroso, K.T.; Peguit, A.D.M.V.; Candidato, R.T. Influence of oh− ion concentration on the surface morphology of Zno-SIO2 nanostructure. J. Nanotechnol. 2015, 2015, 686021. [Google Scholar] [CrossRef]
- Petcu, C.; Nistor, C.L.; Purcar, V.; Cinteză, L.O.; Spătaru, C.-I.; Ghiurea, M.; Ianchiş, R.; Anastasescu, M.; Stoica, M. Facile preparation in two steps of highly hydrophobic coatings on polypropylene surface. Appl. Surf. Sci. 2015, 347, 359–367. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A. Comparison of the surface and anticorrosion properties of SIO2 and TIO2 nanoparticle epoxy coatings. Mater. Teh. 2017, 51, 1043–1046. [Google Scholar] [CrossRef]
- Chakradhar, R.P.S.; Kumar, V.D.; Rao, J.L.; Basu, B.J. Fabrication of superhydrophobic surfaces based on zno–pdms nanocomposite coatings and study of its wetting behaviour. Appl. Surf. Sci. 2011, 257, 8569–8575. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.L. Insulating thermal and water-resistant hybrid coating for fabrics. Coatings 2020, 10, 72. [Google Scholar] [CrossRef]
- Dinu, M.; Hauffman, T.; Cordioli, C.; Vladescu, A.; Braic, M.; Hubin, A.; Cotrut, C.M. Protective performance of zr and cr based silico-oxynitrides used for dental applications by means of potentiodynamic polarization and odd random phase multisine electrochemical impedance spectroscopy. Corros. Sci. 2017, 115, 118–128. [Google Scholar] [CrossRef]
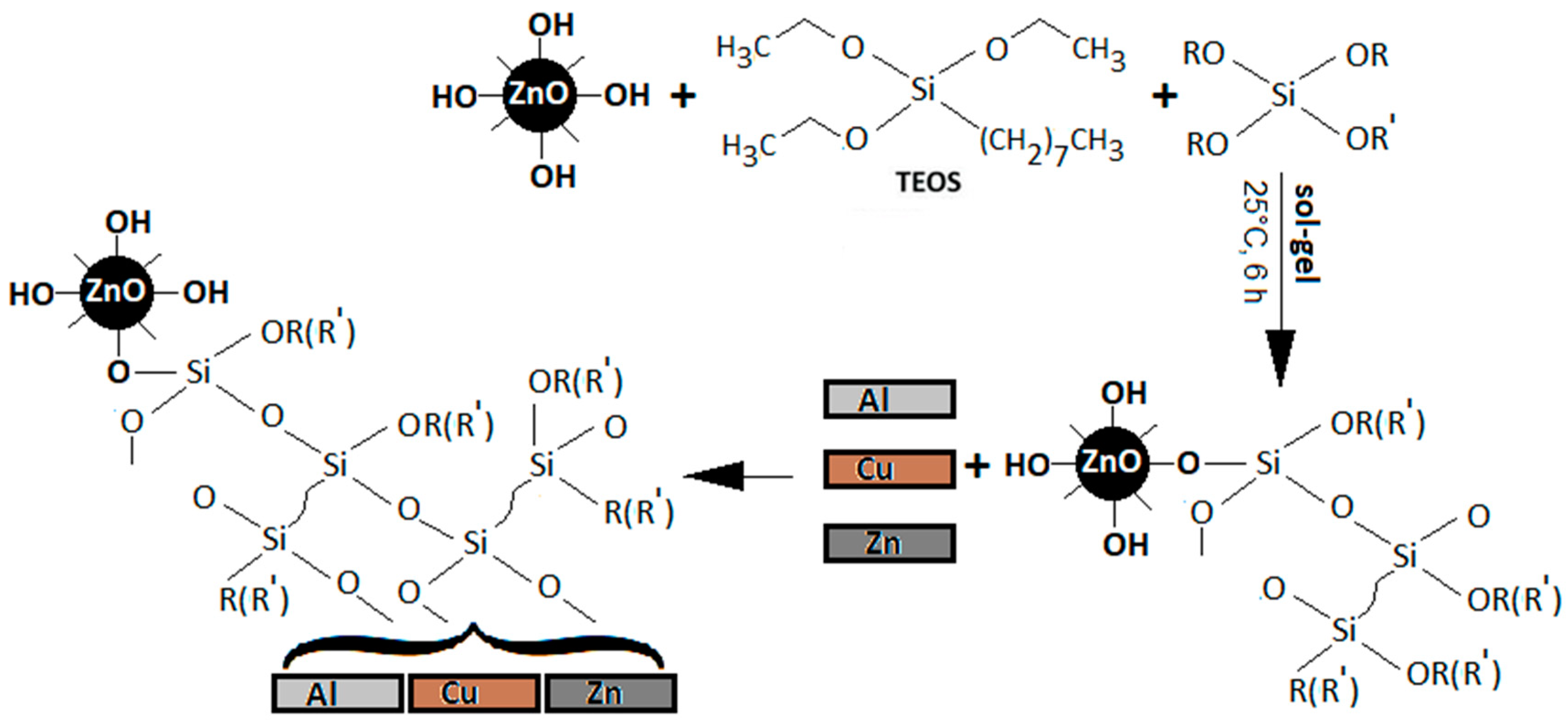

| Sample No. | Silane Precursors | Chemical Formula | |
|---|---|---|---|
| N1 | Octyltriethoxysilane | OTES | 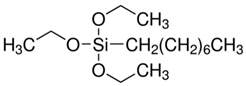 |
| N2 | (3-glycidyloxypropy)trimethoxysilane | GPTMS |  |
| N3 | Octadecyltriethoxysilane | ODTES | 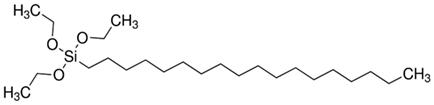 |
| Alkoxysilane Type | Sample No. | Z Average (nm) | P1 (nm) | P2 (nm) | PdI |
|---|---|---|---|---|---|
| - | ZnO | 299 | 268 | - | 0.291 |
| TEOS/OTES | N1 | 437 | 352 | 87 | 0.641 |
| TEOS/GPTMS | N2 | 269 | 226 | - | 0.313 |
| TEOS/ODTES | N3 | 387 | 404 | 32 | 0.573 |
| Metallic Substrate | Sample | Eoc (mV) | Ecorr (mV) | icorr (nA/cm2) | βc (mV) | βa (mV) | Rp (kΩxcm2) |
|---|---|---|---|---|---|---|---|
| Cu | N1 | –222 | –197 | 2.397 | 274.25 | 47.37 | 7.32 |
| N2 | –231 | –227 | 812.97 | 370.30 | 74.61 | 33.21 | |
| N3 | –244 | –229 | 1.935 | 343.13 | 56.79 | 10.94 | |
| Al | N1 | –764 | –755 | 109.66 | 166.60 | 65.84 | 187.10 |
| N2 | –779 | –757 | 130.31 | 145.25 | 37.31 | 99.05 | |
| N3 | –844 | –871 | 155.26 | 124.94 | 115.75 | 168.26 | |
| Zn | N1 | –996 | –1.059 | 5.013 | 97.72 | 95.82 | 4.19 |
| N2 | –1.005 | –1.021 | 2.380 | 156.01 | 70.15 | 8.84 | |
| N3 | –981 | –985 | 5.710 | 242.58 | 80.90 | 4.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somoghi, R.; Purcar, V.; Alexandrescu, E.; Gifu, I.C.; Ninciuleanu, C.M.; Cotrut, C.M.; Oancea, F.; Stroescu, H. Synthesis of Zinc Oxide Nanomaterials via Sol-Gel Process with Anti-Corrosive Effect for Cu, Al and Zn Metallic Substrates. Coatings 2021, 11, 444. https://doi.org/10.3390/coatings11040444
Somoghi R, Purcar V, Alexandrescu E, Gifu IC, Ninciuleanu CM, Cotrut CM, Oancea F, Stroescu H. Synthesis of Zinc Oxide Nanomaterials via Sol-Gel Process with Anti-Corrosive Effect for Cu, Al and Zn Metallic Substrates. Coatings. 2021; 11(4):444. https://doi.org/10.3390/coatings11040444
Chicago/Turabian StyleSomoghi, Raluca, Violeta Purcar, Elvira Alexandrescu, Ioana Catalina Gifu, Claudia Mihaela Ninciuleanu, Cosmin Mihai Cotrut, Florin Oancea, and Hermine Stroescu. 2021. "Synthesis of Zinc Oxide Nanomaterials via Sol-Gel Process with Anti-Corrosive Effect for Cu, Al and Zn Metallic Substrates" Coatings 11, no. 4: 444. https://doi.org/10.3390/coatings11040444
APA StyleSomoghi, R., Purcar, V., Alexandrescu, E., Gifu, I. C., Ninciuleanu, C. M., Cotrut, C. M., Oancea, F., & Stroescu, H. (2021). Synthesis of Zinc Oxide Nanomaterials via Sol-Gel Process with Anti-Corrosive Effect for Cu, Al and Zn Metallic Substrates. Coatings, 11(4), 444. https://doi.org/10.3390/coatings11040444












